Abstract
When an ambiguous stimulus is viewed for a prolonged time, perception alternates between the different possible interpretations of the stimulus. The alternations seem haphazard, but closer inspection of their dynamics reveals systematic properties in many bistable phenomena. Parametric manipulations result in gradual changes in the fraction of time a given interpretation dominates perception, often over the entire possible range of zero to one. The mean dominance durations of the competing interpretations can also vary over wide ranges (from less than a second to dozens of seconds or more), but finding systematic relations in how they vary has proven difficult. Following the pioneering work of W. J. M. Levelt (1968) in binocular rivalry, previous studies have sought to formulate a relation in terms of the effect of physical parameters of the stimulus, such as image contrast in binocular rivalry. However, the link between external parameters and “stimulus strength” is not as obvious for other bistable phenomena. Here we show that systematic relations readily emerge when the mean dominance durations are examined instead as a function of “percept strength,” as measured by the fraction of dominance time, and provide theoretical rationale for this observation. For three different bistable phenomena, plotting the mean dominance durations of the two percepts against the fraction of dominance time resulted in complementary curves with near-perfect symmetry around equi-dominance (the point where each percept dominates half the time). As a consequence, the alternation rate reaches a maximum at equi-dominance. We next show that the observed behavior arises naturally in simple double-well energy models and in neural competition models with cross-inhibition and input normalization. Finally, we discuss the possibility that bistable perceptual switches reflect a perceptual “exploratory” strategy, akin to foraging behavior, which leads naturally to maximal alternation rate at equi-dominance if perceptual switches come with a cost.
Keywords: perceptual organization, motion—2D, computational modeling
Introduction
When a stimulus that has two (or more) distinct interpretations is presented for extended time, observers experience alternations between perceiving one interpretation and the other(s). It has been observed in a host of such bistable (or multistable) phenomena that the fraction of time each percept dominates can be affected by parameters of the stimulus (Bonneh, Cooperman, & Sagi, 2001; Hupé & Rubin, 2003; Levelt, 1967, 1968; Moreno-Bote, Shpiro, Rinzel, & Rubin, 2008; Mueller & Blake, 1989; Vallortigara & Bressan, 1991). Furthermore, the fraction of time percept A dominates, denoted fA, can vary over the entire possible range of 0 ≤ fA ≤ 1 (or most of it)—that is, alternations occur not only when the competing percepts are approximately equally probable but also when they are largely unbalanced. Here, we refer the percept that dominates for a larger fraction of the time as the “prevalent” or the “strongest” percept.
Finding how a particular stimulus parameter affects the fraction of time observers spend in each interpretation may not fully characterize the effect of that parameter. This is because for an ambiguous stimulus that gives rise to two bistable percepts, A and B, the mean dominance durations of each of the percepts, denoted TA and TB, may change independently of each other. Thus, although fA and fB must sum to 1, the system has another degree of freedom (in principle), specified by TA or TB. The value of one determines the other (for a given fA), since they are related through TA/[TA + TB] ≅ fA. (Note that both the fractions of dominance and the mean dominance durations have been found to be stable over time for both binocular rivalry and ambiguous motion displays and can therefore serve as reliable experimental measures; Hupé & Rubin, 2003; Mamassian & Goutcher, 2005; Merk & Schnakenberg, 2002; Rubin & Hupé, 2004.)
