Abstract
Seasonal variation in bone mineral density (BMD) has been documented in humans, and has been attributed to changes in 25-hydroxyvitamin D [25(OH)D] synthesis. To test the hypothesis that seasonal changes in bone mass occur in laboratory mice, we measured body composition, femoral bone phenotypes, and serum bone markers in 16-week old male and female C57BL/6 (B6) mice during the summer (June–August) and winter (December-February) months at The Jackson Laboratory in Bar Harbor, Maine. Both male and female B6 mice had higher volumetric (v)BMD in the summer than winter. Females showed reduced trabecular bone, while males showed changes in bone volume. Males, but not females, had higher IGF-I in summer than in winter, and only males showed an increase in body weight during the winter. No seasonal differences in serum TRAP5b, osteocalcin, or 25(OH)D were noted for either sex. We conclude that seasonal variation in skeletal and body composition parameters in B6 mice is significant and must be considered when performing longitudinal phenotyping of the skeleton. Further studies are needed to determine the environmental factors that cue seasonal changes in body composition and the mechanisms that produce these changes.
Keywords: seasonal, bone mineral density, gender, bone compartment, laboratory mouse
Introduction
In humans, seasonal fluctations in bone mineral density (BMD) [1–3], 25-hydroxyvitamin D [25(OH)D] [4] and other markers of bone metabolism [1, 5, 6] have been well established. This pattern is more pronounced at northern than at equatorial latitudes, where the angle of the sun is reduced during the winter months and a corresponding decrease occurs in endogenous vitamin D production in the skin [4]. However, a direct cause and effect relationship between the decline in serum 25(OH)D and change in bone mineral density (BMD) has been difficult to ascertain, in part because of the many variables inherent in population studies [7].
The genomic homogeneity of inbred mouse strains provides an excellent animal model for dissecting the genetic determinants of complex traits like BMD. Gender specific differences in mouse BMD and bone turnover have been well established [8, 9]. Less clear are the environmental determinants that interact with genetic polymorphisms to alter phenotypes such as weight, body fat, tail length and BMD. Thus, even though individual mice within an inbred strain are genetically identical, there is significant intra- as well as inter-strain variation. Laboratory mice are housed under controlled conditions, including constant light:dark cycles, bedding materials, animal density, dietary constituents, and room temperatures. Given this apparent lack of fluctuating exogenous cues, little consideration has been given to the possibility of circannual rhythms of bone turnover in standard laboratory mouse strains. However, both anecdotal and published data of husbandry records show clear seasonal fluctuations in mouse fecundity. The magnitude of this seasonal effect seems to vary by inbred strain, but in general litter sizes and frequency are lower in the winter months than summer [10].
In our studies of congenic mouse strains investigating the genetic effect of single QTL for bone mass, as well as in a large-scale cross-sectional survey of inbred mouse strains, we recently noted significant variation in areal bone density (aBMD) among C57BL/6J (B6) mice that appeared to be related to the time of measurement. Hence, we hypothesized that seasonal bone loss also occurred in laboratory mice, despite relatively constant environmental factors. To test that hypothesis, we measured BMD, bone turnover, bone architectural properties, and body composition in both male and female B6 mice during winter and summer at The Jackson Laboratory in Bar Harbor, Maine.
Materials and Methods
Animals
C57BL/6JBm (B6) female and male 16 week old mice (N = 20–24 per group) were measured during two seasons; summer (July 2006) and winter (February 2007). All mice were housed in the same mouse room, at an ambient temperature of 70±2°F and under a constant 14:10 hrs light dark cycle. Mice were fed a standard rodent diet (Lab Diet JL Rat & Mouse/ Irr 6F, catalogue number 5LG4) that contained 6% fat by weight (but 16% by kcalorie) and 19% protein by weight, and containing various vitamins and minerals. Of particular interest are the vitamin D (4.0 IU/gm, added to the diet), calcium content (1.13%), and potassium content (0.63%). Ad libitum access was provided to water that contained 0.4 mg/ml of vitamin K (menadione Na bisulfite) and was acidified to pH 2.5 with HCl to retard bacterial growth. At the time of necropsy, body weights (g) were recorded, then trunk blood was collected on ice to be centrifuged 2–4 hours later. Serum was aliquoted and stored at −80°C. Whole body scans were obtained with a PIXImus DEXA instrument, then lower limbs were removed and fixed in 95% ethanol for a minimum of 3–4 weeks. After fixation, femurs were dissected free of surrounding tissue and scanned for both pQCT and MicroCT-40 data. All mouse procedures were approved by the ACUC at The Jackson Laboratory in Bar Harbor, Maine, USA.
