Abstract
Activation of β-catenin, the principal mediator of canonical Wnt signaling, is a common pathologic finding in a wide variety of chronic kidney diseases (CKD). While β-catenin is induced predominantly in renal tubular epithelium in CKD, surprisingly, depletion of tubular β-catenin had little effect on the severity of renal fibrosis. Interestingly, less apoptosis was detected in interstitial fibroblasts in knockout mice, which was accompanied by a decreased expression of Bax and Fas ligand (FasL). Tubule-specific knockout of β-catenin diminished renal induction of matrix metalloproteinase (MMP-7), which induced FasL expression in interstitial fibroblasts and potentiated fibroblast apoptosis in vitro. These results demonstrate that loss of tubular β-catenin resulted in enhanced interstitial fibroblast survival due to decreased MMP-7 expression. Our studies uncover a novel role of the tubular β-catenin/MMP-7 axis in controlling the fate of interstitial fibroblasts via epithelial-mesenchymal communication.
Renal fibrosis is the common final outcome of a wide variety of progressive chronic kidney diseases (CKD). After initial insults, tissue fibrogenesis occurs as a dynamic process consisting of four overlapping phases: priming, activation, execution and progression, in which fibroblast activation is a key step1. In normal adult kidneys, fibroblasts are quiescent in nature and scarce in numbers, and are situated in the interstitial space between the capillaries and tubular epithelium. Upon activation by profibrotic cytokines and/or mechanical stress in vivo, fibroblasts acquire a myofibroblast phenotype by expressing α-smooth muscle actin (α-SMA) and produce a large amount of extracellular matrix (ECM) components1,2,3. Studies indicate that activated fibroblasts in diseased kidneys could derive from different sources via diverse mechanisms, including activation of interstitial fibroblasts and pericytes, phenotypic conversion of tubular epithelia and endothelial cells, and recruitment of circulating fibrocytes4,5,6,7,8,9. Conceivably, the size of the fibroblast population, defined by the dynamic balance of fibroblast generation and depletion, is a major determinant of renal fibrotic lesions and kidney dysfunction. Despite significant advances in our understanding of the origins and activation of matrix-producing fibroblasts in recent years, little is known on the regulation of fibroblast fate in diseased kidneys. Furthermore, it remains completely elusive as to the mediators and mechanisms controlling fibroblast apoptosis and depletion in vivo.
Wnt/β-catenin is a highly conserved signaling cascade associated with a myriad of biologic actions10,11,12. Upon binding to their plasma membrane receptors, Wnts trigger a series of signaling events that result in stabilization of β-catenin, which then enters the nucleus to control the expression of its target genes by interacting with the T cell factor (TCF)/lymphoid enhancer factor (LEF) family of transcription factors. One downstream target gene of Wnt/β-catenin signaling is matrix metalloproteinase-7 (MMP-7), a secreted, zinc- and calcium-dependent endopeptidase that degrades a broad range of ECM substrates13,14. MMP-7 is also known to cleave non-ECM proteins such as E-cadherin and Fas Ligand (FasL), and therefore plays important roles in the regulation of diverse biologic processes such as epithelial-mesenchymal transition (EMT) and cell apoptosis15,16,17. We have previously shown that urinary MMP-7 is a surrogate marker that predicts renal Wnt/β-catenin activity in diseased kidneys18.
Activation of Wnt/β-catenin signaling is a common pathologic finding in a wide variety of CKDs19,20,21,22. Of interest, β-catenin is predominantly up-regulated in renal tubular epithelium of the fibrotic kidneys, suggesting that tubular cells are the major targets of canonical Wnt signaling. Earlier studies indicate that pharmacologic inhibition of Wnt/β-catenin signaling by different approaches is renoprotective, leading to amelioration of renal fibrosis19,23,24. In vitro, activation of β-catenin in tubular epithelial cells induces EMT, as well as the expression of several fibrosis-related genes such as Snail1, plasminogen activator inhibitor 1 (PAI-1), MMP-7 and fibronectin18,25,26. These results suggest that activation of tubular β-catenin probably plays a critical role in the pathogenesis and progression of renal fibrosis. However, the exact role of tubular β-catenin in renal fibrogenesis in vivo remains poorly understood.
In this study, by using conditional knockout mice in which β-catenin is genetically ablated in a tubule-specific fashion, we investigated the role of tubular β-catenin in a mouse model of renal fibrosis induced by unilateral ureteral obstruction (UUO). Surprisingly, we found that loss of tubular β-catenin did not result in any difference in the severity and magnitude of renal fibrosis after UUO. However, we show that tubular β-catenin signaling controls interstitial fibroblast apoptosis through epithelial-mesenchymal communication (EMC) mediated by MMP-7 and FasL. As such, tubular β-catenin plays a pivotal role in fibroblast homeostasis.
Results
Tubule-specific ablation of β-catenin does not affect renal fibrosis
We first sought to confirm that endogenous β-catenin is depleted in a tubule-specific fashion in conditional β-catenin knockout mice (Ksp-β-cat-/-). As shown in Figure 1a, β-catenin was induced predominantly in renal tubular epithelium at 7 days after unilateral ureteral obstruction (UUO) in control mice, as previously reported19. However, β-catenin induction was largely abolished in the tubular epithelium of Ksp-β-cat-/- mice (Figure 1, a and b). Similar results were obtained by Western blot analyses of whole kidney lysates (Figure 1, c and d). These data confirm an efficient depletion of endogenous β-catenin in a tubule-specific fashion.
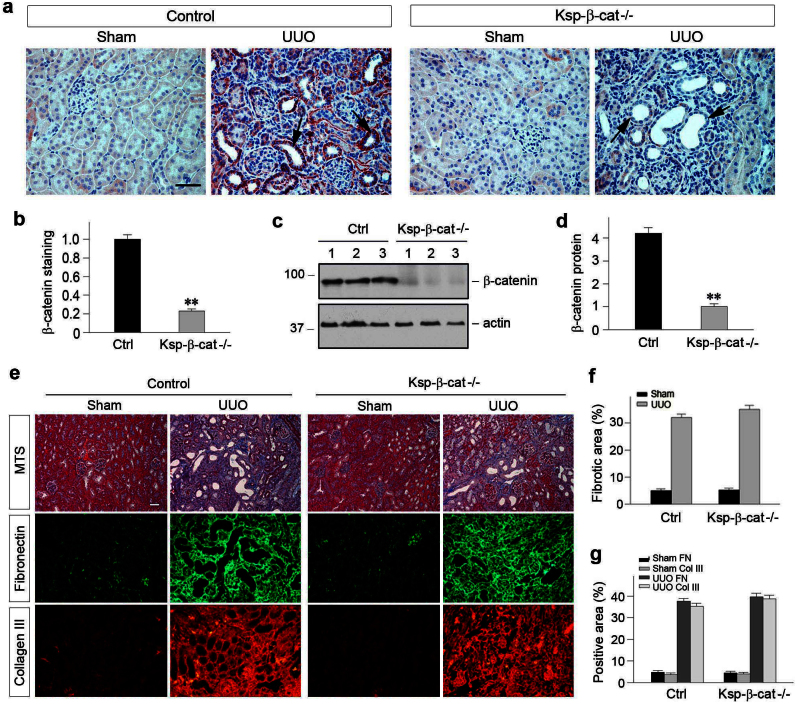
Figure 1. Tubule-specific ablation of β-catenin does not affect the severity of renal fibrosis after obstructive injury.