Consider a bistable stimulus for which, during prolonged presentations, observers spend equal amounts of time, on average, in each of the percepts: fA = fB = 0.5; we will refer to the percepts as “equi-dominant.” Assume that the mean duration is Teq; we therefore have TA = TB = Teq. Figure 1a shows a schematic representation of the time course of perception as observers view this stimulus, alternating between equal durations of percepts A and B (we use regular alternations for illustration purposes). Since these particular values of f and T (0.5 and Teq, respectively) were obtained for a particular parametric setup, changing the value of one (or more) of the parameters of the stimulus may therefore change them. Let us denote by μB a parameter that affects perception such that reducing its value causes a decrease in fB. Now, if TB indeed represents an additional degree of freedom of the system, then the decrease in fB could come about in many ways, corresponding to different combinations of TA and TB that leads to the same reduction in fB. Three specific possibilities are of particular interest here, and they are illustrated in the three panels of Figure 1b: in the top panel, the decrease in fB takes place via a decrease in TB with no change to TA; in the middle panel, there is both a decrease in TB and an increase in TA; and in the bottom panel, TA increases while TB remains unchanged. What determines which of these cases (or an intermediate situation) will, in fact, occur?
Figure 1.
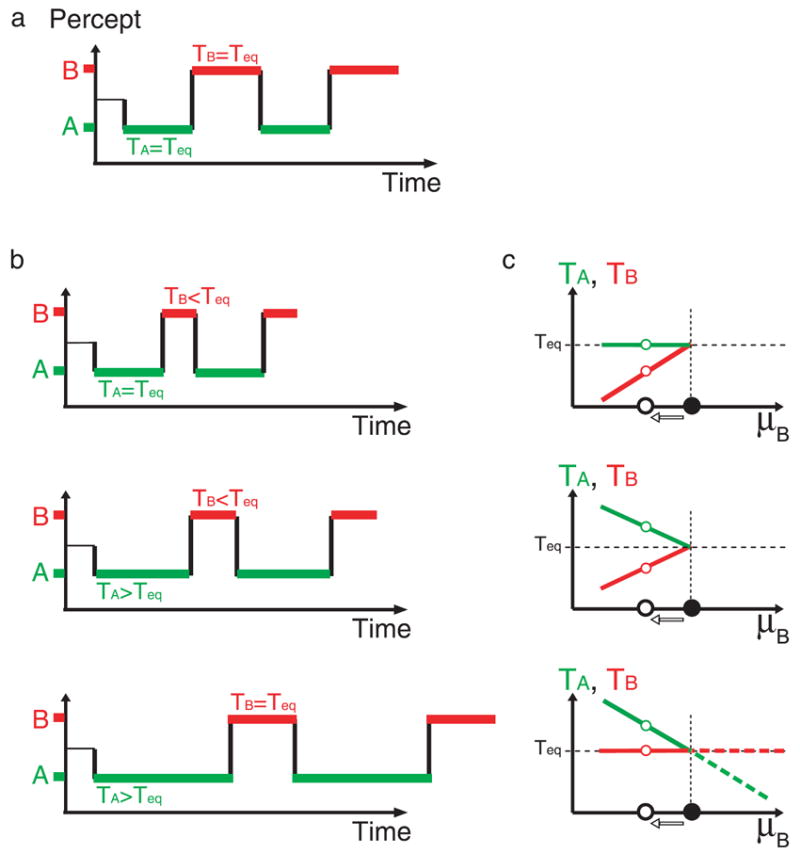
Hypothetical dependences of the mean dominance durations of two percepts occurring during perceptual bistability as a function of the stimulus parameters. (a) An idealized time course of bistable perception reversals when both percepts are equi-dominant with the same mean dominance duration Teq. (b) Three possible ways in which decrements of a stimulus parameter that reduces the fraction of dominance of percept B can result in variations of the mean dominance durations of the two percepts. While the top panel suggests that reducing μB results in a shortening of the mean dominance duration of percept B, TB, the middle suggests both a shortening of TB and a lengthening of TA, and the bottom panel suggests a pure lengthening of TA, the three cases would lead to the same outcome: a reduction of the fraction of dominance of percept B. (c) The corresponding variations of the mean dominance durations as a function of the stimulus parameter being manipulated, μB.