Phenotyping
DEXA scanning by PIXImus
We used PIXImus DEXA (GE-Lunar, Madison, WI) to assess whole body areal BMD (aBMD), whole body bone mineral content (BMC, in g), and percent fat. This methodology has been validated in small animals [11]. PIXImus scanning in mice for BMC and fat is both accurate and precise, although body size must be considered when comparing strains. BMC by PIXImus is highly correlated with mineral content of hydroxyapatite standard of known density (R2= 0.997) [11].
pQCT for femoral volumetric BMD
Volumetric bone mineral density (vBMD) was measured on the entire left femur of each mouse. Isolated femur length was measured with digital calipers (Stoelting, Wood Dale, IL), and then femurs were measured for density using the SA Plus densitometer (Orthometrics, Stratec SA Plus Research Unit, White Plains, NY), for which calibration and methodology has been described previously [9]. Isolated femurs were scanned at 7 locations at 2 mm intervals, beginning 0.8 mm from the distal ends of the epiphyseal condyles. Due to variation in femur lengths, the femoral head and neck could not be scanned at the same location for each bone, and thus were not included in final data. Total vBMD values were calculated by dividing the total mineral content by the total bone volume (bone + marrow) and expressed as mg/mm3. Periosteal and endosteal perimeters were obtained at the mid shaft scan. Precision of the SA Plus for repeated measurement of a single femur has been found to be 1.2% [12].
MicroCT-40 Femoral Cross-Sectional Geometry and Trabecular Morphology
Femurs were scanned using a Microcomputed Tomographic Instrument (MicroCT-40, Scanco Medical AG, Bassersdorf, Switzerland) to evaluate cross-sectional geometry at the femoral mid-shaft and trabecular bone volume fraction and microarchitecture in the secondary spongiosa of the distal femur [13]. The femurs were scanned at low resolution, energy level of 55KeV, and intensity of 145 µA. The MicroCT-40 is calibrated weekly using a phantom provided by Scanco. Eighteen scans were measured at the mid-point of each femur, with an isotropic pixel size of 12 µm and slice thickness of 12 µm, and used to compute the average total cross-sectional area (mm2), bone area (mm2), and marrow area (mm2). For mid-shaft analysis, the cortical shell was contoured by user-defined threshold and iterated across the 18 slices. Trabecular bone volume fraction and microarchitecture were evaluated in the secondary spongiosa, starting proximately at 0.6 mm proximal to the growth plate, and extending proximally 1.5 mm. 100 consecutive slices from a total of 150 scans of the distal femur, acquired at the same parameters as the femoral mid-shaft, were chosen for analysis. All scans were analyzed using manufacturer software (Scanco, version 3.1).
Serum measurements (IGF-I, TRAP5b, OC, 25(OH)D)
The IGF-I, TRAP5b, and OC assays were conducted at the MECORE Laboratory of St. Joseph Healthcare in Bangor, Maine. Each included both internal controls and an aliquot of a normal mouse serum pool on each assay to monitor variability.
Serum insulin-like growth factor 1 (IGF-I) measurements were conducted using a modified radioimmunoassay kit (ALPCO, Windham, NH). IGF binding proteins (IGFBPs) were first separated from the IGF-I by an acid dissociation step. This was followed by the addition of a neutralization buffer containing excess recombinant human IGF-II, allowing the IGF-II to bind to the IGFBPs prior to immunoassay with a human anti-IGF-I polyclonal antibody. The sensitivity of the assay is 0.01 ng/ml IGF-I; the inter assay coefficient of variation based on normal standards and pooled serum of C3H and B6 is approximately 6%. There is no cross reactivity with IGF-II.
