Abstract
For patients with coronary artery disease or limb ischemia, placement of a vein graft as a conduit for a bypass is an important and generally durable strategy among the options for arterial reconstructive surgery. Vein grafts adapt to the arterial environment; limited formation of intimal hyperplasia in the vein graft wall is thought to be an important component of successful vein graft adaptation. However, it is also known that abnormal, or uncontrolled, adaptation may lead to abnormal vessel wall remodeling with excessive neointimal hyperplasia, and ultimately vein graft failure and clinical complications. Therefore, understanding the venous-specific pathophysiological and molecular mechanisms of vein graft adaptation are important for clinical vein graft management. Of particular importance, it is currently unknown whether several specific distinct molecular differences in venous mechanisms of adaptation exist that are distinct from arterial post-injury responses; in particular, the participation of the venous marker Eph-B4 and the vascular protective molecule Nogo-B may be involved in mechanisms of vessel remodeling specific to the vein. In this review, we describe 1) venous biology from embryonic development to the mature quiescent state; 2) sequential pathologies of vein graft neointima formation; and 3) novel candidates for strategies of vein graft management. We believe that the scientific inquiry of venous-specific adaptation mechanisms will ultimately provide improvements in vein graft outcomes.
Keywords: vein, vein graft, adaptation, Eph-B4, Nogo-B
Introduction
Vein is the gold standard for vascular graft conduits, particularly in the setting of arterial reconstructive surgery for patients with coronary artery disease and limb ischemia. Compared to other types of graft conduits, the most distinctive property of the vein graft is the adaptive response to the arterial environment during the post-surgical process; this adaptation is thought to be responsible for the superior performance of vein grafts compared to prosthetic grafts. Vein graft adaptation, commonly known as “arterialization,” is characterized by the process known as intimal hyperplasia (IH), i.e. vessel wall thickening with deposition of smooth muscle cells and extracellular matrix in all layers of the vein graft, and especially in the intima. Vascular remodeling in vein grafts is thought to be a normal and necessary response for environmental adaptation, e.g. to the high wall shear stress and stretch force. However, it is also well-known that current reports show 20-50% of vein grafts are eventually complicated by remodeling, culminating in clinical sequelae to the patient, i.e. vein graft thrombosis and failure [1, 2].
Since vein graft adaptation presents similar physiologic characteristics and clinical sequelae when compared to the post-arterial injury response, it has been hypothesized that they share the same underlying mechanism. Even the term “neointimal hyperplasia (NIH)”, usually applied to pathological remodeling after arterial injury, has been extended to pathological excessive venous remodeling. This observation has lead to a number of current therapies in the post-vein graft surgery patient that are identical to those of the arterial injury patient, though clinical trials have not borne out the efficacy of these managements in the treatment of vein graft complications [3, 4]. Thus, it is likely that vein graft adaptation has specific and distinct differences in its molecular mechanisms, e.g. those specific to venous cells, compared to those processes involved in arterial vessel wall remodeling after arterial injury. This suggestion is not fully understood as of yet, unfortunately, but some recent experimental investigations are beginning to clarify these differences. The purpose of this review is to highlight the underlying physiological and molecular events involved in vein graft adaptation, and to give a spotlight to the recent propositions in the treatment of vein graft wall thickening.
Vein Wall Biology
In this section, we draw attention to aspects of normal vein physiology in order to provide a foundation for understanding vein graft adaptation to the arterial environment. Despite the common basic function of arteries and veins in the transportation of blood, there are many differences in their organic background.
Development
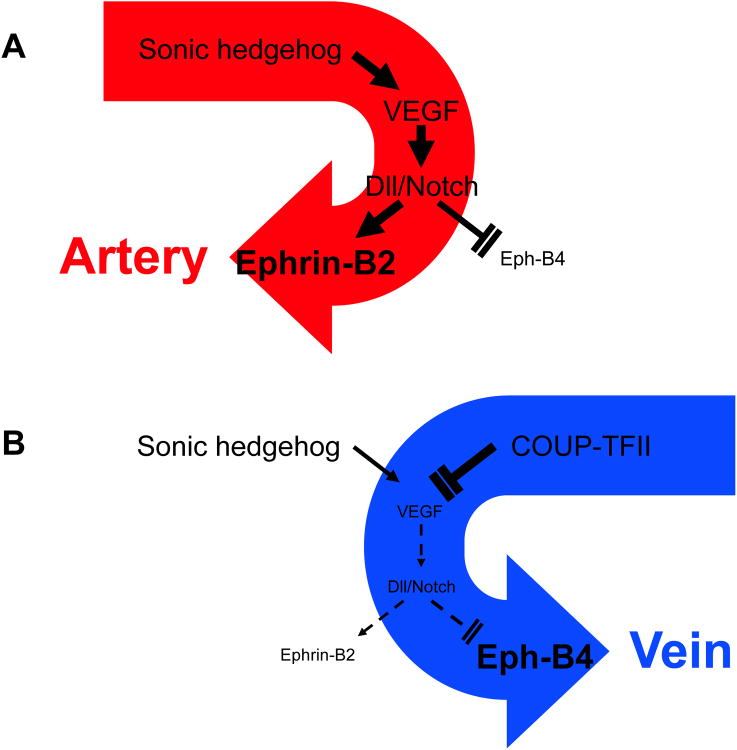
Until the crucial role of transcriptional factor COUP-TFII in embryonic venous development was discovered in 2005 [5], vein was thought to be the default vascular state from which undifferentiated vascular tissue developed, since few distinctive vascular markers had been identified that are specific to the venous lineage [6]. It was known that in the specification stage of embryonic vasculature development, the sonic hedgehog (Shh)-vascular endothelial growth factor (VEGF) cascade, located in arterial endothelial cells, activated the Delta-Notch signaling cascade; this cascade in turn induces Ephrin-B2 expression, which is a distinctive marker of arterial cell fate, and limits Eph-B4 expression, a known venous endothelial marker (Fig. 1A). This cascade was found to be actively blocked by COUP-TFII in the development of veins, inhibiting the VEGF receptor at the initial venous development step. This blockade induces the failure of Delta-Notch activation and Ephrin-B2 expression in venous endothelial cells while simultaneously increases Eph-B4 expression [5, 7] (Fig. 1B). These findings established that environmental factors are not solely responsible for the critical decision of vascular specificity: specific molecular mechanisms are responsible as well.
Fig 1.
Mechanisms of arterial and venous determination during development of the embryonic vasculature. A) In arterial endothelial cells, Sonic hedgehog induces vascular endothelial growth factor (VEGF), which in turn activates Delta-Notch signaling. This cascade induces expression of the arterial-specific marker Ephrin-B2, and simultaneously limits expression of the venous-specific marker Eph-B4. B) In venous endothelial cells, COUP-TFII actively blocks expression of the VEGF receptor, and subsequently expression of the Delta-Notch cascade. The failure of this cascade prevents expression of Ephrin-B2 while simultaneously stimulates Eph-B4 expression.
Morphology and Physiology
The structure of a normal vein wall mimics that of artery as both vessel walls are composed of three distinct layers, the intima, the media, and the adventitia; these three layers are separated by the internal and external elastic layers (Fig. 2). However, in the vein, cellular and fibrous components are significantly limited in number, particularly in the medial and elastic layers, leading to a vessel that is generally thinner than the wall of a comparable anatomic artery. Eph-B4 contributes significantly to this difference in wall thickness, as Eph-B4 limits mural cell recruitment [8], cell proliferation by ERK dephosphorylation [9], and vein wall thickness in mature tissue [10].
Fig 2.
Morphological and physiological features in an artery and a vein. IEL, Internal elastic lamina; EEL, External elastic lamina.
Additionally, veins have a distinctive structure in their lumen: the valve (Fig. 2, right panel). Supporting blood return from peripheral tissues to the cardiac pump, venous valves act to avoid blood reflux in their normal role. However, their presence contributes to impedance of laminar flow through the vein, and to an even greater degree under the increased flow and pressure of the vein graft in the arterial environment. This native disruption in the otherwise cylindrical vascular lumen causes a considerable turbulence in the blood flow that is known to induce vascular endothelial injury, and is thought to be a part of the intimal hyperplasia forming mechanism in arterial interposed vein grafts [11-13].
In their normal physiological environment, the anatomical structure of the vein undergoes constant adaptation to flow and volume (Fig. 2). The venous wall is usually exposed to a low pressure and low flow state; as a consequence, its structure requires high adaptability for a constantly changing volume load. The compliance of the thin venous wall supports this adaptive flexibility to variable local blood volume. Empirically, it is observed that despite a severe disruption of the normal venous blood environment, the vein easily adapts to its new state via a transition of the venous wall structure, as seen in the setting of an arterial vein graft, arteriovenous fistula, or varicose vein. These findings further suggest that the venous structure is actively controlled by molecular elements as well as physiological environments.
The quiescent venous state and important molecules
The main cellular components of a vein wall in its normal state are endothelial cells (EC) and smooth muscle cells (SMC). ECs form a flattened monolayer on the elastic basement membrane, and are thought to play an essential role in venous wall integrity and function. It is well known that healthy ECs produce the vasorelaxant prostacyclin (also known as PGI2) as well as endothelium-derived nitric oxide (NO) [14-16]. PGI2, produced primarily by cyclooxgenase, and NO, produced by nitric oxide synthase (NOS), work in coordinated fashion to prevent platelet activation, adhesion, and aggregation [17, 18]. NO is also known to be a negative conductor for the expression of chemical mediator secretion and inflammatory cell adhesion molecules ICAM-1 and VCAM-1 [19]. These normal EC functions exert an important force in the initial steps of vein graft neointimal formation, as we describe in a later section.