(a) Expression of β-catenin in the obstructed kidneys in Ksp-β-cat-/- mice and their control littermates. Kidney sections were immuno-stained with specific antibody against β-catenin and semi-quantitative analysis is presented (b). (c, d) Western blot analyses demonstrate renal β-catenin levels in Ksp-β-cat-/- and control mice, quantitative data are presented. **P < 0.01 versus controls. (e) Loss of tubular β-catenin resulted in little difference in collagen deposition and renal fibrotic lesions after UUO. Representative micrographs (e) of Masson's Trichrome staining and quantitative determination (f) are presented. Loss of tubular β-catenin did not affect fibronectin and collagen III expression in the obstructed kidneys at 7 days after UUO. Representative micrographs (e) of immunofluorescence staining for fibronectin and collagen III and quantitative determination (g) are presented. Scale bar, 50 μm.
We next examined the effect of tubule-specific depletion of β-catenin on renal interstitial fibrosis induced by obstructive injury. As shown in Figure 1, e through g, loss of tubular β-catenin did not have any significant impact on the severity of renal fibrosis after UUO. Virtually identical levels of collagen deposition and renal fibrotic lesions were observed in the obstructed kidneys in Ksp-β-cat-/- and control mice, as illustrated by Masson's Trichrome staining. Similar results were obtained by immunofluorescence staining for fibronectin and collagen III (Figure 1, e and g).
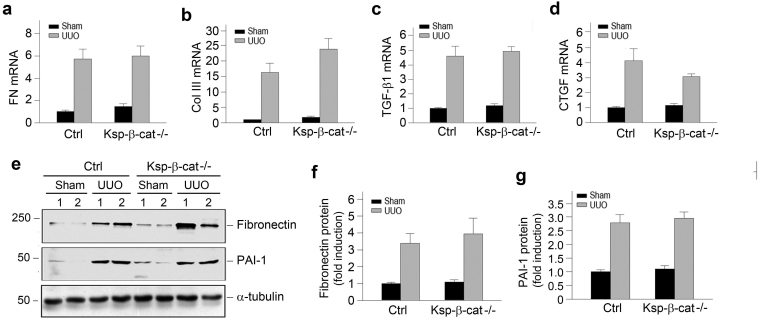
We further examined the expression of various fibrosis-related genes by quantitative, real-time RT-PCR. As shown in Figure 2, a through d, tubule-specific ablation of β-catenin had no effect on renal mRNA expression of numerous fibrosis-related genes such as fibronectin, collagen III, transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF). Similarly, there was no significant difference in renal expression of fibronectin and plasminogen activator inhibitor-1 (PAI-1) proteins between Ksp-β-cat-/- and control mice, as shown by Western blot analyses (Figure 2, e through g). Together, these data indicate that depletion of endogenous tubular β-catenin displays little impact on the development of renal fibrosis.
Figure 2. Loss of tubular β-catenin does not affect fibrosis-related gene expression.
(a through d) Tubule-specific ablation of β-catenin exhibited little effect on renal expression of major fibrosis-related genes including fibronectin (a), collagen III (b), TGF-β1 (c) and connective tissue growth factor (CTGF) (d). No significant difference was found between Ksp-β-cat-/- and control mice. (e) Representative Western blot analyses show protein expression of fibronectin and PAI-1. (f, g) Relative levels of renal fibronectin (f) and PAI-1 (g) in Ksp-β-cat-/- and control mice at 7 days after UUO. No significant difference was found between Ksp-β-cat-/- and control mice.
Depletion of tubular β-catenin reduces epithelial-mesenchymal transition in vivo
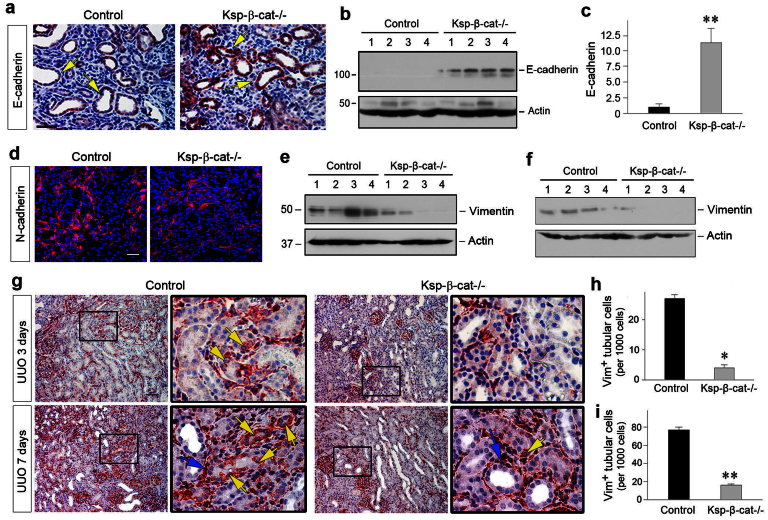
Given that β-catenin signaling is instrumental in mediating epithelial-mesenchymal transition (EMT) in vitro25, we next investigated the effect of tubule-specific loss of β-catenin on this process after obstructive injury. E-cadherin, a tubular epithelial adherens receptor, was largely diminished in the tubular epithelium of control mice at 7 days after UUO27. However, as shown in Figure 3, depletion of β-catenin in Ksp-β-cat-/- mice resulted in an upregulation of E-cadherin expression in renal tubules at 7 days after UUO (Figure 3, a through c). As loss of E-cadherin is an early and key step in EMT28, these data suggest that tubule-specific loss of β-catenin preserves tubular epithelial integrity and prevents EMT after obstructive injury in vivo.