For the domain of binocular rivalry, Levelt (1968) has formulated a set of “propositions” based on experimental observations that offer an answer to the question of how TA and TB change as the parameters of the stimulus change. Interestingly, his results suggest that the answer depends on the particular ways the parameters are manipulated. Specifically, Levelt varied the strength of the image presented to one eye (e.g., by changing its contrast) without changing the strength of the other eye’s image and found that this manipulation affected differentially the mean duration observers spent perceiving each eye’s image. Furthermore, the direction of change was somewhat counterintuitive. Levelt’s (1968) manipulation corresponded to reducing fB by decreasing the contrast of the image shown to eye B (rather than increasing eye A’s contrast). Referring back to Figure 1b, the findings were closest to the bottom panel: i.e., reducing the contrast of eye B primarily affected (increased) the mean duration of the epochs observers spend in eye A, with little or no effect on the mean durations of eye B. The solid red and green lines in the bottom panel of Figure 1c illustrates the same result by plotting TA and TB as a function of the manipulated parameter, denoted μB, over the full range of possible values of fB below the equi-dominance point (i.e., for all fB < 0.5). For the sake of completion, the top and middle panels in Figure 1c represent the other two limiting cases considered in Figure 1b (top and middle panels, respectively) in terms of the (putative) changes to TA and TB as a function of μB.
So far, we have described the experimental manipulation presented above as a reduction of fB from the equi-dominance point, caused by a decrease in μB. However, it is legitimate to describe it as an increase in fB (from a value <0.5) due to an increase in μB. This must be how Levelt (1968) conceptualized the manipulation, since his Proposition II states: “Increasing the stimulus strength in one eye will not affect the average duration of dominance in that eye.” Note that in this phrasing, there is no special consideration of the equi-dominance point. Therefore, Levelt’s (1968) phrasing of Proposition II implies that the further increase of μB (beyond the equi-dominance point) would simply extend the solid red and green lines in Figure 1c (lower panel), as shown by the two corresponding dashed lines. However, in reality, only changes in the range fB < 0.5 were tested directly by Levelt (1968), as well as by several more recent replications (e.g., Bossink, Stalmeier, & De Weert, 1993; Leopold & Logothetis, 1996; Mueller & Blake, 1989).
Recently, Brascamp, Ee, Noest, Jacobs, and Berg (2006) have found that the effect of changing an eye’s contrast on the mean dominance durations of the eyes depends on whether the contrast is lower than the fixed contrast, or larger than that. Varying the contrast below that of the other eye’s fixed contrast produces variations in the mean dominance durations in accord to Levelt’s second proposition. However, if the contrast is changed above the fixed contrast value, the mean dominance duration for the ipsilateral eye is strongly affected, while that for the contralateral eye is weakly affected. In accordance to this result, the authors restated Levelt’s second proposition as follows: “changes in contrast of one eye affect the mean dominance duration of the highest contrast eye” (Brascamp et al., 2006; van Boxtel, van Ee, & Erkelens, 2007; but see Kang, 2009 for the effect of stimulus size on those results). More recently, Klink, van Ee, and van Wezel (2008) showed that a similar behavior holds for another kind of bistable stimulus: they used a rotating random dot sphere and manipulated the dot luminance of one half-sphere. They found that “manipulations of stimulus strength of one perceptual interpretation mainly influence the average dominance duration corresponding to the stronger stimulus.”
In binocular rivalry, the relation between the manipulated parameter (contrast) and “stimulus strength” is intuitive and straightforward. This was the case also for the parameter of dot luminance in the rotating random dot sphere used by Klink et al. (2008). However, for other bistable phenomena the question of how to compare “stimulus strength” for the two competing interpretations is far less straightforward. For example, two superimposed gratings moving in different directions give rise to bistable alternations between the perception of motion transparency and of a single coherent pattern, and varying the angle between the gratings’ directions of motion changes the fraction of dominance time of each percept (Hupé & Rubin, 2003). Does this stimulus manipulation involve change in the “stimulus strength” of the transparent interpretation, the coherent interpretation, or both? The answer is not known. Is it possible to generalize the observations of Brascamp et al. (2006) and Klink et al. (2008) also to bistable stimuli of this kind?