SMCs also play an important role in vessel wall homeostasis. The interaction between SMCs and the extracellular matrix (ECM) promotes a quiescent state in the SMCs in a normal venous environment via TGF-β, heparin, and heparin-like-molecules [14, 20-23]. TGF-β negatively regulates SMC mitogenesis, and stabilizes the ECM, which works as a SMC migration seawall [14, 20]. Heparin is thought to be a neutralizing factor for fibroblast growth factor (FGF), and down-regulates cell proliferation [21-23]. This evidence shows that a quiescent vascular wall state is actively kept by endogenous molecules.
The normal vessel wall is characterized by a low rate of cell turnover. In particular, there are both low rates of cell proliferation and apoptosis. The low rate of cell turnover may promote mechanical vessel integrity during normal environmental conditions; however, after injury, or after a change in flow conditions, vessels can increase their rates of proliferation and apoptosis, reacting to the stimulus.
Algorithm of pathological vein graft wall thickening
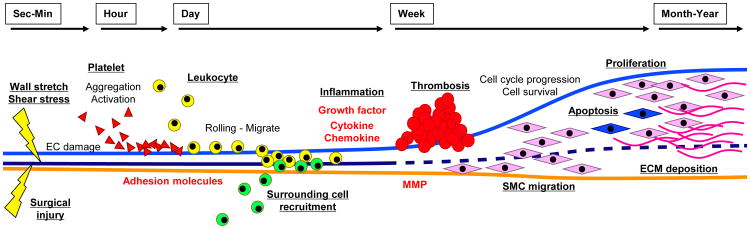
In 2005, Mitra and colleagues reviewed the chronological events in vein graft neointima formation [14]. They broadly classified the events leading to a neointima into 5 steps: 1) platelet activation and correlated events; 2) inflammation with leukocyte recruitment; 3) activation of the coagulation cascade; 4) SMC migration and 5) proliferation. In this section, we describe the pathological algorithm of vein graft adaptation sequentially with their mechanisms and molecular interactions (Fig. 3).
Fig 3.
Time-course of vein graft neointimal formation. EC, endothelial cell; SMC, smooth muscle cell; MMP, matrix metalloproteinase; ECM, extracellular matrix.
Surgical injury
The etiology for all vein graft adaptation is initiated by surgical resection of the vein; the vein is likely to be in a quiescent state prior to surgical harvest. During the vein harvest, the adventitia is damaged when it is disrupted from its blood supply, the vasa vasorum; consequences of this disruption include tissue hypoxia as well as hyponutrition of vessel wall [24]. Vein graft hypoxia leads to the release of the inflammatory cytokines, which have downstream impact in neointimal hyperplasia; IL-6 and IL-8 have been shown to be specifically released in this setting, while other known acute phase reactants have yet to be demonstrated [25]. It has been suggested that the degree of surgical graft adventitial injury would thus be directly related to subsequent graft neointima formation. To avoid adventitial injury, in situ vein graft conduits have been preferentially selected for vascular reconstruction in limb ischemia patients at some institutes [26].
Pulsatile wall stretch and hydrostatic force
When transplanted into an arterial environment, venous tissue is immediately exposed to intense pulsatile stretch forces and wall shear stress. It is known that wall stretch force induces cell apoptosis with subsequent cell proliferation [27]. In some experimental models that supported the vein graft with a vascular external sheath, graft failure was prevented due to reduced direct wall stretch force [28-31]. Data from these physiologic models is bolstered by reports of several molecular interactions induced by this environmental transition. Insulin-like growth factor-1 receptor (IGF-1R) and the tyrosine phosphorylation of its substrate IRS-1 are induced by mechanical stretch, activated Src which is also induced by the same mechanical stretch, and autocrine IGF-1 production [32]. Likewise, SMC derived from human saphenous vein demonstrates ERK phosphorylation, Akt/PKB phosphorylation, and Rho/Rho-kinase activation under cyclic stretch stimulation [33]. These intracellular signaling cascades are known to induce cell phenotype change, induce a transition from the quiescent, or contractile, phenotype, into a synthetic, migrative and proliferative phenotype, and also induce apoptosis, as we describe in a later section.
It is also known that the transition to an arterial environment with its higher wall shear “hydrostatic” stress injures the vein graft endothelium. In early stages of vein graft adaptation, physiological low-concentration NO is known to have an important role for preserving venous identity, and protects the vascular wall from platelet-derived vasoactive substances and SMC relaxation, as well as inflammatory responses [16, 34, 35]. Physiological NO is created by healthy endothelial cells via endothelial NOS (eNOS) at venous levels of shear stress. Under arterial conditions, in which shear stress is comparatively much higher, the venous tissue homeostasis that induced NO production is disrupted due to endothelial cell injury.
The function of NO in limiting vein graft neointimal volume has been confirmed in the rabbit constitutive eNOS overexpression experimental model [36]. On the other hand, highly concentrated pathogenic NO, produced by inducible NOS (iNOS) activation, is known to promote vascular injury and apoptosis via superoxide and peroxinitrite production and subsequently increases vein graft neointima volume [37]. These findings have led to therapeutic experiments in NO manipulation. Recently, the impact of an addition of nitric oxide-donating aspirin (NO-ASA) was examined in a pig vein graft model [38]. The negative neointimal regulation effect of NO-ASA was also confirmed in human saphenous ex vivo experimental model that elicited venous tissue relaxation, cGMP promotion, and SMC proliferation inhibition [39]. Since gastroprotective, antithrombotic, and anti-atherogenic effects were appreciated in the NO-ASA experiments [38], it is thought that this is one of the NO donating applications closest to approaching clinical trials.
Platelet adhesion, aggregation, and activation
An injured endothelium acts as a theater for platelet events quite rapidly, within a minute after vein graft implantation. At the site of endothelial injury, exposed subendothelial matrix following injured endothelium denudation leads to the adherence and aggregation of platelets. Numerous cytokines and other bioactive substances are released by activated platelets [40]; for instance, adenosine diphosphate is known to be released from adhered platelets, and activates the arachidonic acid synthesis pathway which produces thromboxane A2, a potent chemo-attractant and a SMC mitogen [14, 41]. Several other growth factors and cytokines, such as PDGF, TGF-β, IL-1, IL-6, IL-8, and thrombin, are also known to come into play following these events [42]. Therefore, platelet aggregation is thought to be a target for limiting vein graft wall thickening. In a recent report, cilostazol, an inhibitor of cyclic adenosine monophosphate phosphodiesterase III (PDE-III inhibitor), was proposed to have a protective effect for neointimal hyperplasia formation in the rat VG model [43].
Leukocyte recruitment and inflammation
During the subsequent week after implantation, leukocyte recruitment and the inflammatory response play the primary roles in vein graft adaptation. Chronic inflammation in the graft wall is initiated by adherence molecule expression following platelet activation at the injured endothelium site. Leukocytes attach and roll on the endothelial surface while undergoing activation via the interaction of leukocyte surface P-selectin glycoprotein ligand-1 (PSGL-1) and P-selectin on the bound adherent platelet [44, 45]. Activated leukocytes then migrate into the graft vessel wall, an action mediated by adherence molecules such as MCP-1 [46], ICAM [47, 48], Mac-1 [44], and GPIbα [49]. While TGF-β overexpression is sustained until the late phase of vein graft adaptation, MCP-1 works primarily during the early phase of vein graft adaptation and diminishes after 1 week, as observed in an experimental animal model [50]. These findings support the idea that adherence molecule expression and leukocyte recruitment interact significantly, and that these events are essential in the early phase of graft adaptation.
It is known that the degree of leukocyte migration is an important factor in the level of neointimal thickening that a vein graft experiences. Peppel and colleagues showed that 60% of the neointima area of ROSA26 mice vein graft model was derived from cells extrinsic to the graft [51]. They also found that GFP-expressing bone marrow cells transplanted into the C57Bl/6 mouse for recipient showed abundant GFP positive cells in vein graft neointima. Thus, leukocyte migration is thought to be a noteworthy target for vein graft neointimal hyperplasia treatment. Experimentally, by blocking MCP-1 receptor CCR-2 using 7ND-MCP-1 gene transduction in an ApoE3Leiden mouse vein graft model, a 51% volume reduction in neointimal hyperplasia was appreciated [46].
Similarly, recruitment of the surrounding cells and adventitial inflammation also play important roles in neointimal development [14, 52]. Adherence molecule E-selectin has been shown to be induced in the post-injury adventitial layer, and furthermore, mediates subsequent inflammation [53]. Recognition of E-selectin by the cells surround the vein graft is thought to further contribute to the vein graft adaptation; in the ROSA26 transgenic mouse model, isolation of the graft from its surrounding cells resulted in a 90% reduction of smooth muscle cell number in the vein graft neointima [52]. While adherence molecules have been shown to strongly contribute to neointimal hyperplasia, growth factors PDGF-BB and TGF-β1 are also known to influence local adventitial cell accumulation, angiogenesis, ECM deposition, and enhanced generation of reactive oxygen species, and therefore subsequently contribute to neointimal hyperplasia volume [54].