Figure 3. Loss of tubular β-catenin reduces tubular epithelial-mesenchymal transition (EMT) after UUO.
(a) Loss of tubular β-catenin preserves tubular epithelial E-cadherin expression. Immunohistochemical staining showed an increased E-cadherin expression in tubular epithelium in Ksp-β-cat-/- kidneys at 7 days after UUO, compared with controls. Arrows indicate E-cadherin-positive tubules. Scale bar, 50 μm. (b, c) Western blot analyses demonstrate that loss of tubular β-catenin preserved E-cadherin protein in the obstructed kidneys. Representative Western blot (b) and quantitative data (c) are presented. **P < 0.01 versus controls. (d) Loss of tubular β-catenin reduces N-cadherin expression in the obstructed kidneys. Representative micrographs show N-cadherin protein expression in control and Ksp-β-cat-/- kidneys at 7 days after UUO. Scale bar, 50 μm. (e, f) Western blots demonstrate renal vimentin expression in the obstructed kidneys at 3 days (e) or 7 days (f) after UUO. Numbers (1, 2, 3 and 4) denote each individual animal in a given group. (g) Immunohistochemical staining showed reduced tubular vimentin-positive staining in the Ksp-β-cat-/- mice at 3 and 7 days after UUO, respectively. Boxed areas are enlarged. Yellow arrows indicate vimentin-positive tubular cells. Blue arrows indicate vimentin-positive interstitial cells. Scale bar, 50 μm. (h, i) Quantitative data on vimentin-positive tubular cells are presented. Data are presented as vimentin-positive tubular cells per 1000 tubular cells in the obstructed kidneys at 3 days (h) or 7 days (i) after UUO. *P < 0.05, **P < 0.01 versus controls (n = 4–6).
EMT is often accompanied by an E-cadherin/N-cadherin switch29,30. We therefore examined N-cadherin expression by immunofluorescence staining. As shown in Figure 3d, concomitant with an increased E-cadherin, N-cadherin expression was reduced in the obstructed kidneys of Ksp-β-cat-/- mice at 7 days after UUO, compared with the controls. We further investigated the expression and localization of vimentin, an intermediate cytoskeleton protein and a specific marker for mesenchymal cells1, in the obstructed kidneys at different time points after UUO. As shown in Figure 3, e and f, Western blot analyses revealed a substantial reduction of vimentin in Ksp-β-cat-/- kidneys at 3 and 7 days after UUO, respectively, compared to controls. Immunohistochemical staining showed a significant number of tubular cells stained positively for vimentin in the obstructed kidneys of control mice (Figure 3g, yellow arrows). However, tubular expression of vimentin was rare in the Ksp-β-cat-/- kidneys at different time points after UUO. Quantitative data on vimentin-positive tubular cells are presented in Figure 3, h and i. These results illustrate that loss of β-catenin in a tubule-specific fashion reduces EMT in vivo.
Depletion of β-catenin in renal tubules does not alter the size of the fibroblast population
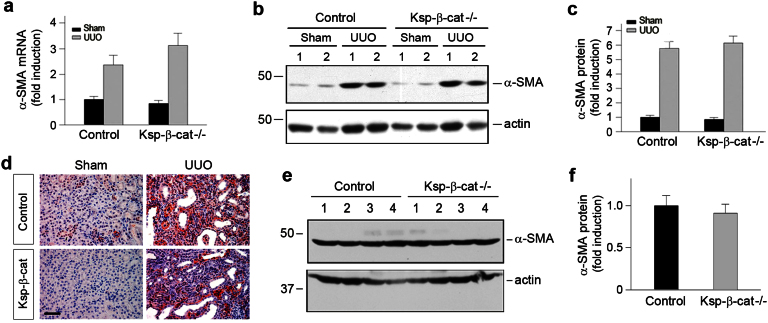
Despite a reduced tubular EMT (Figure 3), we found that depletion of β-catenin in renal tubules did not significantly affect the pool size of renal myofibroblasts, a unique cell population that is believed to be the principal matrix-producing cells in fibrotic tissues1,7,8. As shown in Figure 4a, qRT-PCR demonstrated no significant differences in the expression of α-SMA, the molecular signature of myofibroblasts, between Ksp-β-cat-/- and control kidneys at 7 days after UUO. Similarly, Western blot analyses and immunohistochemical staining also revealed comparable α-SMA protein expression in the obstructed kidneys at 7 days after UUO (Figure 4, b through d), suggesting a similar size of the myofibroblast population. Consistently, no difference in α-SMA expression was found in the obstructed kidneys between control and Ksp-β-cat-/- mice at 3 days after UUO (Figure 4, e and f). These data suggest that despite a reduced contribution by EMT, the pool size of renal myofibroblast remains unchanged in the Ksp-β-cat-/- kidneys after UUO.
Figure 4. Loss of tubular β-catenin does not affect the size of renal myofibroblast population.
(a) Quantitative determination of α-SMA mRNA expression in the obstructed kidneys at 7 days after UUO by qRT-PCR (n = 5). (b, c) Western blot analyses demonstrate no significant difference in renal α-SMA protein expression in Ksp-β-cat-/- and control mice at 7 days after UUO. Representative Western blot (b) and quantitative data (c) are shown (n = 5). (d) Immunohistochemical staining demonstrate little difference in the α-SMA-positive myofibroblasts in Ksp-β-cat-/- and control mice. Representative micrographs are presented. Scale bar, 50 μm. (e, f) Western blot analyses demonstrate that there was no difference in α-SMA protein expression in the obstructed kidneys of Ksp-β-cat-/- and control mice at 3 days after UUO. Representative Western blot (e) and quantitative data (f) are shown. Numbers (1, 2, 3 and 4) denote each individual animal in a given group.