We sought to find a formulation of the effect of parametric changes on mean dominance durations that could be used for all perceptually bistable phenomena (not just those where stimulus strength is easy to infer from the manipulated parameter). The success of such a general formulation will depend on finding a proper measure for the strengths of the percepts, since the parameter manipulations employed to change mean dominance durations can be very different across experiments. We decided to use the fraction of time f that a given percept is dominant as a measure of its strength. Crucially, this measure does not depend on the physical parameters being varied in the stimulus, nor on the arbitrarily chosen units of those parameters. As a natural extension, we defined the stronger (weaker) percept as that with the larger (smaller) f.
We tested three bistable phenomena: binocular rivalry (Blake, 2001; Blake & Logothetis, 2002; Logothetis, 1998; Wheatstone, 1838), ambiguous motion displays (Hupé & Rubin, 2003; Vallortigara & Bressan, 1991; Wallach, 1976; Wilson & Kim, 1994; Wuerger, Shapley, & Rubin, 1996), and gratings’ depth reversals (Moreno-Bote et al., 2008). We find that in all cases the effect of stimulus parameters on the mean dominance durations can be summarized by the proposition: “Parametric manipulations that affect the fraction of dominance of the competing percepts will change the mean dominance duration of the stronger percept more than that of the weaker percept.” This formulation is consistent with those put forward by Brascamp et al. (2006) and Klink et al. (2008), while generalizing them to more bistable stimuli. Furthermore, when plotted against our measure of percept strength (fraction of dominance), the mean dominance times of the two competing percepts show near-perfect symmetry around equi-dominance (the point where each percept dominates half the time). Consequently, the alternation rate (the number of perceptual states reported per unit of time) reaches a maximum at equi-dominance. These results imply that the alternation rate reaches a maximum when the stimulus is maximally ambiguous. Measures of the ambiguity of the stimulus, such as the entropy, are shown to closely correlate with the alternation rate. Finally, we study the behavior of double-well energy models in which the depth of the wells are affected anti-symmetrically by the parameter manipulations, as well as more realistic rate-based models with input normalization, and show that they naturally account for the experimentally observed behaviors.
Methods
Observers
A total of 12 naive observers participated in the experiments; four in Experiment 1 (#1–4; two females), four in Experiment 2 (#5–8; two females), and five in Experiment 3 (#8–12; three females). All observers had normal or corrected-to-normal vision. They were paid $10 per session for their participation and provided informed consent according to the guidelines of the NYU Committee on Activities Involving Human Subjects.
Stimulus
Experiment 1 (binocular rivalry): Two half-square-wave drifting gratings were independently projected to the two eyes of the subjects using custom-made oriented prism glasses, as schematically represented in Figure 2. The gratings were generated on the same screen, one in the top half of the screen, and the second in the bottom half of the screen. Prisms oriented upward and downward and positioned in front of each eye attached to a glass frame collected the images and projected them onto corresponding places of the retinas. The right eye viewed the grating on the top, and the left eye viewed the grating on the bottom. Viewing the stimulus for prolonged periods of time leads to stochastic alternations between perceptual dominance of one grating and perceptual dominance of the other grating, intermingled by short periods of composites. In order to aid stability at the projected images, two concentric annuli (one white with inner radius of 0.92° and outer radius of 1.02°, and a second black with inner radius of 1.02° and outer radius of 1.10°) surrounded the circular aperture of radius 0.55° through which the grating was visible in each eye. Because the surrounding annuli were identical to the two eyes, diplopia was minimized. A central white fixation point of diameter 0.09° and luminance 97 cd/m2 was also added in the center of the two gratings. The contrast (8.6%), the wavelength (0.37°), and speed (1.84°/s) of the grating projected to one eye were fixed, and it moved 45° counterclockwise from the vertical line. Identical parameters were used for the grating moving orthogonally to the other one and presented to the contralateral eye (45° clockwise from the vertical line), except that its contrast varied pseudo-randomly from trial to trial with the values: 3.5, 5.2, 6.9, 8.6, 13.8, 24.5, 53.5, and 100%. The mean luminance of the gratings was 45 cd/m2, which was identical to the luminance of the screen everywhere else. Subjects adjusted their distance to the monitor in order to achieve fusion of the concentric annuli and the fixation point at each grating. Visual angles above were calculated from a subject who sat 57 cm from the screen. Each trial was repeated four times, the fixed parameter grating was presented half of the trials in one eye and the other half in the contralateral eye, in a randomized order.