Leukocyte recruitment is accelerated by immune responsive substances secreted from graft wall component cells and the leukocytes themselves. IL-8 is known neutrophil chemoattractant. IL-1, IL-6 and TNF-α, in association with reactive oxygen species, numerous growth factors, and proteolytic enzymes, modulate inflammatory responses [55]. These bioattractive agents play an important role in the late phase of vein graft neointimal formation following adherence molecule expression [50]. The degree of wall shear stress is also known to be a trigger for cytokine secretion. A low shear stress model of vein graft adaptation was observed to have an increased intimal volume due to the secretion of the pro-inflammatory cytokine IL-1β, whereas a high shear stress model was found to limit wall thickening via the secretion of the anti-inflammatory cytokine IL-10 [56].
Given the role of inflammation in the formation of the neointima, suppression of the immune reaction is a theoretical option in the treatment of pathologies associated with neointimal hyperplasia. For example, the vaccinia virus protein 35K, which possesses an anti-inflammatory effect, was analyzed in ex vivo adenoviral transfection; it was discovered to reduce rabbit vein graft neointimal hyperplasia by limiting macrophage infiltration [57]. Flavinoid, which is known to have anti-inflammatory, anti-allergic, and anti-oxidant effects, also attenuates rat vein graft neointima formation through the dowregulation of PDGF-BB and TNF-α [58]. Another immunosuppressive agent, FK778, is known to limit neointimal hyperplasia in the rat VG model [59].
Rapamycin, the most well analyzed immunosuppressive agent, acts via mTOR inhibition in the Akt signaling pathway, and is anticipated to have an anti-proliferation effect clinically. Experimentally, rapamycin was shown to inhibit LPS-dependent TNFα release from human saphenous-vein-derived-SMC in culture [60]. In vivo perivascular rapamycin application by pluronic gel shows dose dependent reduction of neointimal hyperplasia due to decrease inflammatory cell infiltration and increase cell apoptosis [61, 62]. The inflammatory cytokine TNF has a unique mechanism for vein graft neointimal regulation: TNF regulates SMC mitogenesis via complementary co-stimulation with other growth factors during vein graft intimal hyperplasia formation [63]. In a genetic knockout experimental model, TNF receptor-1 amplifies neointimal hyperplasia volume and up-regulates leukocyte adherence molecules such as MCP-1 [64]. On the other hand, TNF signaling via TNF receptor-2 reduces vein graft neointimal hyperplasia by the suppression of adhesion molecule VCAM-1 and attenuation of EC apoptosis [65].
Coagulation cascade and thrombosis
Endothelial injury also activates the coagulation cascade. Thrombus formation at the site of injured vein graft endothelium is well known to contribute not only to promotion of acute phase reactants but also to intimal hyperplasia formation in vein graft. Tissue factor (TF) is the key mediator of coagulation at the site of vessel wall injury when subendothelium is exposed to the circulating blood [14]. TF, a glycoprotein found in vessel walls and mononuclear cells, binds to coagulation factor VII/VIIa to initiate the coagulation cascade. Downstream from VII/VIIa activation, coagulation factor Xa and PDGF release from activated platelets play a role in SMC mitogenesis [66]. Circulating TF is incorporated into the forming thrombus and activates platelets, which accelerates thrombin generation, and is additionally a known SMC proliferation agonist [67, 68]. Thus, studies have been undertaken to inhibit TF function in a rabbit model, and these have demonstrated limitation of vein graft neointima formation [69].
Since vessel wall thrombus induces SMC proliferation, it is thought that anti-coagulant drugs may have potential vein graft wall-limiting effects. For example, local aspirin application in a mouse vein graft model attenuates acute phase graft thrombosis [70]. However, in examination of human coronary grafts, aspirin did not reduce the incidence of long term vein graft failure [71]. Likewise, anticoagulant proteins thrombomodulin and endothelial cell protein C receptor have been shown to reduce early stage vein graft thrombosis, but they do not contribute to long-term prevention of neointimal hyperplasia [72]. Another anticoagulant, Tissue plasminogen activator (tPA), is described as a regulator of ECM remodeling in vein grafts [73]. In one animal model, perivascular treatment of tPA modified ECM gradients and prevented neointimal hyperplasia due to alterations in SMC migration.
The role of growth factors in SMC migration and proliferation
The common result of the multiple forms of vascular injury as described above is an alteration in the character of the vessel wall SMCs . By one week to one month after vein graft implantation, SMCs change phenotype from the quiescent contractile state to the synthetic motile state, and migrate into the neointima from the medial layer [14]. Synthetic state SMCs subsequently undergo rapid proliferation and growth to form the neointimal lesion. Simultaneously, vascular injury itself is known to accelerate cell apoptosis in the neointimal layer. In this mitogenic, synthetic, and apoptotic complex state, numerous extracellular stimuli and intracellular signal transduction cascades are presumed to be involved.
In animal models, several growth factors have been well described in the process of vein graft neointimal formation, i.e. transforming/tumor growth factor-bata (TGF-β, vascular endothelial cell growth factor (VEGF), basic-fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), and platelet derived growth factor (PDGF). The majority of growth factor receptors are cell membrane-type tyrosine kinases that trigger downstream signal cascades including mitogen-activating protein kinase (MAPK) pathway and phosphatidyl-inositol 3 kinase (PI3K) – Akt/PKB pathway. These pathways involve proliferation, survival, differentiation and migration of SMCs, therefore growth factors are thought to be effective targets for controlling vein graft wall thickening.
VEGF is known to be a negative regulator of neointimal formation in vein graft animal models [10, 74]. The mechanism for this regulation is thought to be accelerated re-endothelialization of the injured vessels [75], increased NO production [76], and communication with other growth factor pathways [77]. VEGF is also known to be a key initiator molecule for vascular determination [5, 10]; thus, VEGF manipulation in vein grafts may protect venous-specific biology during adaptation.
IGF-1 is known to play a pivotal role for SMC proliferation in vascular system. In venous SMC, stimulation of stretch force induces expression of IGF-1 and its receptor IGF-1R, and activates downstream intracellular signaling cascades that are both of IGF-1 dependent and independent [32]. Since the IGF-1-PI3K-Akt/PKB cascade regulates SMC cell cycle progression [78, 79], therefore, it may an effective target of pharmacological manipulation for management of vein graft failure.
TGF-β is often described as a positive regulator of neointima formation, consistent with its role in formation and stabilization of the ECM. Anti-sense (blocking) TGF-β1 mRNA prevented collagen accumulation in a rat vein graft model by reducing TIMP-1 (tissue inhibitor of mettaloproteinase-1) and collagen expression, and changing the proportions of the SMC and immune cell type populations [80]. Ex vivo organ culture model of human saphenous vein also demonstrates that a TGF-β1 antagonist reduces collagen content and degree of intimal hyperplasia [81]. Contrarily, Activin-A which is also in the TGF-β super family, induces low SMC proliferation rates and reduces neointimal hyperplasia in rat vein grafts [82].
bFGF also effects positive regulation during vein graft neointimal formation. In a rabbit vein graft model, antisense bFGF coding adenovirus limited neointimal hyperplasia by a ERK-dependent mechanism [83]. PDGF has been shown to accelerate SMC proliferation in both human arteries and veins as well [84]. Positive regulation of SMC proliferation by PDGF is also confirmed in vein graft models. Blockade of PDGF-MAPK-AP1 signaling attenuates neointimal formation in mouse vein grafts [85], and PDGF-R inhibitor reduces cell proliferation and subsequent neointimal hyperplasia in a rabbit model [86]. Midkine, which is a heparin-binding growth factor, has been recently described as a further positive regulator of neointimal hyperplasia via its role in inflammation and cell proliferation; siRNA blockade of its activity has been shown to reduce neointimal hyperplasia in an animal model [87].
Intracellular signaling
Several cytokines and growth factors are involved in varied intracellular signaling cascades during vein graft adaptation; therefore, they are commonly proposed molecules for potential targeted therapy of neointimal hyperplasia. The MAPK signal transduction pathway is a well known part of cellular mitogenic responses, and demonstrates an important role during vein graft adaptation. A canine vein graft model showed that ERK1/2, a key molecule in the MAPK cascade, accelerates medial layer cell proliferation, suppress apoptosis, and induces inflammatory cell infiltration [88]. The MAPK signaling cascade is also known to control MMPs and TIMP-2 expression in vein grafts [89].
The PI3K-Akt/PKB pathway is also important in cell proliferation and additionally contributes to cell survival in the vein graft neointima. Modulation of the PI3K signaling through PTEN, which is downstream regulator of PI3K, prevents PDGF dependent Akt/PKB phosphorylation and limits intimal hyperplasia due to decreased SMC proliferation [90]. PI3K-Akt/PKB is also known to induce p53, a proapoptotic transcriptional factor, and accelerate apoptosis [79, 91, 92]. Overexpression of p53 induces apoptosis of SMCs, and demonstrates improvement graft lumen diameter at moderate-term follow-up in a porcine vein graft model [91]. Perivascular treatment of Rapamycin also accelerates apoptosis in mouse vein grafts, due to PI3K-Akt/PKB signal activation via mTOR inhibition [61]. Additionally, in vitro, SMCs derived from human saphenous vein show higher Akt/PKB phosphorylation rates than arterial SMCs [93]. These findings support Akt's importance in cell survival during venous neointimal formation.