Tubular depletion of β-catenin promotes interstitial fibroblast survival
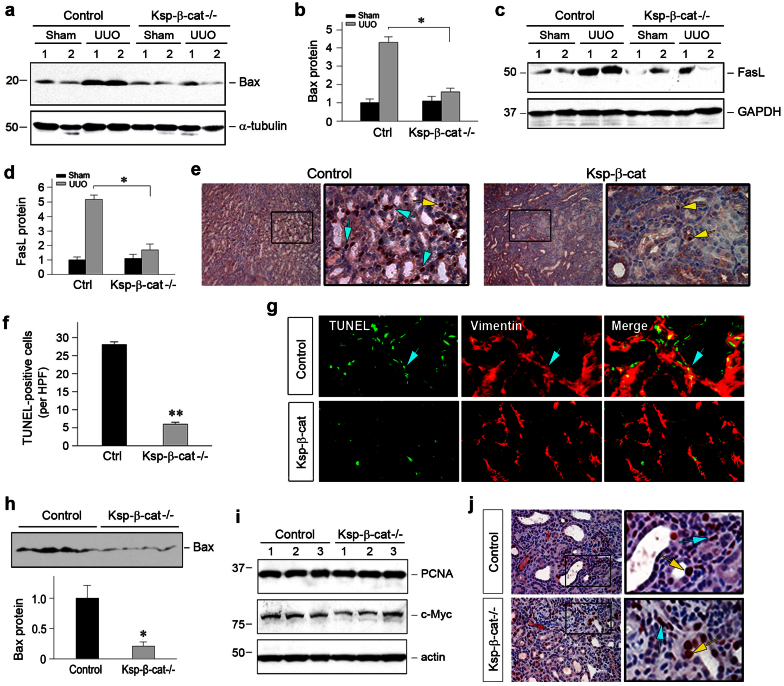
The size of the myofibroblast population is conceivably determined by the balance of the generation and depletion of these cells. Therefore, we examined cell apoptosis. Earlier studies indicate that β-catenin is an important player in tubular cell survival after acute kidney injury31,32. Surprisingly, we found that apoptosis was substantially reduced in Ksp-β-cat-/- mice after UUO, compared to the controls. As shown in Figure 5, a through d, Western blot analyses of whole kidney lysates showed an overall reduction of pro-apoptotic Bax and FasL protein expression in the obstructed kidneys in Ksp-β-cat-/- mice. Consistently, TUNEL staining revealed significant numbers of apoptotic cells in the obstructed kidneys of control mice at 7 days after UUO, which were largely localized to the interstitial compartment (Figure 5e, blue arrows in boxed area). In Ksp-β-cat-/- mice, apoptotic cells in renal interstitium were decreased, compared to control mice, while tubular cell apoptosis was unchanged (Figure 5e, yellow arrows in the boxed area). To further characterize the identity of apoptotic cells, kidney sections were co-stained with TUNEL and anti-vimentin antibody. As shown in Figure 5g, fewer fibroblasts underwent apoptosis in Ksp-β-cat-/- mice after obstructive injury, compared to control mice. Similar data on Bax expression were also obtained in the obstructed kidneys at 3 days after UUO (Figure 5h). These results suggest that loss of tubular β-catenin promotes interstitial fibroblast survival. Of note, loss of tubular β-catenin did not affect cell proliferation, as no or little difference in renal expression of proliferating cell nuclear antigen (PCNA) and c-Myc was found in Ksp-β-cat-/- and control mice (Figure 5, i and j).
Figure 5. Loss of tubular β-catenin promotes interstitial fibroblast survival.
(a through d) Loss of tubular β-catenin reduced renal expression of Bax and FasL after obstructive injury. Representative Western blot (a, c) and quantitative data (b, d) are presented. Numbers (1, 2) denote each individual animal in a given group. *P < 0.05 versus control mice (n = 5). (e, f) TUNEL staining demonstrated a reduced apoptosis in the obstructed kidneys in Ksp-β-cat-/- mice at 7 days after UUO. Apoptotic cells were largely localized in renal tubulointerstitium (blue arrows). Tubular cell apoptosis was indicated by yellow arrows. Representative micrographs (e) and quantitative data (f) are presented. (g) Double staining showed decreased fibroblast apoptosis after obstructive injury in Ksp-β-cat-/- mice. Vimentin-positive, TUNEL-positive cells were indicated by blue arrows. (h) Loss of tubular β-catenin reduced renal expression of Bax at 3 days after UUO. Western blot (top panel) and quantitative data (bottom panel) are presented. Loading control of the Western blot, as shown by immunoblotting for actin, was presented in Figure 4e. *P < 0.05 versus control mice (n = 4). (i, j) Loss of tubular β-catenin did not affect cell proliferation. Western blot analyses (i) showed no difference in renal expression of proliferating cell nuclear antigen (PCNA) and c-Myc. Numbers (1, 2 and 3) indicate each individual animal in a given group (n = 5). (j) Immunohistochemical staining showed no significant change of PCNA expression in both tubular (yellow arrows) and interstitial compartments (blue arrows) between control and Ksp-β-cat-/- kidneys at 7 days after UUO.
Tubular depletion of β-catenin abolishes renal MMP-7 induction
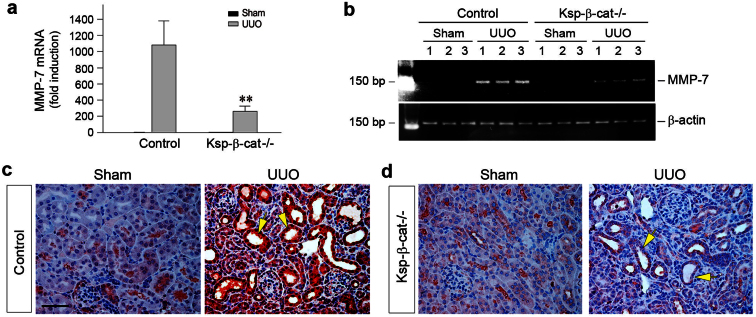
The observation that loss of tubular β-catenin promotes interstitial fibroblast survival clearly suggests an active epithelial-mesenchymal communication (EMC) in the injured kidneys. We hypothesized that such an EMC may be mediated by secreted factors that are regulated by β-catenin. To test this hypothesis, we examined the expression of MMP-7, a direct transcriptional target of β-catenin that is implicated in regulating cell survival. As shown in Figure 6, qRT-PCR analyses revealed a marked reduction of MMP-7 mRNA expression in the obstructed kidneys of Ksp-β-cat-/- mice at 7 days after UUO, compared to control mice. Immunohistochemical staining demonstrated that MMP-7 protein was predominantly induced in the tubular epithelium of the obstructed kidneys after UUO. However, tubule-specific depletion of β-catenin resulted in a dramatic loss of MMP-7 protein expression in Ksp-β-cat-/- mice, consistent with the notion that MMP-7 is a direct target of β-catenin.
Figure 6. Loss of tubular β-catenin reduces MMP-7 expression in obstructive nephropathy.