Figure 2.
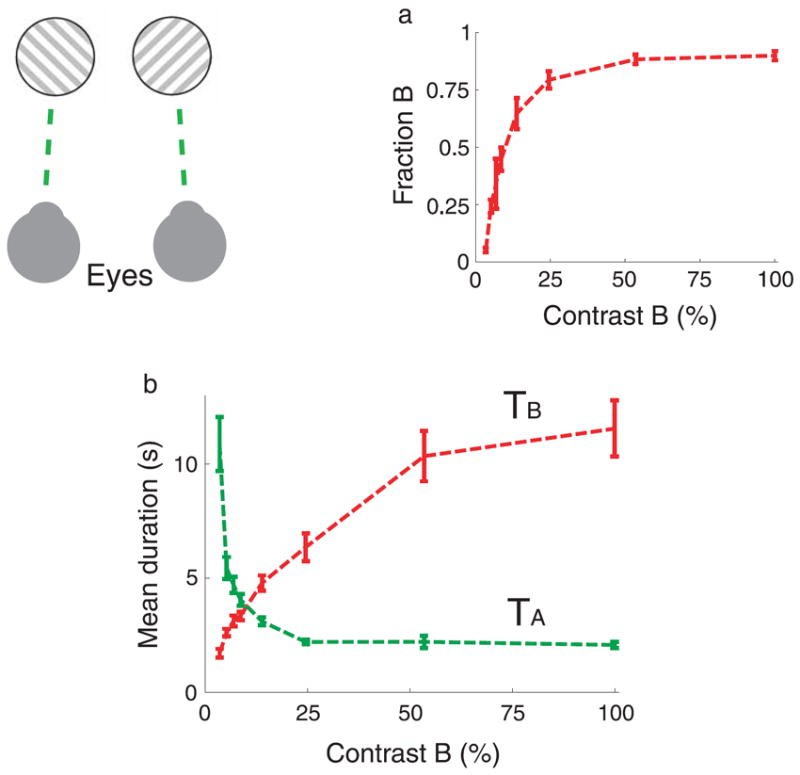
Dependence of (a) fraction of dominance and (b) mean dominance durations on parameter manipulations in binocular rivalry. The contrast of image B is varied from trial to trial while the contrast of image A is fixed.
Experiment 2 (ambiguous plaid motion): The stimulus consisted of two superimposed rectangular-wave gratings moving at different directions, as shown schematically in Figure 3. This stimulus is perceptually bistable as to the number of perceived surfaces: either the two constituent gratings move coherently in a single direction (coherent percept), or the two gratings move in different directions (transparent percept). The luminance of the bars was 30 cd/m2, and that of the background was 76 cd/m2. The gratings have duty cycle equal to 0.2 and wavelength of 2.7°, and move with a speed equal to 5.4°/s. The positions at which the two gratings overlaid (bars intersections) had a luminance of 15 cd/m2 to favor transparent motion (lower luminance that than that of the bars; Stoner, Albright, & Ramachandran, 1990). The angles between the directions of motion of the two gratings were α = 10, 70, 90, 110, 130, 145, 165, 175, 179°. In half of the trials, the plaid moved upward, and in the other half, it moved downward, in a randomized order.
Figure 3.