Small guanosine triphosphate-binding proteins (small G protein) have an important role in cytoskeletal dynamics, which are a vital component of cell migration. Statins, commonly used clinically for serum lipid reduction as well as plaque stabilization, are HMG-CoA reductase inhibitors and additionally have vascular protective effects through inhibition of Rho and Rho-associated kinase activity. As described previously, Rho/Rho-kinase are induced under pulsatile stretch conditions in venous tissue, and statins specifically inhibit this sequence and thus reduce SMC proliferation [33]. Limitation of Rho-kinase activity increases eNOS expression as well, and it is thought the part of the mechanism that limits neointimal hyperplasia [94, 95]. Furthermore, simvastatin down-regulates the RhoA/ROCK pathway, and reduces MMP-9 secretion [96]. In 2005, Komori and colleagues found that pravastatin treatment limits growth factor production and neointimal hyperplasia in a rabbit vein graft model [94, 97].
Transcription factors
Vein graft implantation results in a hyperproliferative state where stimulation of growth factors induces accelerated transcription factor activation and subsequent cell cycle entry and protein turnover. Therefore, numerous transcription factors have been investigated as targets for neointimal hyperplasia prevention and treatment. For instance, Rb (retinoblastoma) protein, which inhibits E2F, shows significant reduction of neointimal hyperplasia formation in a human saphenous vein organ culture model [98]. E2F is the first transcription factor targeted in humans to prevent vein graft neointimal proliferation [99]. Ex vivo intravascular application of decoy-ODN to vein grafts was implemented prior to implantation. Although late phase clinical trials resulted in no significant improvement in graft patency in the experimental group in either coronary or peripheral vein grafts [3, 4], this trial has advanced the possibilities of vein graft management.
In the human ex vivo organ culture model, angiotensin II induces MAPK, which activates transcription factor AP-1 (activator protein-1). AP-1 increases connexin-43 expression which positively regulates neointimal hyperplasia in saphenous veins via SMC proliferation and migration [100, 101]. Additionally, endothelin-1 triggers SMC proliferation and acts via endothelin A/B receptors. Decoy oligodeoxynucleotide (ODN) against AP-1 reduces neointimal hyperplasia in a rabbit vein graft model via this endothelin A/B receptor downregulation [102]. Endothelin-1A receptor antagonist treatment reduces neointimal hyperplasia in pig vein graft model as well [103]. Thus, AP-1 may be a future candidate for neointimal hyperplasia prevention [102].
Other transcription factors cause distortion of vascular phenotype in experimental models. p53 null mice have increased neointimal hyperplasia lesions in vein grafts due to accelerated cell proliferation, migration, MMP expression, and reduced apoptosis [92]. c-Jun, a shear-responsive transcription factor, induces SMC proliferation, MMP-2 secretion, and subsequently increases neointima in explanted human saphenous vein and rabbit vein graft model [104]. NFκ-B, which is a positive regulator of neointimal hyperplasia and is activated by TNF [63], is known to control cytokine and adhesion molecule gene expression. NFκ-B treatment of vein grafts by decoy ODN causes suppression of neointima formation in multiple animal models [105, 106]. PPARγ is also described as a transcription factor with potential to limit neointimal hyperplasia, as it is involved with regulation of the cell cycle, cellular senescence, and apoptosis [107].
ECM deposition
During vein graft adaptation, ECM degradation is a necessary process for SMC migration into the neointimal layer. Matrix metalloproteinases (MMPs) are key enzymes in ECM degradation and remodeling in injured vessel walls, therefore inhibition of these molecules is another potential target to limit vein graft neointima formation [108]. For example, via alteration in ECM components, MMP-9 induces SMC proliferative phenotypes and subsequently accelerates neointimal hyperplasia [109, 110]. c-Jun induces MMP-2, which also indirectly stimulates SMC proliferation phenotypes during vein graft adaptation [104]. Furthermore, tissue inhibitor of metalloproteinase-2 (TIMP-2) treatment of vein grafts consistently attenuates neointima formation in a mouse model [111]. Interestingly, in vivo adenoviral MMP-3 transfection reduces rabbit vein graft stenosis and neointimal hyperplasia [112]. Therefore, the functions of MMPs are complex, with overlapping or bidirectional interactions, and comprehensive clarification is necessary for true understanding of the specifics of MMP activity during vein graft wall remodeling.
Novel targets for management of vein graft thickening: Spotlights for the future
Although there are numerous potential experimental treatments for vein graft wall thickening that are successful in animal models, as described above (Table), no accepted strategy for management of vein graft neointimal hyperplasia has translated into clinical use for human patients. This section introduces novel concepts for vein graft neointimal hyperplasia management and cutting edge drug/gene delivery systems.
Table.
Experiments that have Reported Manipulation of Vein Graft Neointimal Hyperplasia.
| Mechanism | Manipulated factor | Vein graft model | Ref. |
|---|---|---|---|
| EC damage, Platelet aggregation, and Thrombosis | |||
| eNOS | eNOS gene | Rabbit | 36 |
| Superoxide | Celiprolol | Rabbit | 37 |
| NO | NO-donating aspirin | Procine | 38 |
| PDE-III | Cilostazol | Rat | 43 |
| Tissue factor | rTFPI * | Rabbit | 69 |
| Thrombosis, EC damage | Aspirin | Mouse | 70 |
| Tissue plasminogen activator | microsperes releasing tPA | Rabbit | 73 |
| Immunoresponse | |||
| MCP-1 | 7ND-MCP-1 | Mouse | 46 |
| ICAM-1 | ICAM-1 knockout mouse | Mouse | 48 |
| TGFβ, MCP-1 (monocyte accumulation) | Lip-Clod ** | Rat | 50 |
| IL-6, IL-8 | N-acetylcysteine | Rat | 25 |
| Chemokine | vaccinia virus protein 35K | Rabbit | 57 |
| Immunoresponse | FK778 | Rat | 59 |
| TNF | TNF receptor knockout mouse | Mouse | 64, 65 |
| TNFα, PDGF-BB | Flavinoid | Rat | 58 |
| Growth factors | |||
| VEGF | recombinalnt VEGF | Rabbit | 74 |
| VEGF - Eph-B4 | VEGF siRNA | Rat | 10 |
| TGFβ | Anti-sense TGFβ | Rat | 80 |
| TGFβ | Activin-A | Rat | 82 |
| bFGF | Anti-sense bFGF | Rabbit | 83 |
| PDGF | Suramin | mouse | 85 |
| PDGF receptor | Imatinib mesylate | Rabbit | 86 |
| Midkine | Midkine siRNA | Rabbit | 87 |
| Intracellular signalings | |||
| ERK-1/2 | ERK-1/2 inhibitor | Canine | 88, 89 |
| mTOR | Rapamycin | Mouse | 61, 62 |
| PTEN | PTEN adenovirus | Canine | 90 |
| Rho | Statin | Rabbit | 94, 97 |
| Transcriptional factors | |||
| p53 | p53 adenovirus | Procine | 91 |
| p53 | p53 knockout mouse | Mouse | 92 |
| AP-1 | AP-1 decoy ODN | Rabbit | 102 |
| cJun | cJun DNAzyme | Rabbit | 104 |
| NFκB | NFκB decoy ODN | Rabbit | 105 |
| NFκB | NFκB decoy ODN | Canine | 106 |
| Extracellular matrix deposition | |||
| MMPs | TIMP-1 adenovirus | Rabbit | 57 |
| MMPs | TIMP-1 plasmid DNA | Mouse | 109 |
| MMPs | TIMP-2 adenovirus | Mouse | 111 |
| MMP-3 | MMP-3 adenovirus | Rabbit | 112 |
| Others | |||
| Nogo-B | Nogo-B adenovirus | Procine | 114 |
| Endothelin | Endothelin-1A receptor antagonist | Procine | 103 |
recombinant human tissue factor pathway inhibitor,
Liposomally encapsulated dichloromethylene bisphosphonate
Novel molecular mechanism involved in vein graft adaptation
As described in the first section, there are molecular differences in the development of the arterial and venous embryonic systems, including varied molecular determinant molecules [5]. In 2007, Kudo and colleagues described involvement of the venous specific molecule Eph-B4 during vein graft adaptation [10]. The authors reported that Eph-B4 is functional not only in embryonic development but also in mature, adult venous tissue. This suggests that VEGF - Delta/Notch - Ephrin-B2/Eph-B4 cascade has some potential to regulate or induce a “venous specific” mechanism of neointimal hyperplasia.
Another molecule, Nogo-B, which was discovered in 2004 to be a molecule that limits arterial injury, and thus is protective for the arterial wall [113], presents an interesting expression pattern in human and rat venous neointimal SMCs [10]. Nogo-B expression is diminished in arteries after wire injury in mouse models. However, the vein graft neointima demonstrates Nogo-B upregulation, particularly in the SMC layer. Though Nogo-B expression in vein grafts and after arterial injury show opposite patterns, overexpression is known to attenuate neointimal hyperplasia of both arterial injury sites and vein grafts [113, 114]. Since specific mechanisms of Nogo-B function are not currently understood, it may be a formidable option to regulate neointimal formation. However, these findings support the hypothesis that there are different mechanisms involved in the formation of arterial and venous neointima.