Serum tartrate-resistant acid phosphatase (TRAP5b) measurements were obtained using an ELISA kit (IDS Ltd., Fountain Hills, AZ). 25µL of serum per well was assayed in duplicate following the manufacturer’s instructions. The interassay CV for the TRAP5b ELISA was 7%.
Osteocalcin (OC) was measured with an immunoradiometric assay kit (ALPCO, Windham, NH), using 10ul of serum. The interassay CV for the mouse osteocalcin kit was 7%.
The total 25(OH)D levels were determined by liquid chromatography mass spectroscopy as previously described [14].
Statistics
Data are expressed as mean ± SEM in tables and figures. Statistical evaluation was performed using the JMP v.6 (SAS, Cary, NC) software program. Each sex was separately compared between seasons using ANCOVA, with the size measures of body weight and femur length included as covariates where appropriate for bone phenotypes [15].
Results
PIXImus parameters, body weight, and femur length
Male B6 mice had a higher overall body weight in winter versus summer, despite no significant changes by season in percent body fat, whole body aBMD, BMC, or femur length (Table 1). Females did not show any seasonal differences in body weight, percent fat, aBMD, BMC, or femur length (Table 1).
Table 1.
Morphometric measurements and PIXImus body composition phenotypes in B6 mice, by gender 2 and season (mean value +/− SE).
| Sex | Female | Male | ||
|---|---|---|---|---|
| Season | Winter | Summer | Winter | Summer |
| N | 23 | 20 | 18 | 21 |
| Body weight (g) | 21.08 +/− 0.44 | 21.84 +/− 0.47 | 29.17 +/− 0.55a | 27.17 +/− 0.51 |
| Femur length (mm) | 15.64 +/− 0.05 | 15.60 +/− 0.05 | 16.00 +/− 0.06 | 15.97 +/− 0.06 |
| aBMD (g/cm2) | 0.0484 +/− 0.0003 | 0.0488 +/− 0.0003 | 0.0519 +/− 0.0005 | 0.0511 +/− 0.0005 |
| BMC (g) | 0.463 +/− 0.005 | 0.458 +/− 0.006 | 0.531 +/− 0.011 | 0.521 +/− 0.010 |
| % fat | 19.70 +/− 0.60 | 20.15 +/− 0.64 | 19.44 +/− 0.95 | 18.31 +/− 0.88 |
p<0.05
IGF-I, TRAP5b, and 25(OH)D serum measurements by season
Male B6 mice had significantly higher IGF-I levels in the summer than the winter season, whereas females did not significantly differ by season for this serum growth factor. TRAP5b levels did not differ by season for females or for males. Osteocalcin levels showed a non-significant trend towards being higher in females during the winter (p=0.10) but did not differ by season in males (p=0.70). Serum 25(OH)D levels did not differ by season for either females (p=0.1521) or males(p=0.8670) (see Table 2).
Table 2.
Serum IGF-I, TRAP5b, osteocalcin, and 25-(OH)D measurements in B6 mice, by gender and 2 season (mean values +/− SE).
| Sex | Female | Male | ||
|---|---|---|---|---|
| Season | Winter | Summer | Winter | Summer |