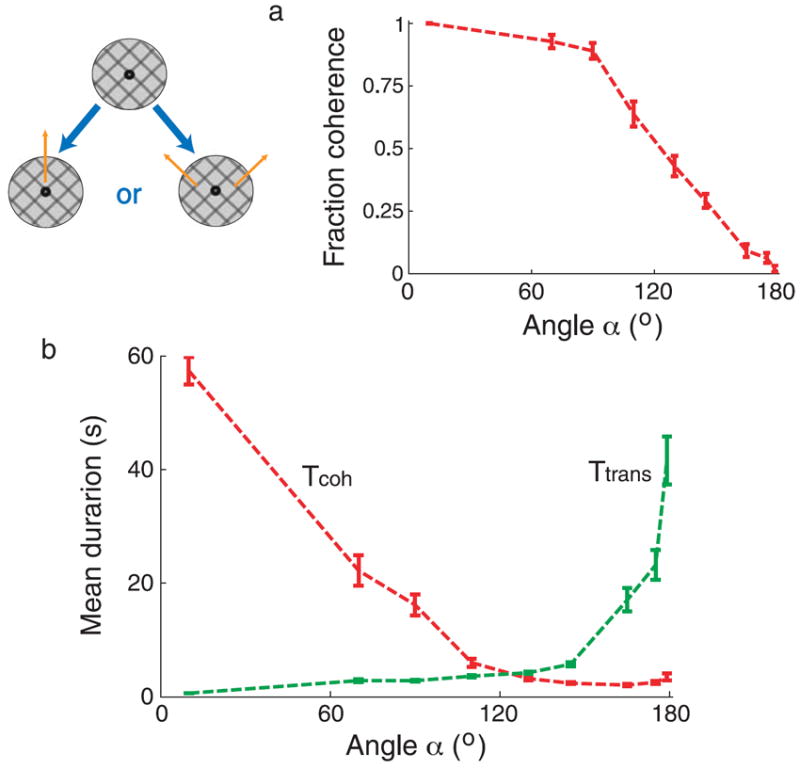
Dependence of (a) fraction of dominance and (b) mean dominance durations on parameter manipulations in bistable motion perception. The angle between the directions of motion of the constituent gratings of a moving plaid is varied.
Experiment 3 (grating’s depth reversals): The stimulus consisted of two superimposed rectangular-wave gratings moving at an angle of 160° between their directions of motion (±80 from the vertical°), as shown schematically in Figure 4. This stimulus is ambiguous as to the depth ordering of the two gratings: one grating can be seen as being behind the other one, or the reversed ordering. One of the two gratings had its wavelength fixed at λ = 2.7°, while the wavelength of the other grating took one of the following values in each trial: λ = 0.9, 1.35, 1.8, 2.25, 2.7, 3.24, 4.05, 5.4, 8.1°. The luminance of the bars (and intersections between grating bars) was 40 cd/m2, and that of the background was 89 cd/m2. Other parameters of the gratings were identical to those in Experiment 2. The global directions of motion of the two gratings were randomized (up-right, up-left, down-right, and down-left; always ±80° from the vertical each; the global directions of motion did not produce any significant effect).
Figure 4.