Gene/Drug delivery for vein graft management
An important concept in the development of potential therapies for vein graft neointimal hyperplasia is the insight that the pathology is localized to the graft itself. As such, development of a local gene/drug delivery system would be a valuable tool, allowing increased local concentrations of drug delivery with minimal systemic side effects. In the past, direct ex vivo transfection or drug treatments have been used for gene or drug delivery, i.e. directly exposing the explanted vein to the drug on the back table of the operating room prior to surgical implantation of the treated vein [3, 4, 99]. The advantage of this method is ease of application, however, the disadvantage is that treatment effects may be short and insufficient. Current animal models of vein grafts most commonly use local reagent delivery by perivascular application with pluronic gel [10, 43, 61, 62, 70] and atelocollagen [87]. Additionally, gelatin hydrogel graft wrapping for bFGF delivery was recently reported with good effect in a rat vein graft model [115]. Nanoparticle and microsphere-mediated drug delivery systems have also been reported in animal vein graft models [73, 86]. These slow release methods might be additional useful options for direct drug delivery to the graft.
The safety and efficacy of gene administration vectors is currently controversial. In most studies, replication-deficient recombinant adenoviral vectors are used for gene transfection in venous tissue. Adenoviral vectors require simple handling techniques, easy viral concentration, and the ability to transfect the gene of interest into quiescent cells. RGD-4C integrin-targeting-peptide inserted adenovirus is being developed to improve efficiency of vascular EC and SMC specific gene delivery [116]. The main disadvantage of adenoviral vector delivery is the transient nature of transfection. The transfected gene is not able to insert itself into the host genome and is thus diluted with cell proliferation. Adeno-associated virus has also been reported as a useful delivery vector, with reduced immunogenicity [117]. Lentivirus vector, which is a member of the retrovirus vector family, has advantages over adenovirus vectors. Lentivirus provides not only high transfective efficiency into quiescent state cells but also permanent expression of the transfected gene, as the target gene is inserted into the host genomic DNA. Third-generation lentivirus, with an improved safety profile over earlier generation lentiviruses, has high transfection efficiency in human venous vascular cells [118], thus it may be the optimal tool for gene transduction into vein grafts.
Conclusion
Since Kunlin first described the use of autogenous veins as grafts in arterial repair in the early 1950's [119, 120], patients with ischemic disease received benefit from veins used as reconstructive conduits for arterial surgery. Over the past 60 years, numerous physiologic and molecular mechanisms of vein graft adaptation have been discovered. However, this complex process has yet to be clarified, and we do not yet have the ability to therapeutically control graft wall thickening. The current progress in vascular biology is exciting; one recent example with potential therapeutic applications, is the recently discovered microRNAs that are involved in the growth and differentiation of vascular SMCs [121]. This development is attracting significant attention by researchers in the field of vascular biology [122, 123] and their role in vein graft adaptation will likely be clarified in the near future.
For the improvement of vein graft clinical outcomes, it may be necessary to regulate both negative and positive wall thickening. The proper balance of positive and negative remodeling may be one of the most difficult clinical problems in understanding vein graft adaptation, as it is critical to balance the outward adaptation necessary to carry arterial blood flow to the distal tissues with the inward forces preventing vein graft deterioration and failure. As such, we believe that study of the underlying venous-specific mechanisms of remodeling is critical. And it is hoped that this scientific inquiry will be able to provide improved care and quality of life for patients with vascular disease.
Acknowledgments
This review study was supported in part by the National Institute of Health grant R01-HL095498-01, the American Vascular Association William J. von Liebig Award, as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, Conn
References
- 1.Veith FJ, Gupta SK, Ascer E, White-Flores S, Samson RH, Scher LA, Towne JB, Bernhard VM, Bonier P, Flinn WR, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986 Jan;3(1):104–14. doi: 10.1067/mva.1986.avs0030104. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996 Sep;28(3):616–26. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 3.Conte MS, et al. Results of PREVENT III: A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 4.Alexander JH, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 5.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005 May 5;435(7038):98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 6.Adams RH. Molecular control of arterial-venous blood vessel identity. J Anat. 2003 Jan;202(1):105–12. doi: 10.1046/j.1469-7580.2003.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009 Mar 13;104(5):576–88. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 8.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006 Jan 13;124(1):161–73. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002 Jul;3(7):475–86. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 10.Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, Westvik TS, Frattini JC, Breuer CK, Cha CH, Nishibe T, Tellides G, Sessa WC, Dardik A. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. 2007 Jul;27(7):1562–71. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- 11.Vesti BR, Primozich J, Bergelin RO, Strandness E., Jr Follow-up of valves in saphenous vein bypass grafts with duplex ultrasonography. J Vasc Surg. 2001 Feb;33(2):369–74. doi: 10.1067/mva.2001.111744. [DOI] [PubMed] [Google Scholar]
- 12.Singh RN. Flow disturbance due to venous valves: a cause of graft failure. Cathet Cardiovasc Diagn. 1986;12(1):35–8. doi: 10.1002/ccd.1810120109. [DOI] [PubMed] [Google Scholar]
- 13.Lajos TZ, Robicsek F, Thubrikar M, Urschel H. Improving patency of coronary conduits “valveless” veins and/or arterial grafts. J Card Surg. 2007 Mar-Apr;22(2):170–7. doi: 10.1111/j.1540-8191.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- 14.Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006 Apr;84(2):115–24. doi: 10.1111/j.1440-1711.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- 15.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11-17;327(6122):524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 16.Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep. 1999 Aug;19(4):235–51. doi: 10.1023/a:1020537506008. [DOI] [PubMed] [Google Scholar]
- 17.Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–46. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–8. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 19.Bath PM, Hassall DG, Gladwin AM, Palmer RM, Martin JF. Nitric oxide and prostacyclin. Divergence of inhibitory effects on monocyte chemotaxis and adhesion to endothelium in vitro. Arterioscler Thromb. 1991 Mar-Apr;11(2):254–60. doi: 10.1161/01.atv.11.2.254. [DOI] [PubMed] [Google Scholar]
- 20.Reilly CF, McFall RC. Platelet-derived growth factor and transforming growth factor-beta regulate plasminogen activator inhibitor-1 synthesis in vascular smooth muscle cells. J Biol Chem. 1991;266:9419–27. [PubMed] [Google Scholar]
- 21.Snow AD, Bolender RP, Wight TN, Clowes AW. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990;137:313–30. [PMC free article] [PubMed] [Google Scholar]
- 22.Clowes AW, Karnowsky MJ. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977;265:625–6. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- 23.Lindner V, Olson NE, Clowes AW, Reidy MA. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest. 1992;90:2044–9. doi: 10.1172/JCI116085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGeachie JK, Meagher S, Prendergast FJ. Vein-to-artery grafts: the long-term development of neo-intimal hyperplasia and its relationship to vasa vasorum and sympathetic innervation. Aust NZJ Surg. 1989;59:59–65. doi: 10.1111/j.1445-2197.1989.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 25.de Graaf R, Tintu A, Stassen F, Kloppenburg G, Bruggeman C, Rouwet E. N-acetylcysteine prevents neointima formation in experimental venous bypass grafts. Br J Surg. 2009 Aug;96(8):941–50. doi: 10.1002/bjs.6659. [DOI] [PubMed] [Google Scholar]