| IGF-I (ng/ml) | N = 24 275 +/− 8.6 |
N = 14 292 +/− 11.3 |
N = 18 199 +/− 7.6 a |
N = 18 234 +/− 7.6 |
| TRAP5b (IU/L) | N = 10 13.73 +/− 1.2 |
N = 10 13.84 +/− 1.2 |
N = 11 12.00 +/− 0.83 |
N = 11 11.02 +/− 0.89 |
| Osteocalcin (ng/ml) | N = 10 134.2 +/− 7.6 |
N = 9 115.2 +/− 8.0 |
N = 10 91.4 +/− 8.0 |
N = 10 95.8 +/− 8.0 |
| 25(OH)D (ng/ml) | N = 8 88.6 +/− 27.1 |
N = 10 72.0 +/− 19.8 |
N = 9 86.8+/− 19.7 |
N = 9 85.5 +/− 11.5 |
p<0.05
pQCT phenotypes
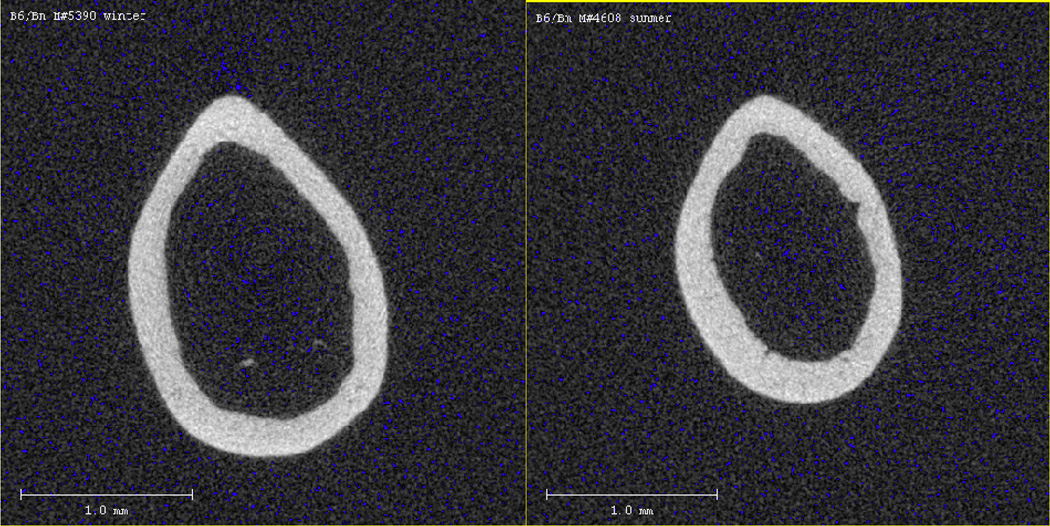
Both male and female B6 mice had significantly higher femoral vBMD in the summer than in the winter seasons (Table 3). However, the mechanism by which this increase occurred differed by sex. For females, total mineral trended towards being lower in the winter than in summer (p = 0.058) without accompanying volume changes. On the other hand, males had greater total bone volume during the winter, with no changes in the mineral content. This was reflected by measurements in the mid-shaft circumference measurements; i.e. males had larger periosteal and endosteal circumferences in winter than in summer; females showed no differences in either bone envelope by season (Table 3).
Table 3.
Total femoral bone density and mid-shaft pQCT measurements in B6 mice, by gender and 2 season (mean values +/− SE).
| Sex | Female | Male | ||
|---|---|---|---|---|
| Season | Winter | Summer | Winter | Summer |
| N | 24 | 20 | 18 | 21 |
| Total vBMD (mg/cm3) | 0.580 +/− 0.005 | 0.599 +/− 0.006 a | 0.581 +/− 0.006 | 0.604 +/− 0.006 b |
| Total mineral (mg) | 11.55 +/− 0.12 | 11.91 +/− 0.14 * | 13.88 +/− 0.20 | 13.86 +/− 0.19 # |
| Total volume (cm3) | 19.89 +/− 0.11 | 19.94 +/− 0.12 | 23.92 +/− 0.24 | 22.92 +/− 0.22 b # |
| Periosteal circumference (mm) | 4.94 +/− 0.02 | 4.93 +/− 0.02* | 5.66 +/− 0.03 | 5.44 +/− 0.03 c # |
| Endosteal circumference (mm) | 3.75 +/− 0.03 | 3.77 +/− 0.02 * | 4.44 +/− 0.03 | 4.23 +/− 0.03 c * # |
p<0.05,
p<0.01,
p<0.0001
body weight covariate
femur length covariate
MicroCT-40 femoral phenotypes
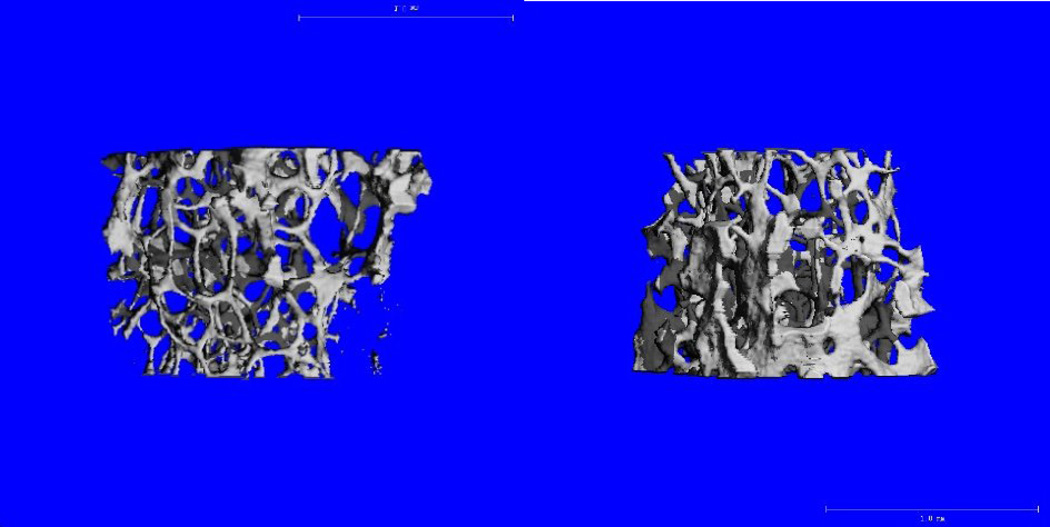
Similar to our measurements for total volumetric BMD by pQCT, femoral trabecular bone volume fraction and architecture as measured by MicroCT-40 also varied by season and gender. At the distal femur, females in the summer season showed a significant increase in trabecular BV/TV, and an associated increase in trabecular number compared to female mice measured in the winter (Table 4). In contrast, males did not exhibit seasonal differences in trabecular architectural parameters at the distal femur, rather, male mice measured in the summer had an increase in total bone area (i.e. femoral midshaft bone area/total area) compared to those in the winter season (Table 4). Cortical thickness did not differ by season for females or for males.