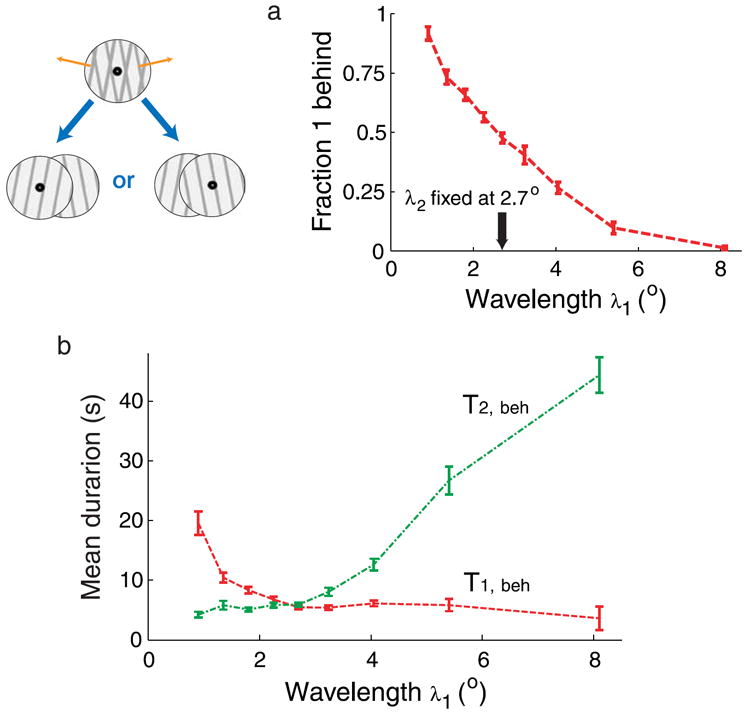
Dependence of (a) fraction of dominance and (b) mean dominance durations on parameter manipulations in bistable depth perception. The wavelength of grating number 1 is varied from trial to trial while the wavelength of grating number 2 is kept fixed.
In Experiments 2 and 3, the stimulus appears within a circular aperture of diameter 12.5°. Luminance outside the aperture was 18 cd/m2. A circular fixation point (radius 0.18°, luminance 58 cd/m2) was overlaid on a small homogeneous circular region (radius 0.9°, luminance 0.2 cd/m2) that covered the center of the display. All lines were anti-aliased (i.e., intermediate luminance values were used for the pixels at their edges). Observers sat at a distance of 57 cm from the screen.
Apparatus
The stimuli were generated by an Intel-based PC running a C program and using the OpenGL graphics library and displayed on a 19″ CRT screen at 75 Hz with a resolution of 1280 × 1024 pixels.
Experimental procedure
Observers sat in front of a computer screen with their heads supported by a chin rest. They were asked to continually report their percept by holding down one of two designated keys (i.e., grating moving right up or left up in Experiment 1, motion coherency or transparency in Experiment 2, and directions of motion (right or left) of the grating that they perceived as being behind the other in Experiment 3). Observers were given passive viewing instructions (not to try to perceive one possibility more than the other, just to report the spontaneous changes) and were instructed to not press either key if the percept was unclear (this option was used 11% of the time, on average, in the binocular rivalry stimulus—likely corresponding to perception of composites—and in less than 5% in the rest of experiments). Observers fixated the central spot during the whole 1-min duration of each trial. In each session (two sessions were run), each combination of the stimulus parameters was repeated four times. Observers ran a total of 36 trials of 1 min each in a single session; they were instructed to take a 10- to 30-s rest between the trials.
Analysis
We define dominance duration of one percept as the time between the onset and offset of exclusive visibility of that percept. Durations shorter than 300 ms (cutoff duration) were excluded from the analysis. For each combination of the stimulus parameters, we computed from the data the durations , defined as the jth dominance duration of percept i within a particular trial k. The index i takes the values 1 or 2 (two percepts, either A or B), and k takes the values of the repeated trials (1 to 8) for the same stimulus parameters. Index j is random, as the number of dominances observed varies from trial to trial.
We measured the fraction of dominance time and the mean dominance duration of each percept. The fraction of time that percept i dominated is defined as fi = (the cumulative time percept i was reported as dominant)/(the total time that either of the percepts was reported as dominant). This fraction is a number between zero and one, with a value of 0.5 indicating that the two possible percepts were equally likely. More explicitly, the fraction of dominance of percept i is computed as
| (1) |
In the figures, the fraction of dominance is averaged across subjects.