- 26.Conte MS. Technical factors in lower-extremity vein bypass surgery: how can we improve outcomes? Semin Vasc Surg. 2009 Dec;22(4):227–33. doi: 10.1053/j.semvascsurg.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Liu SQ, Ruan YY, Tang D, Li YC, Goldman J, Zhong L. A possible role of initial cell death due to mechanical stretch in the regulation of subsequent cell proliferation in experimental vein grafts. Biomech Model Mechanobiol. 2002 Jun;1(1):17–27. doi: 10.1007/s10237-002-0003-2. [DOI] [PubMed] [Google Scholar]
- 28.Angelini GD, Lloyd C, Bush R, Johnson J, Newby AC. An external, oversized, porous polyester stent reduces vein graft neointima formation, cholesterol concentration, and vascular cell adhesion molecule 1 expression in cholesterol-fed pigs. J Thorac Cardiovasc Surg. 2002 Nov;124(5):950–6. doi: 10.1067/mtc.2002.127004. [DOI] [PubMed] [Google Scholar]
- 29.Dashwood MR, Angelini GD, Wan S, Yim A, Mehta D, Izzat MB, Jeremy JY. Does external stenting reduce procine vein-graft occlusion via an action on vascular nerves? J Card Surg. 2002 Nov-Dec;17(6):556–60. doi: 10.1046/j.1540-8191.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- 30.Jeremy JY, Bulbulia R, Johnson JL, Gadsdon P, Vijayan V, Shukla N, Smith FC, Angelini GD. A bioabsorbable (polyglactin), nonrestrictive, external sheath inhibits porcine saphenous vein graft thickening. J Thorac Cardiovasc Surg. 2004 Jun;127(6):1766–72. doi: 10.1016/j.jtcvs.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 31.Vijayan V, Smith FC, Angelini GD, Bulbulia RA, Jeremy JY. External supports and the prevention of neointima formation in vein grafts. Eur J Vasc Endovasc Surg. 2002 Jul;24(1):13–22. doi: 10.1053/ejvs.2002.1676. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol. 2007 Aug;27(8):1744–51. doi: 10.1161/ATVBAHA.107.147371. [DOI] [PubMed] [Google Scholar]
- 33.Kozai T, Eto M, Yang Z, Shimokawa H, Lüscher TF. Statins prevent pulsatile stretch-induced proliferation of human saphenous vein smooth muscle cells via inhibition of Rho/Rho-kinase pathway. Cardiovasc Res. 2005 Dec 1;68(3):475–82. doi: 10.1016/j.cardiores.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995 Mar 1;91(5):1314–9. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 35.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004 Jun 11;94(11):1408–17. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 36.Ohta S, Komori K, Yonemitsu Y, Onohara T, Matsumoto T, Sugimachi K. Intraluminal gene transfer of endothelial cell-nitric oxide synthase suppresses intimal hyperplasia of vein grafts in cholesterol-fed rabbit: a limited biological effect as a result of the loss of medial smooth muscle cells. Surgery. 2002 Jun;131(6):644–53. doi: 10.1067/msy.2002.124878. [DOI] [PubMed] [Google Scholar]
- 37.Hattori K, Yamanouchi D, Banno H, Kobayashi M, Yamamoto K, Kajikuri J, Itoh T, Komori K. Celiprolol reduces the intimal thickening of autogenous vein grafts via an enhancement of nitric oxide function through an inhibition of superoxide production. J Vasc Surg. 2007 Jul;46(1):116–23. doi: 10.1016/j.jvs.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 38.Wan S, Shukla N, Angelini GD, Yim AP, Johnson JL, Jeremy JY. Nitric oxide-donating aspirin (NCX 4016) inhibits neointimal thickening in a pig model of saphenous vein-carotid artery interposition grafting: a comparison with aspirin and morpholinosydnonimine (SIN-1) J Thorac Cardiovasc Surg. 2007 Oct;134(4):1033–9. doi: 10.1016/j.jtcvs.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Shukla N, Angelini GD, Ascione R, Talpahewa S, Capoun R, Jeremy JY. Nitric oxide donating aspirins: novel drugs for the treatment of saphenous vein graft failure. Ann Thorac Surg. 2003 May;75(5):1437–42. doi: 10.1016/s0003-4975(02)04892-0. [DOI] [PubMed] [Google Scholar]
- 40.Lee MS, David EM, Makkar RR, Wilentz JR. Molecular and cellular basis of restenosis after percutaneous coronary intervention: the intertwining roles of platelets, leucocytes, and the coagulation-fibrinolysis system. J Pathol. 2004;203:861–70. doi: 10.1002/path.1598. [DOI] [PubMed] [Google Scholar]
- 41.Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994;81:1254–69. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 42.Ishiwata S, Tukada T, Nakanishi S, Nishiyama S, Seki A. Postangioplasty restenosis: platelet activation and the coagulation-fibrinolysis system as possible factors in the pathogenesis of restenosis. Am Heart J. 1997;133:387–92. doi: 10.1016/s0002-8703(97)70178-9. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto K, Onoda K, Sawada Y, Fujinaga K, Imanaka-Yoshida K, Yoshida T, Shimpo H. Locally applied cilostazol suppresses neointimal hyperplasia and medial thickening in a vein graft model. Ann Thorac Cardiovasc Surg. 2007 Oct;13(5):322–30. [PubMed] [Google Scholar]
- 44.Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–57. [PubMed] [Google Scholar]
- 45.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leucocyte recruitment. J Clin Invest. 1997;100:S97–103. [PubMed] [Google Scholar]
- 46.Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, de Vries CJ, Egashira K, van Bockel JH, Quax PH. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006 Sep;26(9):2063–9. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- 47.Diacovo TG, deFougerolles AR, Bainton DF, Springer TA. A functional integrin ligand on the surface of platelets: intercellular adhesion molecule-2. J Clin Invest. 1994;94:1243–51. doi: 10.1172/JCI117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou Y, Hu Y, Mayr M, Dietrich H, Wick G, Xu Q. Reduced neointima hyperplasia of vein bypass grafts in intercellular adhesion molecule-1-deficient mice. Circ Res. 2000 Mar 3;86(4):434–40. doi: 10.1161/01.res.86.4.434. [DOI] [PubMed] [Google Scholar]
- 49.Simon DI, Chen Z, Xu H, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leucocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolff RA, Tomas JJ, Hullett DA, Stark VE, van Rooijen N, Hoch JR. Macrophage depletion reduces monocyte chemotactic protein-1 and transforming growth factor-beta1 in healing rat vein grafts. J Vasc Surg. 2004 Apr;39(4):878–88. doi: 10.1016/j.jvs.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Freedman NJ, Brian L, Peppel K. Graft-extrinsic cells predominate in vein graft arterialization. Arterioscler Thromb Vasc Biol. 2004 Mar;24(3):470–6. doi: 10.1161/01.ATV.0000116865.98067.31. [DOI] [PubMed] [Google Scholar]
- 52.Fogelstrand P, Osterberg K, Mattsson E. Reduced neointima in vein grafts following a blockage of cell recruitment from the vein and the surrounding tissue. Cardiovasc Res. 2005 Aug 1;67(2):326–32. doi: 10.1016/j.cardiores.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 53.Gotoh R, Suzuki J, Kosuge H, et al. E-selectin blockade decreases adventitial inflammation and attenuates intimal hyperplasia in rat carotid arteries after balloon injury. Arterioscler Thromb Vasc Biol. 2004;24:2063–8. doi: 10.1161/01.ATV.0000145942.31404.20. [DOI] [PubMed] [Google Scholar]
- 54.Siow RC, Churchman AT. Adventitial growth factor signalling and vascular remodelling: potential of perivascular gene transfer from the outside-in. Cardiovasc Res. 2007 Sep 1;75(4):659–68. doi: 10.1016/j.cardiores.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Wainwright CL, Miller AM, Wadsworth RM. Inflammation as a key event in the development of neointima following vascular balloon injury. Clin Exp Pharmacol Physiol. 2001;28:891–5. doi: 10.1046/j.1440-1681.2001.03543.x. [DOI] [PubMed] [Google Scholar]
- 56.Jiang Z, Berceli SA, Pfahnl CL, Wu L, Goldman D, Tao M, Kagayama M, Matsukawa A, Ozaki CK. Wall shear modulation of cytokines in early vein grafts. J Vasc Surg. 2004 Aug;40(2):345–50. doi: 10.1016/j.jvs.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 57.Puhakka HL, Turunen P, Gruchala M, Bursill C, Heikura T, Vajanto I, Greaves DR, Channons K, Ylä-Herttuala S. Effects of vaccinia virus anti-inflammatory protein 35K and TIMP-1 gene transfers on vein graft stenosis in rabbits. In Vivo. 2005 May-Jun;19(3):515–21. [PubMed] [Google Scholar]
- 58.Cayci C, Wahlquist TC, Seckin SI, Ozcan V, Tekinay AB, Martens TP, Oz MC, Ascherman JA. Naringenin inhibits neointimal hyperplasia following arterial reconstruction with interpositional vein graft. Ann Plast Surg. 2010 Jan;64(1):105–13. doi: 10.1097/SAP.0b013e31819b03cd. [DOI] [PubMed] [Google Scholar]
- 59.de Graaf R, Kloppenburg G, Tintu A, Rouwet E, Kitslaar P, van Hooff J, Bruggeman C, Stassen F. The new immunosuppressive agent FK778 attenuates neointima formation in an experimental venous bypass graft model. Vascul Pharmacol. 2009 Mar-Apr;50(3-4):83–8. doi: 10.1016/j.vph.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Adkins JR, Castresana MR, Wang Z, Newman WH. Rapamycin inhibits release of tumor necrosis factor-alpha from human vascular smooth muscle cells. Am Surg. 2004 May;70(5):384–7. 387–8. discussion. [PubMed] [Google Scholar]