(a) Quantitative determination of MMP-7 mRNA expression in obstructed kidneys by qRT-PCR. Data are presented as fold induction over sham. **P < 0.01 versus control mice. (b) Representative RT-PCR showed a marked reduction of MMP-7 mRNA expression in the obstructed kidneys of Ksp-β-cat-/- mice at 7 days after UUO. (c, d) Immunohistochemical staining showed MMP-7 protein expression and localization. Representative micrographs showed MMP-7 staining in control and Ksp-β-cat-/- kidneys at 7 days after UUO. Yellow arrows indicate renal tubules. Scale bar, 50 μm.
MMP-7 induces FasL expression and potentiates fibroblast apoptosis
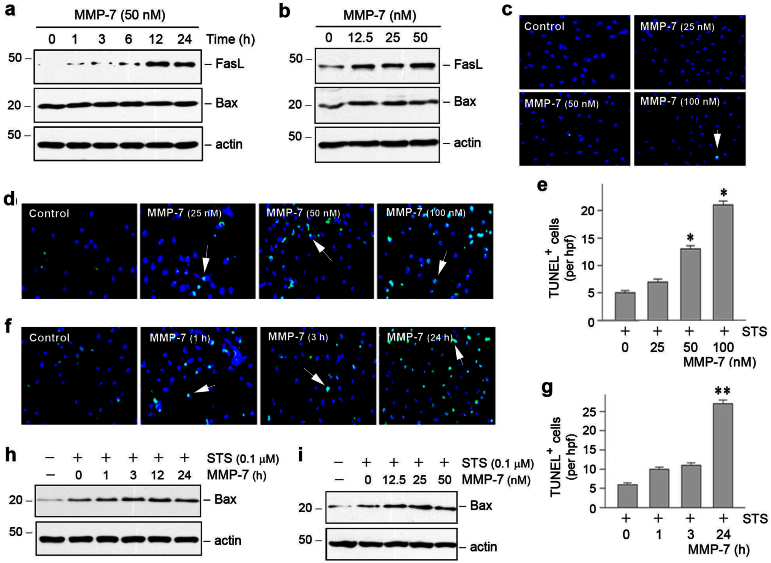
To investigate the role of tubule-derived MMP-7 in controlling interstitial fibroblast apoptosis, we examined the potential of recombinant MMP-7 in regulating key cell survival/apoptosis pathways in vitro. As shown in Figure 7, A and B, MMP-7 induced FasL expression in normal rat kidney interstitial fibroblasts (NRK-49F) in a time- and dose-dependent manner. As FasL is a key player in the extrinsic, receptor-mediated apoptosis pathway33,34, these results suggest that tubule-derived MMP-7 can directly regulate fibroblast survival. Notably, renal FasL expression was markedly induced in the obstructed kidneys after UUO in control mice (Figure 5, c and d). However, tubule-specific depletion of β-catenin abolished FasL induction, presumably due to repression of MMP-7 expression in Ksp-β-cat-/- kidneys.
Figure 7. MMP-7 induces Fas ligand expression in renal interstitial fibroblasts and potentiates fibroblast apoptosis.
(a, b) Recombinant MMP-7 induced Fas ligand (FasL) expression in a time- and dose-dependent manner. Normal rat kidney interstitial fibroblast (NRK-49F) cells were incubated with 50 nM of MMP-7 for various periods of time (a), or different doses of MMP-7 for 24 h (b). Cell lysates were immunoblotted with antibodies against FasL, Bax and actin, respectively. (c) MMP-7 alone did not significantly induce fibroblast apoptosis. (d through g) MMP-7 potentiated fibroblast apoptosis induced by staurosporine (STS). NRK-49F cells were incubated with different doses of MMP-7 for 24 h (d, e) or 50 nM of MMP-7 for various periods of time (f, g) in the presence of 0.1 μM staurosporine. Representative micrographs (d, f) and quantitative data (e, g) are presented. (h, i) MMP-7 promoted Bax induction in renal interstitial fibroblasts. NRK-49F cells were incubated with 50 nM of MMP-7 for various periods of time (h) or different doses of MMP-7 for 24 h (i) in the presence of 0.1 μM staurosporine. Cell lysates were immunoblotted with antibodies against Bax and actin, respectively.
To ascertain the direct involvement of tubule-derived MMP-7 in controlling the fate of interstitial fibroblasts, we examined the ability of exogenous MMP-7 to induce fibroblast apoptosis in vitro. As shown in Figure 7, a through c, incubation of NRK-49F cells with MMP-7 alone did not significantly influence cell survival/apoptosis, as Bax expression and apoptosis detected by TUNEL staining were not significantly changed. However, MMP-7 markedly potentiated staurosporine-triggered fibroblast apoptotic cell death. In the presence of staurosporine, MMP-7 promoted renal interstitial fibroblast apoptosis in a time- and dose-dependent manner, as illustrated by TUNEL staining (Figure 7, d through g). Consistently, MMP-7 also induced pro-apoptotic Bax protein expression in NRK-49F cells in the presence of staurosporine (Figure 7, h and i). These results demonstrate that tubule-derived MMP-7 mediates an active epithelial-mesenchymal communication, thereby controlling interstitial fibroblast fate in fibrotic kidneys.