Table 4.
MicroCT-40 femoral distal trabecular and midshaft phenotypes in B6 mice, by gender and 2 season (mean values +/− SE).
| Sex | Female | Male | ||
|---|---|---|---|---|
| Season | Winter | Summer | Winter | Summer |
| N | 10 | 14 | 10 | 10 |
| Trabecular BV/TV | 0.0738 +/− 0.0043 | 0.0873 +/− 0.0036 a | 0.2195 +/− 0.0080 | 0.2224 +/− 0.0110 |
| Trabecular number | 3.875 +/− 0.049 | 4.030 +/− 0.041 a | 5.375 +/− 0.061 | 5.536 +/− 0.061 # |
| Midshaft BA/TA | 0.498 +/− 0.004 | 0.503 +/− 0.004 | 0.463 +/− 0.006 | 0.493 +/− 0.006 a # |
| Cortical thickness (mm) | 0.204 +/− 0.002 | 0.209 +/− 0.002 | 0.212 +/− 0.003 | 0.217 +/− 0.003 |
p<0.05
femur length covariate
Discussion
In this paper we report for the first time, significant seasonal changes in femoral bone mass for B6 male and female laboratory mice. Although there have been anecdotal reports of seasonal fluctuations in various aspects of mouse husbandry, the BMD changes that occurred in mice housed under standard controlled laboratory conditions are surprising, yet provocative. Male and female adult B6 mice sacrificed during winter had a markedly lower femoral vBMD compared to those sacrificed in the summer. Furthermore, the femoral density differences were due to changes in distinct compartments and were gender dependent. For example, in the winter, females trended towards reduced total femoral mineral content, while males had greater total femoral volume but the same mineral. These gender specific volumetric differences were also mirrored by changes in the trabecular bone compartment. Corresponding to a higher vBMD in females during summer, we found increased femoral distal trabecular BV/TV and greater trabecular number, but no differences in other volumetric parameters. Males, on the other hand, had increased mid-shaft periosteal and endosteal circumferences during the winter months, but no changes in femoral BV/TV, cortical thickness, or trabecular thickness. These findings would suggest that gender must play a critical role in the adaptation to environmental signals causing bone loss.
Though our earliest observations of seasonal bone changes were noted by aBMD and BMC obtained by PIXImus, no such differences were found in the current set of B6 mice. Earlier observations from the large-scale cross sectional study involved younger animals (i.e. 14 weeks old) and included all four seasons, which may account for this difference. The more fine-scale measures obtained here using pQCT and MicroCT-40 did show seasonal changes that perhaps the PIXImus may not have been sensitive enough to detect.