- 61.Schachner T, Oberhuber A, Zou Y, Tzankov A, Ott H, Laufer G, Bonatti J. Rapamycin treatment is associated with an increased apoptosis rate in experimental vein grafts. Eur J Cardiothorac Surg. 2005 Feb;27(2):302–6. doi: 10.1016/j.ejcts.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Schachner T, Zou Y, Oberhuber A, Tzankov A, Mairinger T, Laufer G, Bonatti JO. Local application of rapamycin inhibits neointimal hyperplasia in experimental vein grafts. Ann Thorac Surg. 2004 May;77(5):1580–5. doi: 10.1016/j.athoracsur.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Peppel K, Zhang L, Orman ES, Hagen PO, Amalfitano A, Brian L, Freedman NJ. Activation of vascular smooth muscle cells by TNF and PDGF: overlapping and complementary signal transduction mechanisms. Cardiovasc Res. 2005 Feb 15;65(3):674–82. doi: 10.1016/j.cardiores.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Peppel K, Brian L, Chien L, Freedman NJ. Vein graft neointimal hyperplasia is exacerbated by tumor necrosis factor receptor-1 signaling in graft-intrinsic cells. Arterioscler Thromb Vasc Biol. 2004 Dec;24(12):2277–83. doi: 10.1161/01.ATV.0000147766.68987.0d. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Sivashanmugam P, Wu JH, Brian L, Exum ST, Freedman NJ, Peppel K. Tumor necrosis factor receptor-2 signaling attenuates vein graft neointima formation by promoting endothelial recovery. Arterioscler Thromb Vasc Biol. 2008 Feb;28(2):284–9. doi: 10.1161/ATVBAHA.107.151613. [DOI] [PubMed] [Google Scholar]
- 66.Bretschneider E, Braun M, Fischer A, Wittpoth M, Glusa E, Schrör K. Factor Xa acts as a PDGF-independent mitogen in human vascular smooth muscle cells. Thromb Haemost. 2000 Sep;84(3):499–505. [PubMed] [Google Scholar]
- 67.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–22. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 68.Giesen PL, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci USA. 1999;96:2311–15. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huynh TT, Davies MG, Thompson MA, Ezekowitz MD, Hagen P, Annex BH. Local treatment with recombinant tissue factor pathway inhibitor reduces the development of intimal hyperplasia in experimental vein grafts. J Vasc Surg. 2001;33:400–407. doi: 10.1067/mva.2001.111989. [DOI] [PubMed] [Google Scholar]
- 70.Torsney E, Mayr U, Zou Y, Thompson WD, Hu Y, Xu Q. Thrombosis and neointima formation in vein grafts are inhibited by locally applied aspirin through endothelial protection. Circ Res. 2004 Jun 11;94(11):1466–73. doi: 10.1161/01.RES.0000129570.06647.00. [DOI] [PubMed] [Google Scholar]
- 71.Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Kern KB, Sethi G, Sharma GV, Khuri S. Long-term graft patency (3 years) after coronary artery surgery. Effects of aspirin: results of a VA Cooperative study. Circulation. 1994 Mar;89(3):1138–43. doi: 10.1161/01.cir.89.3.1138. [DOI] [PubMed] [Google Scholar]
- 72.Kim AY, Walinsky PL, Kolodgie FD, Bian C, Sperry JL, Deming CB, Peck EA, Shake JG, Ang GB, Sohn RH, Esmon CT, Virmani R, Stuart RS, Rade JJ. Early loss of thrombomodulin expression impairs vein graft thromboresistance: implications for vein graft failure. Circ Res. 2002 Feb 8;90(2):205–12. doi: 10.1161/hh0202.105097. [DOI] [PubMed] [Google Scholar]
- 73.Amabile PG, Wang DS, Kao EY, Lee J, Elkins CJ, Yuksel E, Hilfiker PR, Waugh JM, Dake MD. Directed migration of smooth muscle cells to engineer plaque-resistant vein grafts. J Endovasc Ther. 2005 Dec;12(6):667–75. doi: 10.1583/04-1268Ra.1. [DOI] [PubMed] [Google Scholar]
- 74.Luo Z, Asahara T, Tsurumi Y, Isner JM, Symes JF. Reduction of vein graft intimal hyperplasia and preservation of endothelium-dependent relaxation by topical vascular endothelial growth factor. J Vasc Surg. 1998 Jan;27(1):167–73. doi: 10.1016/s0741-5214(98)70304-0. [DOI] [PubMed] [Google Scholar]
- 75.Lahtinen M, Blomberg P, Baliulis G, Carlsson F, Khamis H, Zemgulis V. In vivo h-VEGF165 gene transfer improves early endothelialisation and patency in synthetic vascular grafts. Eur J Cardiothorac Surg. 2007 Mar;31(3):383–90. doi: 10.1016/j.ejcts.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 76.Mayr U, Zou Y, Zhang Z, Dietrich H, Hu Y, Xu Q. Accelerated arteriosclerosis of vein grafts in inducible NO synthase(-/-) mice is related to decreased endothelial progenitor cell repair. Circ Res. 2006 Feb 17;98(3):412–20. doi: 10.1161/01.RES.0000201957.09227.6d. [DOI] [PubMed] [Google Scholar]
- 77.Heydarkhan-Hagvall S, Helenius G, Johansson BR, Li JY, Mattsson E, Risberg B. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J Cell Biochem. 2003 Aug 15;89(6):1250–9. doi: 10.1002/jcb.10583. [DOI] [PubMed] [Google Scholar]
- 78.Jia G, Mitra AK, Gangahar DM, Agrawal DK. Regulation of cell cycle entry by PTEN in smooth muscle cell proliferation of human coronary artery bypass conduits. J Cell Mol Med. 2009 Mar;13(3):547–54. doi: 10.1111/j.1582-4934.2008.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitra AK, Jia G, Gangahar DM, Agrawal DK. Temporal PTEN inactivation causes proliferation of saphenous vein smooth muscle cells of human CABG conduits. J Cell Mol Med. 2009 Jan;13(1):177–87. doi: 10.1111/j.1582-4934.2008.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolff RA, Malinowski RL, Heaton NS, Hullett DA, Hoch JR. Transforming growth factor-beta1 antisense treatment of rat vein grafts reduces the accumulation of collagen and increases the accumulation of h-caldesmon. J Vasc Surg. 2006 May;43(5):1028–36. doi: 10.1016/j.jvs.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 81.Ranjzad P, Salem HK, Kingston PA. Adenovirus-mediated gene transfer of fibromodulin inhibits neointimal hyperplasia in an organ culture model of human saphenous vein graft disease. Gene Ther. 2009 Sep;16(9):1154–62. doi: 10.1038/gt.2009.63. [DOI] [PubMed] [Google Scholar]
- 82.Kloppenburg GT, Grauls GE, Bruggeman CA, Stassen FR. Adenoviral activin A expression prevents vein graft intimal hyperplasia in a rat model. Interact Cardiovasc Thorac Surg. 2009 Jan;8(1):31–4. doi: 10.1510/icvts.2008.182329. [DOI] [PubMed] [Google Scholar]
- 83.Yamashita A, Hanna AK, Hirata S, Dardik A, Sumpio BE. Antisense basic fibroblast growth factor alters the time course of mitogen-activated protein kinase in arterialized vein graft remodeling. J Vasc Surg. 2003 Apr;37(4):866–73. doi: 10.1067/mva.2003.130. [DOI] [PubMed] [Google Scholar]
- 84.Huang B, Dreyer T, Heidt M, Yu JC, Philipp M, Hehrlein FW, Katz N, Al-Fakhri N. Insulin and local growth factor PDGF induce intimal hyperplasia in bypass graft culture models of saphenous vein and internal mammary artery. Eur J Cardiothorac Surg. 2002 Jun;21(6):1002–8. doi: 10.1016/s1010-7940(02)00111-2. [DOI] [PubMed] [Google Scholar]
- 85.Hu Y, Zou Y, Dietrich H, Wick G, Xu Q. Inhibition of neointima hyperplasia of mouse vein grafts by locally applied suramin. Circulation. 1999 Aug 24;100(8):861–8. doi: 10.1161/01.cir.100.8.861. [DOI] [PubMed] [Google Scholar]
- 86.Kimura S, Egashira K, Nakano K, Iwata E, Miyagawa M, Tsujimoto H, Hara K, Kawashima Y, Tominaga R, Sunagawa K. Local delivery of imatinib mesylate (STI571)-incorporated nanoparticle ex vivo suppresses vein graft neointima formation. Circulation. 2008 Sep 30;118(14 Suppl):S65–70. doi: 10.1161/CIRCULATIONAHA.107.740613. [DOI] [PubMed] [Google Scholar]
- 87.Banno H, Takei Y, Muramatsu T, Komori K, Kadomatsu K. Controlled release of small interfering RNA targeting midkine attenuates intimal hyperplasia in vein grafts. J Vasc Surg. 2006 Sep;44(3):633–41. doi: 10.1016/j.jvs.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 88.Pintucci G, Saunders PC, Gulkarov I, Sharony R, Kadian-Dodov DL, Bohmann K, Baumann FG, Galloway AC, Mignatti P. Anti-proliferative and anti-inflammatory effects of topical MAPK inhibition in arterialized vein grafts. FASEB J. 2006 Feb;20(2):398–400. doi: 10.1096/fj.05-4114fje. [DOI] [PubMed] [Google Scholar]
- 89.Sharony R, Pintucci G, Saunders PC, Grossi EA, Baumann FG, Galloway AC, Mignatti P. Matrix metalloproteinase expression in vein grafts: role of inflammatory mediators and extracellular signal-regulated kinases-1 and -2. Am J Physiol Heart Circ Physiol. 2006 Apr;290(4):H1651–9. doi: 10.1152/ajpheart.00530.2005. [DOI] [PubMed] [Google Scholar]
- 90.Hata JA, Petrofski JA, Schroder JN, Williams ML, Timberlake SH, Pippen A, Corwin MT, Solan AK, Jakoi A, Gehrig TR, Kontos CD, Milano CA. Modulation of phosphatidylinositol 3-kinase signaling reduces intimal hyperplasia in aortocoronary saphenous vein grafts. J Thorac Cardiovasc Surg. 2005 Jun;129(6):1405–13. doi: 10.1016/j.jtcvs.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 91.Wan S, George SJ, Nicklin SA, Yim AP, Baker AH. Overexpression of p53 increases lumen size and blocks neointima formation in porcine interposition vein grafts. Mol Ther. 2004 May;9(5):689–98. doi: 10.1016/j.ymthe.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Mayr U, Mayr M, Li C, Wernig F, Dietrich H, Hu Y, Xu Q. Loss of p53 accelerates neointimal lesions of vein bypass grafts in mice. Circ Res. 2002 Feb 8;90(2):197–204. doi: 10.1161/hh0202.103715. [DOI] [PubMed] [Google Scholar]