Discussion
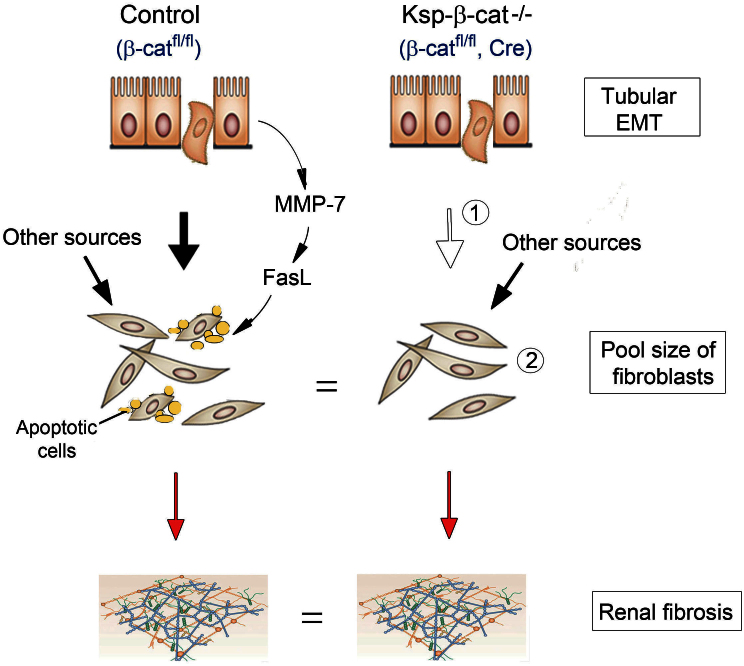
Given that β-catenin is predominantly induced in renal tubular epithelium in fibrotic kidneys and that pharmacological inhibition of β-catenin signaling is renal protective19,25, we originally speculated that genetic ablation of tubular β-catenin protects kidneys against development of fibrotic lesions. To our surprise, knockout of β-catenin in a tubule-specific fashion did not alter the severity of renal fibrosis after obstructive injury. Furthermore, the size of the myofibroblast population, reflected by renal α-SMA expression, was similar between control and Ksp-β-cat-/- mice at different time points after UUO. As illustrated in Figure 8, in control mice, tubular activation of β-catenin after injury leads to EMT (solid arrow) and upregulates tubular MMP-7 expression and secretion18,25,35. As a result, secreted MMP-7 induces FasL expression in interstitial fibroblasts and potentiates their apoptosis. However, in Ksp-β-cat-/- mice, despite presumably less fibroblasts being generated from tubular EMT in the absence of β-catenin (empty arrow), interstitial fibroblasts have a prolonged life-span due to reduced apoptosis. Therefore, the net size of the interstitial myofibroblast pool in control and Ksp-β-cat-/- kidneys is actually unchanged, which results in similar fibrotic lesions. These data demonstrate that tubular β-catenin controls interstitial fibroblast fate via MMP-7-mediated epithelial-mesenchymal communication.
Figure 8. Schematic model depicts that tubular β-catenin controls interstitial fibroblast fate via epithelial-mesenchymal communication.
In control mice, tubular activation of β-catenin after injury leads to EMT (solid arrow) and upregulates tubular MMP-7 expression and secretion. This induces FasL expression in fibroblasts and potentiates their apoptosis. However, in Ksp-β-cat-/- mice, despite less EMT (empty arrow), fibroblasts have a prolonged life-span due to diminished apoptosis. Therefore, the pool size of interstitial fibroblasts in Ksp-β-cat-/- is unchanged, which results in similar fibrotic lesions. It is assumed that loss of tubular β-catenin does not affect fibroblast generation from other sources.
Activated fibroblasts, characterized by α-SMA and type I collagen expression, are the principal matrix-producing cells that play a central role in the scar formation of kidney parenchyma1,3. While the origins and activation processes of interstitial fibroblasts have been extensively studied in recent years4,5,6,36,37, little is known about the fate of interstitial fibroblasts after activation in diseased kidneys. In this regard, the present study represents the first report on the regulation of interstitial fibroblast fate in vivo, and provides significant insights into the mediators and mechanisms underlying fibroblast apoptosis. The fact that the survival or death of interstitial fibroblasts is dictated by the β-catenin signaling in renal tubules demonstrates an active crosstalk between tubular epithelium and interstitial fibroblasts during renal fibrogenesis, consistent with a recent report that tubule-derived sonic hedgehog (shh) targets interstitial fibroblasts for their activation38. It should be pointed out that despite a reduced rate of apoptosis, the volume of fibroblast population in Ksp-β-cat-/- kidneys after UUO is similar to that in controls (Figure 8). This suggests a possible deficiency in fibroblast generation in the absence of tubular β-catenin in Ksp-β-cat-/- mice, as the size of the fibroblast pool is determined by the balance between its generation and its depletion via apoptosis. Although activated fibroblasts could originate from different sources such as quiescent fibroblasts, pericytes, or tubular epithelial cells via distinct pathways1,6,7, it is conceivable that a diminished tubular EMT may account for less efficiency in fibroblast generation in Ksp-β-cat-/- kidneys. This notion is supported by the observation that β-catenin activation induces tubular EMT in vitro25. Consistently, tubular epithelial cell E-cadherin is largely preserved and de novo expression of vimentin in renal tubules is prevented in Ksp-β-cat-/- kidneys after obstructive injury (Figure 3). Taken together, these results are in harmony with the notion that loss of β-catenin reduces tubular EMT in vivo.
The present study has identified secreted MMP-7 as the intercellular agent that mediates tubular epithelial-interstitial fibroblast communication. This notion is supported by several lines of evidence. First, MMP-7 is a direct transcriptional target of canonical Wnt/β-catenin signaling18,39, and is induced specifically in renal tubular epithelium (Figure 6c). Second, ablation of tubular β-catenin abolishes MMP-7 induction in renal tubules after UUO in vivo (Figure 6d). Third, MMP-7 potentiates interstitial fibroblast apoptosis in vitro (Figure 7). Finally, loss of tubular β-catenin/MMP-7 results in decreased fibroblast apoptosis in vivo (Figure 5). Collectively, these findings strongly implicate MMP-7 in regulating fibroblast fate via epithelial-mesenchymal communication, although we cannot exclude other possibilities.
It should be noted that MMP-7 could also influence renal fibrosis by controlling EMT via a multitude of mechanisms13,18. First, MMP-7 induces proteolytic degradation of E-cadherin15,18, an epithelial cell adhesion receptor that is essential for the maintenance of tubular epithelial integrity. As destruction of E-cadherin is the initial step during EMT28, MMP-7-mediated E-cadherin ectodomain shedding undoubtedly contributes to the tubular epithelial injury and promotes EMT in diseased kidneys. Furthermore, as MMP-7 degrades collagen IV and laminin, the major components of tubular basement membrane (TBM), it is conceivable that increased MMP-7 would aggravate TBM damage, which facilitates tubular EMT in the fibrotic kidneys13. Consistently, tubule-specific knockout of β-catenin leads to suppression of MMP-7 expression and preservation of E-cadherin, as well as inhibition of tubular vimentin expression. This provides a rational explanation for the reduced EMT observed in Ksp-β-cat-/- kidneys after UUO.