The explanation of seasonal bone differences in laboratory mice is not apparent from the biochemical measures of bone turnover that we investigated (see Table 2). For example, three markers (Trap5b, 25(OH)D, and osteocalcin) did not differ by season for either gender. Serum IGF-I levels were lower in winter than in summer for both males and females, but these differences were only statistically significant in the males. Although it was surprising that the markers did not show seasonal differences, it is conceivable that the turnover indices we chose were not sensitive enough to detect change, or that there may be a lag between changes at the cellular level and macroscopic differences in bone mass.
It is possible that seasonal changes in levels of nutrient intake or in dietary composition might be responsible for these differences in bone density. One possibility is that behavioral changes induced by innate circannual rhythms might lead to reduced food consumption during a particular season, resulting in lower IGF-I levels, for example. Conversely, dietary intake may have been constant by season, but the non-nutritive composition per unit of food may have changed. Most animal facilities use commercially available standard grain-based laboratory diets consisting of various agricultural products. These diets are defined for nutrition content and proportions, contain ground plant matter (i.e. corn, oats, alfalfa meal, soybean meal, and ground wheat), may or may not contain animal protein (i.e. fish meal), and are supplemented with the addition of vitamins and minerals [16]. An important, yet disregarded, facet of these products is the non-nutritive portions that are largely undefined. Of particular relevance to this study is the variable level of dietary phytoestrogens, i.e. nonsteroidal estrogens that originate in plant matter and bind weakly to the estrogen receptor that are often found in laboratory diets [17]. In fact, the proportion of phytoestrogens in diets can vary tremendously from lot to lot, though the nutritive content is carefully controlled [16]. Since the mice in this study were not fed a fixed refined diet, this possibility needs to be explored further.
There are several limitations to this study. First, we did not perform paired feedings, nor did we directly measure the amount of food consumed per day. It is conceivable that the increased body weight in males during the winter is a function of reduced physical activity and/or greater food consumption. To address this possibility, we are currently conducting a larger study in B6 mice using a fixed and refined diet, and are measuring intake during each season. Second, although we performed skeletal phenotyping by several means (DXA, pQCT and micro CT) in this pilot, we did not evaluate bone turnover by dynamic histomorphometry. This would be important to appreciate subtle changes in remodeling by season, and is also planned for our larger study. Finally, as noted, although bone turnover markers in mice are imprecise, we found little evidence for significant changes by season. Still, it is possible that we did not select appropriate serum or urine markers to detect what might be minimal changes in the remodeling unit.
Whatever the mechanism(s), the seasonal differences noted in the current study are quite compelling and have important implications for research involving laboratory animals. Subtle differences in various phenotypes could easily be masked or confounded by the influence of season. Further exploration is required to characterize the extent to which seasonal variation occurs in different inbred strains of laboratory mice. Interestingly, B6 laboratory mice have a loss of function mutation in the melatonin generating enzyme, serotonin N-acetyltransferase (arylalkylamine N-acetyltransferase, AANAT) [18, 19], which may have been inadvertently selected for along with the accompanying year-round breeding it promotes [20]. It is possible that the absence of melatonin in B6 would affect not only the circadian cycle, but also any interaction that may entrain circannual rhythms. Not surprising, seasonal variation can occur in several metabolic phenotypes not only in free-ranging animals, but also among captive mammals [21]. However, the genetic, environmental, hormonal and nutritional cues that entrain circannual rhythms when light, temperature and food intake remain constant have yet to be identified. This remains a major challenge not only for scientists studying laboratory mice, but also for investigators examining the determinants of bone loss in humans.
In summary, we found significant seasonal variation in several skeletal and body composition parameters of the B6 laboratory mouse that were gender and compartment specific. Understanding how circannual and circadian rhythms regulate skeletal homeostasis should provide further insight into the pathogenesis of bone loss in humans.
Figure 1.
Trabecular bone in 16-week old female B6 mice during winter (left) and summer (right) 2 seasons.
Figure 2.
Mid-shaft cortical bone in 16 week-old B6 male mice during winter (left) and summer (right) 2 seasons.