- 93.Frischknecht K, Greutert H, Weisshaupt C, Kaspar M, Yang Z, Luscher TF, Carrel TP, Tanner FC. Different vascular smooth muscle cell apoptosis in the human internal mammary artery and the saphenous vein. Implications for bypass graft disease. J Vasc Res. 2006;43(4):338–46. doi: 10.1159/000093606. [DOI] [PubMed] [Google Scholar]
- 94.Yamanouchi D, Banno H, Nakayama M, Sugimoto M, Fujita H, Kobayashi M, Kuwano H, Komori K. Hydrophilic statin suppresses vein graft intimal hyperplasia via endothelial cell-tropic Rho-kinase inhibition. J Vasc Surg. 2005 Oct;42(4):757–64. doi: 10.1016/j.jvs.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 95.Ming XF, Viswambharan C, Barandier C, Ruffieux K, Kaibuchi K, Rusconi S, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxidase synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Turner NA, O'Regan DJ, Ball SG, Porter KE. Simvastatin inhibits MMP-9 secretion from human saphenous vein smooth muscle cells by inhibiting the RhoA/ROCK pathway and reducing MMP-9 mRNA levels. FASEB J. 2005 May;19(7):804–6. doi: 10.1096/fj.04-2852fje. [DOI] [PubMed] [Google Scholar]
- 97.Fujita H, Banno H, Yamanouchi D, Kobayashi M, Yamamoto K, Komori K. Pitavastatin inhibits intimal hyperplasia in rabbit vein graft. J Surg Res. 2008 Aug;148(2):238–43. doi: 10.1016/j.jss.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 98.Lamfers ML, Aalders MC, Grimbergen JM, de Vries MR, Kockx MM, van Hinsbergh VW, Quax PH. Adenoviral delivery of a constitutively active retinoblastoma mutant inhibits neointima formation in a human explant model for vein graft disease. Vascul Pharmacol. 2002 Dec;39(6):293–301. doi: 10.1016/s1537-1891(03)00043-0. [DOI] [PubMed] [Google Scholar]
- 99.Mann MJ, Conte MS. Transcription factor decoys for the prevention of vein bypass graft failure. Am J Cardiovasc Drugs. 2003;3(2):79–85. doi: 10.2165/00129784-200303020-00001. Review. [DOI] [PubMed] [Google Scholar]
- 100.Jia G, Cheng G, Gangahar DM, Agrawal DK. Involvement of connexin 43 in angiotensin II-induced migration and proliferation of saphenous vein smooth muscle cells via the MAPK-AP-1 signaling pathway. J Mol Cell Cardiol. 2008 May;44(5):882–90. doi: 10.1016/j.yjmcc.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jia G, Mitra AK, Cheng G, Gangahar DM, Agrawal DK. Angiotensin II and IGF-1 regulate connexin43 expression via ERK and p38 signaling pathways in vascular smooth muscle cells of coronary artery bypass conduits. J Surg Res. 2007 Sep;142(1):137–42. doi: 10.1016/j.jss.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Kusch B, Waldhans S, Sattler A, Wagner A, Hecker M, Moosdorf R, Vogt S. Inhibition of carotis venous bypass graft disease by intraoperative nucleic acid-based therapy in rabbits. Thorac Cardiovasc Surg. 2006 Sep;54(6):388–92. doi: 10.1055/s-2006-924410. [DOI] [PubMed] [Google Scholar]
- 103.Wan S, Yim AP, Johnson JL, Shukla N, Angelini GD, Smith FC, Dashwood MR, Jeremy JY. The endothelin 1A receptor antagonist BSF 302146 is a potent inhibitor of neointimal and medial thickening in porcine saphenous vein-carotid artery interposition grafts. J Thorac Cardiovasc Surg. 2004 May;127(5):1317–22. doi: 10.1016/j.jtcvs.2003.06.018. [DOI] [PubMed] [Google Scholar]
- 104.Ni J, Waldman A, Khachigian LM. c-Jun regulates shear- and injury-inducible Egr-1 expression, vein graft stenosis after autologous end-to-side transplantation in rabbits, and intimal hyperplasia in human saphenous veins. J Biol Chem. 2010 Feb 5;285(6):4038–48. doi: 10.1074/jbc.M109.078345. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Miyake T, Aoki M, Shiraya S, Tanemoto K, Ogihara T, Kaneda Y, Morishita R. Inhibitory effects of NFkappaB decoy oligodeoxynucleotides on neointimal hyperplasia in a rabbit vein graft model. J Mol Cell Cardiol. 2006 Sep;41(3):431–40. doi: 10.1016/j.yjmcc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 106.Shintani T, Sawa Y, Takahashi T, Matsumiya G, Matsuura N, Miyamoto Y, Matsuda H. Intraoperative transfection of vein grafts with the NFkappaB decoy in a canine aortocoronary bypass model: a strategy to attenuate intimal hyperplasia. Ann Thorac Surg. 2002 Oct;74(4):1132–7. 1137–8. doi: 10.1016/s0003-4975(02)03921-8. discussion. [DOI] [PubMed] [Google Scholar]
- 107.Gizard F, Bruemmer D. Transcriptional Control of Vascular Smooth Muscle Cell Proliferation by Peroxisome Proliferator-Activated Receptor-gamma: Therapeutic Implications for Cardiovascular Diseases. PPAR Res 2008. 2008:429123. doi: 10.1155/2008/429123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turner NA, Hall KT, Ball SG, Porter KE. Selective gene silencing of either MMP-2 or MMP-9 inhibits invasion of human saphenous vein smooth muscle cells. Atherosclerosis. 2007 Jul;193(1):36–43. doi: 10.1016/j.atherosclerosis.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 109.Eefting D, de Vries MR, Grimbergen JM, Karper JC, van Bockel JH, Quax PH. In vivo suppression of vein graft disease by nonviral, electroporation-mediated, gene transfer of tissue inhibitor of metalloproteinase-1 linked to the amino terminal fragment of urokinase (TIMP-1.ATF), a cell-surface directed matrix metalloproteinase inhibitor. J Vasc Surg. 2010 Feb;51(2):429–37. doi: 10.1016/j.jvs.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 110.Thomas AC, Newby AC. Effect of Matrix Metalloproteinase-9 Knockout on Vein Graft Remodelling in Mice. J Vasc Res. 2009 Dec 16;47(4):299–308. doi: 10.1159/000265564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu Y, Baker AH, Zou Y, Newby AC, Xu Q. Local gene transfer of tissue inhibitor of metalloproteinase-2 influences vein graft remodeling in a mouse model. Arterioscler Thromb Vasc Biol. 2001 Aug;21(8):1275–80. doi: 10.1161/hq0801.093658. [DOI] [PubMed] [Google Scholar]
- 112.Kallenbach K, Salcher R, Heim A, Karck M, Mignatti P, Haverich A. Inhibition of smooth muscle cell migration and neointima formation in vein grafts by overexpression of matrix metalloproteinase-3. J Vasc Surg. 2009 Mar;49(3):750–8. doi: 10.1016/j.jvs.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, Tempst P, Strittmatter SM, Sessa WC. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004 Apr;10(4):382–8. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- 114.Kritz AB, Yu J, Wright PL, Wan S, George SJ, Halliday C, Kang N, Sessa WC, Baker AH. In vivo modulation of Nogo-B attenuates neointima formation. Mol Ther. 2008 Nov;16(11):1798–804. doi: 10.1038/mt.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haraguchi T, Okada K, Tabata Y, Maniwa Y, Hayashi Y, Okita Y. Controlled release of basic fibroblast growth factor from gelatin hydrogel sheet improves structural and physiological properties of vein graft in rat. Arterioscler Thromb Vasc Biol. 2007 Mar;27(3):548–55. doi: 10.1161/01.ATV.0000254811.11741.2b. [DOI] [PubMed] [Google Scholar]
- 116.Work LM, Reynolds PN, Baker AH. Improved gene delivery to human saphenous vein cells and tissue using a peptide-modified adenoviral vector. Genet Vaccines Ther. 2004 Oct 8;2(1):14. doi: 10.1186/1479-0556-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kilian EG, Eifert S, Beiras-Fernandez A, Daebritz S, Reichenspurner H, Reichart B. Adeno-associated virus-mediated gene transfer in a rabbit vein graft model. Circ J. 2008 Oct;72(10):1700–4. doi: 10.1253/circj.cj-07-0921. [DOI] [PubMed] [Google Scholar]
- 118.Dishart KL, Denby L, George SJ, Nicklin SA, Yendluri S, Tuerk MJ, Kelley MP, Donahue BA, Newby AC, Harding T, Baker AH. Third-generation lentivirus vectors efficiently transduce and phenotypically modify vascular cells: implications for gene therapy. J Mol Cell Cardiol. 2003 Jul;35(7):739–48. doi: 10.1016/s0022-2828(03)00136-6. [DOI] [PubMed] [Google Scholar]
- 119.King Peter, Jhon P. Royle. Autogenous vein grafting in atheromatous rabbits. Cardiovasc Res. 1971;6:627–633. doi: 10.1093/cvr/6.6.627. [DOI] [PubMed] [Google Scholar]
- 120.Wyatt AP, Rothnie NG, Taylor GW. The vascularization of vein-grafts. Brit J Surg. 1964;51(5):378–381. doi: 10.1002/bjs.1800510522. [DOI] [PubMed] [Google Scholar]
- 121.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs Are Necessary for Vascular Smooth Muscle Growth, Differentiation, and Function. Arterioscler Thromb Vasc Biol. 2010 Apr 8; doi: 10.1161/ATVBAHA.109.200873. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bonauer A, Boon RA, Dimmeler S. Vascular microRNAs. Curr Drug Targets. 2010 Apr 26; doi: 10.2174/138945010791591313. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 123.Weber C, Schober A, Zernecke A. MicroRNAs in Arterial Remodelling, Inflammation and Atherosclerosis. Curr Drug Targets. 2010 Apr 26; doi: 10.2174/138945010791591377. Epub ahead of print. [DOI] [PubMed] [Google Scholar]