In addition to regulating EMT, we report here a novel function for MMP-7 in controlling fibroblast survival and apoptosis in diseased kidneys. Apoptosis is a programmed cell death regulated by either intrinsic, mitochondria-dependent or extrinsic, receptor-mediated mechanisms33,40,41. Of the extrinsic apoptosis pathways, the engagement of Fas and FasL on adjacent cells is an important pathway in controlling cell death, and earlier studies demonstrate that MMP-7 protects tumor cells against apoptosis by proteolytically cleaving FasL16,42. However, we found that MMP-7 induces FasL expression in renal interstitial fibroblasts (Figure 7). Consistently, depletion of tubular β-catenin in Ksp-β-cat-/- mice leads to MMP-7 suppression and abolishes renal FasL induction (Figure 5), which results in the selective protection of interstitial fibroblasts from apoptosis, leading to a prolonged life-span of fibroblasts in Ksp-β-cat-/- kidneys. Of note, although MMP-7 induces FasL expression in vitro, it alone does not significantly trigger fibroblast apoptosis in vivo, as illustrated by Bax expression and TUNEL staining (Figure 7). Nevertheless, it potentiates staurosporine-induced fibroblast death. As staurosporine triggers cell apoptosis via an intrinsic, mitochondria-dependent pathway43,44, these results suggest that fibroblast depletion in vivo probably requires a synergy between extrinsic and intrinsic apoptosis pathways.
The present study also shows that tubular depletion of β-catenin does not significantly affect tubular cell apoptosis after UUO (Figure 5). This finding is not in line with early reports suggesting that β-catenin plays a role in promoting tubular cell survival after acute kidney injury (AKI)31,32. Such a discrepancy could be related to the distinct nature of the injuries in AKI versus CKD. Therefore, the impact of β-catenin on renal cell survival/apoptosis varies depending on the type of cells being studied as well as the nature of injury being induced. Along this line, it should also be pointed out that despite the insignificant impact on fibrosis after genetic ablation of tubular β-catenin (Figures 1 and 2), numerous studies indubitably demonstrate that blockade of Wnt/β-catenin via different approaches is effective in preventing fibrotic lesions after kidney injury19,23,24,25. As the up-regulation of Wnt/β-catenin is a common finding in virtually all kinds of CKD20,21, whether the result of genetic knockout of β-catenin prior to injury is relevant to clinical setting is uncertain. Furthermore, we also cannot exclude the possibility that these results could be due to the specific model used and might not be applicable to other models of fibrosis. Due to these limitations, one should exercise cautions when interpret the data. Nonetheless, our studies unravel a novel mechanism by which tubular β-catenin signaling controls the fate of interstitial fibroblasts via MMP-7/FasL pathway. This study also illustrates that fibroblast apoptosis/depletion, in addition to its generation, is another major determinant of the net mass of the fibroblast population and the severity of renal fibrosis in diseased kidneys.
Methods
Animal model
Generation and genotyping of conditional knockout mice (Ksp-β-cat-/-) with tubule-specific ablation of β-catenin was described previously31. The breeding protocol generated offspring with 50% Ksp-β-cat-/- mice (genotype β-catfl/fl, Cre) and 50% control mice (genotype β-catfl/fl) within the same litters. All animals were born at the expected Mendelian frequency; and they were normal in size and did not display any gross physical or behavioral abnormalities. Age- and sex- matched Ksp-β-cat-/- and control mice were used. Unilateral ureteral obstruction (UUO) was performed as described previously38. At 3 or 7 days after surgery, groups of mice were euthanized, and kidney tissues collected for subsequent analyses. Animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Histology and immunohistochemical staining
Paraffin-embedded mouse kidney sections (3-μm thickness) were prepared by a routine procedure. The sections were stained with hematoxylin-eosin (HE), periodic acid-Schiff (PAS) and Masson's Trichrome staining reagents by standard protocol, respectively. Immunohistochemical staining was performed according to the established protocol as described previously23. The antibodies against β-catenin (ab15180; Abcam, Cambridge, MA), α-SMA (Ab5694-100; Abcam), E-cadherin (#3195; Cell Signaling Technology, Danvers, MA), vimentin (#5741s; Cell Signaling Technology), PCNA (sc-56), Bax (sc-493; Santa Cruz Biotechnology, Santa Cruz, CA) and MMP-7 (#104658; GeneTex Inc., Irvine, CA) were used.
Immunofluorescence staining and confocal microscopy
Kidney cryosections were fixed with 3.7% paraformaldehyde for 15 min at room temperature and immersed in 0.2% Triton X-100 for 10 min. After blocking with 10% donkey serum in PBS for 1 h, slides were immunostained with following antibodies: anti-fibronectin (F3648; Sigma, St. Louis. MO), anti-collagen III (#234189; EMD Millipore, Billerica, MA), anti-vimentin (#5741s; Cell Signaling Technology) and anti-N-cadherin (610920; BD Transduction Laboratories, San Jose, CA). To visualize the primary antibodies, slides were stained with cyanine Cy2- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Stained slides were viewed under a Leica TCS-SL confocal microscope equipped with a digital camera (Buffalo Grove, IL).
Detection of apoptotic cells
Apoptotic cell death was determined by using terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) staining with DeadEnd Colorimetric or Fluorometric Apoptosis Detection System (Promega, Madison, WI), as described previously31.
Real-time RT-PCR
Total RNA isolation and real-time RT-PCR were carried out by the procedures described previously38. Briefly, first strand cDNA synthesis was carried out by using a reverse transcription system kit according to the instructions of the manufacturer (Promega). Quantitative, real-time RT-PCR (qRT-PCR) was performed on an ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA) as described previously. The sequences of the primer pairs for mouse fibronectin, collagen III and α-SMA were reported previously25. The primer pairs for TGF-β1, CTGF and MMP7 were as follows: mouse TGF-β1, 5′-GTGGAAATCAACGGGATCAG-3′ (sense) and 5′-GTTGGTATCCAGGGCTCTCC-3′ (anti-sense); mouse CTGF, 5′-CCTGGTCCAGACCACAGAGT-3′ (sense) and 5′-TTTTCCTCCAGGTCAGCTTC-3′ (anti-sense); mouse MMP-7, 5′- TAGGCGGAGATGCTCACTTT -3′ (sense) and 5′- TTCTGAATGCCTGCAATGTC -3′ (anti-sense). PCR was run by using standard conditions. The mRNA levels of various genes were calculated after normalizing with β-actin.
Western blot analysis
Kidney tissues were lysed with radioimmunoprecipitation assay (RIPA) buffer containing 1% NP-40, 0.1% SDS, 100 μg/ml PMSF, 1% protease inhibitor cocktail, and 1% phosphatase I and II inhibitor cocktail (Sigma) in PBS on ice. The supernatants were collected after centrifugation at 13,000 × g at 4°C for 15 min. Protein expression was analyzed by Western blot analysis as described previously25. The primary antibodies used were as follows: anti-β-catenin (#610154; BD Transduction Laboratories, San Jose, CA), anti-fibronectin (F3648; Sigma), anti-α-SMA (A2547; Sigma), anti-PAI-1 (sc-5297), anti-E-cadherin (#3195; Cell Signaling Technology, Danvers, MA), anti-vimentin (V2258; Sigma, St. Louis, MO), anti-Bax (sc-493), anti-FasL (sc-6237), anti-PCNA (sc-56), anti-c-Myc (sc-40s) and anti-actin (sc-1616) (Santa Cruz Biotechnology), anti-α-tubulin (T9026; Sigma) and anti-GAPDH (AM4300; Ambion, Austin, TX).