Acknowledgements
This work has been supported by NIH grant AR055633, AR053853 and AR043618.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Storm D, Eslin R, Smith Porter E, Musgrave K, Vereault D, Patton C, Kessenich C, Mohan S, Chen T, Holick MF. Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: a randomized placebo-controlled trial. Journal of Clinical Endocrinology and Metabolism. 1998;83:3817–3825. doi: 10.1210/jcem.83.11.5289. [DOI] [PubMed] [Google Scholar]
- 2.Gerdhem P, Mallmin H, Akesson K, Obrant K. Seasonal variation in bone density in postmenopausal women. Journal of Clinical Densitometry. 2006;7:93–100. doi: 10.1385/JCD:7:1:93. [DOI] [PubMed] [Google Scholar]
- 3.Rapuri PB, Kinyamu HK, Gallagher JC, Haynatzka V. Seasonal changes in calciotropic hormones, bone markers, and bone mineral density in elderly women. The Journal of Clinical Endocrinology and Metabolism. 2002;87:2024–2032. doi: 10.1210/jcem.87.5.8475. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. The New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Need AG, Horowitz M, Morris HA, Moore R, Nordin RC. Seasonal changes in osteoid thickness and mineralization lag time in ambulant patients. Journal of Bone and Mineral Research. 2007;22:757–761. doi: 10.1359/jbmr.070203. [DOI] [PubMed] [Google Scholar]
- 6.Armas L, Heaney RP, Recker RR. Seasonal variation in bone histomorphometry. Journal of Bone and Mineral Research. 2008;23:301. doi: 10.1359/jbmr.071026. [DOI] [PubMed] [Google Scholar]
- 7.Woitge HW, Knothe A, Witte K, Schmidt-Gayk H, Zeigler R, Lemmer B, Seibel MJ. Circaannual rhythms and interactions of vitamin D metabolites, parathyroid hormone, and biochemical markers of skeletal homeostasis: a prospective study. J Bone Miner Res. 2000;15:2443–2450. doi: 10.1359/jbmr.2000.15.12.2443. [DOI] [PubMed] [Google Scholar]
- 8.Orwoll EC, Belknap JK, Klein RF. Gender specificity in the genetic determinants of peak bone mass. J Bone Miner Res. 2001;16:1962–1971. doi: 10.1359/jbmr.2001.16.11.1962. [DOI] [PubMed] [Google Scholar]
- 9.Beamer WG, Shultz KL, Ackert-Bicknell CL, Horton LG, Delahunty KM, Coombs HF, III, Donahue LR, Canalis E, Rosen CJ. Genetic dissection of mouse distal Chromosome 1 reveals three linked BMD QTL with sex dependent regulation of bone phenotypes. Journal of Bone and Mineral Research. 2007;22:1187–1196. doi: 10.1359/jbmr.070419. [DOI] [PubMed] [Google Scholar]
- 10.Pritchett KR, Taft R. Reproductive biology of the laboratory mouse, v3. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. The Mouse in Biomedical Research. 2nd Edn. Boston: Academic Press, Elsevier; 2007. pp. 91–122. [Google Scholar]
- 11.Nagy TR, Clair A-L. Precision and accuracy of dual-energy x-ray absorptiometry for determining in vivo body composition in mice. Obesity Res. 2000;8:392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 12.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 13.Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. Journal of Bone and Mineral Reseach. 2005;20:1085–1092. doi: 10.1359/JBMR.050307. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, dePapp AE. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. Journal of Clinical Endocrinology & Metabolism. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 15.Sokal RR, Rohlf FJ. Biometry. New York: W.H. Freeman and Company; 2000. [Google Scholar]
- 16.Thigpen JE, Setchell KDR, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Laboratory Animal Science. 1999;49:530–536. [PubMed] [Google Scholar]
- 17.Thigpen JE, Setchell KDR, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR Journal. 2004;45:401–416. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- 18.Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- 19.Roseboom PH, Aryan Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. Natural melatonin 'knockdown' in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Molecular Brain Research. 1998;63:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 20.Olson MV. When less is more: gene loss as an engine of evolutionary change. American Journal of Human Genetics. 1999;64:18–23. doi: 10.1086/302219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18:4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]