Cell culture and transfection
Normal rat kidney interstitial fibroblast (NRK-49F) cells were obtained from ATCC (Manassas, VA). Cells were maintained as described previously25. Serum-starved NRK-49F cells were treated with recombinant MMP-7 (#444270; EMD Chemicals, Gibbstown, NJ) for various periods of time at the concentrations as indicated. For some experiments, NRK-49F cells were also treated with 0.1 μM staurosporine (S4400; Sigma) and MMP-7 for various periods of time as indicated, and then subjected to TUNEL staining and Western blot analyses, respectively.
Statistical analyses
All data were expressed as mean ± SEM. Statistical analysis of the data was performed using SigmaStat software (Jandel Scientific Software, San Rafael, CA). Comparison between groups was made using one-way ANOVA, followed by the Student-Newman-Keuls test. P < 0.05 was considered significant.
Author Contributions
D.Z. and Y.L. conceived the study and designed the experiments; D.Z., R.J.T., L.Z. and Y.L. performed the experiments; D.Z. and Y.L. analyzed the data; D.Z., R.J.T. and Y.L. wrote the manuscript, and all authors reviewed the manuscript.
Acknowledgments
We thank Ms. L. Lin for her excellent technical assistance in genotyping the mice. This work was supported by NSFC grant 81130011, National Institutes of Health grants DK064005 and DK091239.
References
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7, 684–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M. & Neilson E. G. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21, 1819–1834 (2010). [DOI] [PubMed] [Google Scholar]
- Boor P., Ostendorf T. & Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6, 643–656 (2010). [DOI] [PubMed] [Google Scholar]
- Iwano M. et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110, 341–350 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176, 85–97 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M. & Duffield J. S. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol 21, 1247–1253 (2010). [DOI] [PubMed] [Google Scholar]
- Grgic I., Duffield J. S. & Humphreys B. D. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr Nephrol 27, 183–193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz F. How many different roads may a cell walk down in order to become a fibroblast? J Am Soc Nephrol 19, 2246–2248 (2008). [DOI] [PubMed] [Google Scholar]
- Herzog E. L. & Bucala R. Fibrocytes in health and disease. Exp Hematol 38, 548–556 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. & Nusse R. Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 (2012). [DOI] [PubMed] [Google Scholar]
- MacDonald B. T., Tamai K. & He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17, 9–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S. & Moon R. T. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10, 468–477 (2009). [DOI] [PubMed] [Google Scholar]
- Tan R. J. & Liu Y. Matrix metalloproteinases in kidney homeostasis and disease. Am J Physiol Renal Physiol 302, F1351–F1361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T., Jung A., Dag S., Hlubek F. & Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 155, 1033–1038 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J. K., Li Q. & Parks W. C. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 162, 1831–1843 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades N., Yu W. H., Poulaki V., Tsokos M. & Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res 61, 577–581 (2001). [PubMed] [Google Scholar]
- Li M., Yamamoto H., Adachi Y., Maruyama Y. & Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med 231, 20–27 (2006). [DOI] [PubMed] [Google Scholar]
- He W. et al. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol 23, 294–304 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W. et al. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20, 765–776 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Toerne C. et al. Wnt pathway regulation in chronic renal allograft damage. Am J Transplant 9, 2223–2239 (2009). [DOI] [PubMed] [Google Scholar]
- Nelson P. J., von Toerne C. & Grone H. J. Wnt-signaling pathways in progressive renal fibrosis. Expert Opin Ther Targets 15, 1073–1083 (2011). [DOI] [PubMed] [Google Scholar]
- Dai C. et al. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20, 1997–2008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Kang Y. S., Dai C. & Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury in adriamycin nephropathy. J Am Soc Nephrol 22, 90–103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran K., Schiavi S. & Hruska K. A. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16, 2373–2384 (2005). [DOI] [PubMed] [Google Scholar]
- Hao S. et al. Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 22, 1642–1653 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W. et al. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J Biol Chem 285, 24665–24675 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. & Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 159, 1465–1475 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15, 1–12 (2004). [DOI] [PubMed] [Google Scholar]
- Zeisberg M. & Neilson E. G. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119, 1429–1437 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Acloque H., Huang R. Y. & Nieto M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009). [DOI] [PubMed] [Google Scholar]
- Zhou D. et al. Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury. Kidney Int 82, 537–547 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. et al. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol 20, 1919–1928 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenschwender M. & Wajant H. The role of FasL and Fas in health and disease. Adv Exp Med Biol 647, 64–93 (2009). [DOI] [PubMed] [Google Scholar]
- Villa-Morales M. & Fernandez-Piqueras J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin Ther Targets 16, 85–101 (2012). [DOI] [PubMed] [Google Scholar]
- Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21, 212–222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaggin S. E. & Kapus A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int 80, 41–50 (2011). [DOI] [PubMed] [Google Scholar]
- Wada T. et al. Involvement of bone-marrow-derived cells in kidney fibrosis. Clin Exp Nephrol 15, 8–13 (2011). [DOI] [PubMed] [Google Scholar]
- Ding H. et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol 23, 801–813 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran K., Simon T. C., Liapis H. & McGuire J. K. Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int 65, 2212–2222 (2004). [DOI] [PubMed] [Google Scholar]
- Havasi A. & Borkan S. C. Apoptosis and acute kidney injury. Kidney Int 80, 29–40 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Wang C. Y., Huang S., Yang T. & Dong Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. American Journal of Physiology - Renal Physiology 296, F983–993 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth S. J. et al. IL-13 and TH2 cytokine exposure triggers matrix metalloproteinase 7-mediated Fas ligand cleavage from bronchial epithelial cells. J Allergy Clin Immunol 126, 366–374 (2010). [DOI] [PubMed] [Google Scholar]
- Hu K. et al. tPA protects renal interstitial fibroblasts and myofibroblasts from apoptosis. J Am Soc Nephrol 19, 503–514 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roucou X., Antonsson B. & Martinou J. C. Involvement of mitochondria in apoptosis. Cardiol Clin 19, 45–55 (2001). [DOI] [PubMed] [Google Scholar]