The mean dominance duration of percept i is the mean value of the dominance durations of that percept averaged over trials, and it is calculated as
| (2) |
where ni is the total number of recorded durations for percept i. Note that the fractions and mean dominance durations are approximately related through fi ≅ Ti/[TA + TB], where Ti is the mean dominance duration of percept i. Means and error bars for the mean dominance durations are computed, respectively, as the mean and standard error of the dominance durations across durations in trials and across subjects. The same qualitative results to those described in the main text were observed in binocular rivalry for each subject, for each eye and when the cutoff durations were made shorter (see Figures SM1, SM2, and SM3, respectively, in the Supplementary material). The same qualitative features were also present in the other two experiments for each subject and were also largely insensitive to the chosen cutoff durations (not shown).
We define the alternation rate as the number of perceptual switches per unit time:
| (3) |
where T is the total accumulated time (8 trials × 60 s = 480 s). Alternation rates and error bars are calculated as the means and standard error, respectively, of the alternation rates across trials and subjects. Note that the alternation rate cannot be expressed directly in terms of the fraction of dominance and the mean dominance durations of each percept, since it is possible to have two or more consecutive epochs with the same percepts. Therefore, the alternation rate should be then considered as a measure independent of the fractions of dominance and mean dominance durations. However, as an approximation, Rate ≅ 1/[TA + TB].
Neuronal competition models
In this section, we describe briefly the double-well energy models for perceptual bistability introduced in Moreno-Bote, Rinzel, and Rubin (2007) and the rate-based models described in Moreno-Bote et al. (2007), Shpiro, Curtu, Rinzel, and Rubin (2007), and Shpiro, Moreno-Bote, Rubin, and Rinzel (2009). A more detailed description of these models is provided in the Supplementary material.
Double-well potential model
In a double-well energy model, the dynamics of a single variable r (representing, e.g., the difference in firing rate of two competing populations) is described. The variable r obeys
| (4) |
where τ = 10 ms is the timescale of the dynamics, the currents IA and IB measure the stimulus strength in favor of percept A or B, respectively, and n(t) is a noise term. Equation 4 has two stationary solutions close to r = ±1. Dominance of percept A corresponds to the case r ~ 1, while dominance of percept B corresponds to the case r ~ −1. A transition occurs when r crosses zero. The dynamics of Equation 4 can be viewed as a noisy descent over the energy landscape (Figure 9a). The effect of increasing stimulus strength for, e.g., percept B is to add a straight line with positive slope to the energy landscape, increasing the energy well for percept B while reducing the energy well for percept A. This leads to an increase of the mean dominance duration of percept B and a reduction of the mean dominance duration of percept A.
Figure 9.
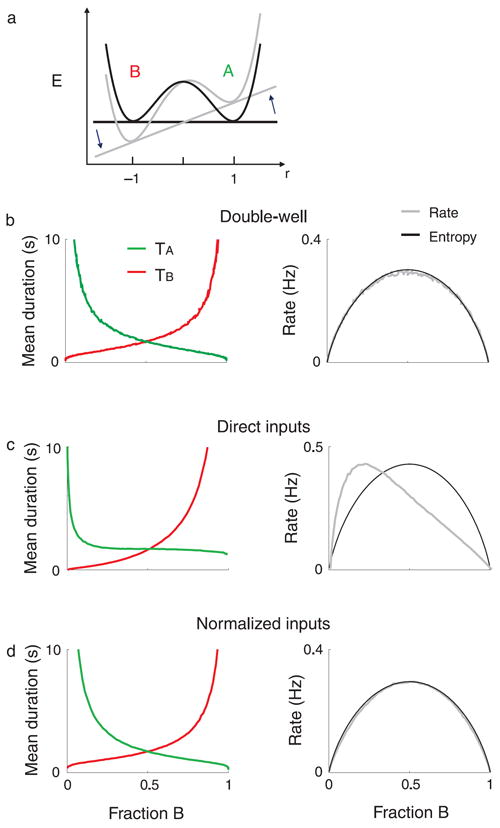
Models with gain normalization reproduce experimental results. Simulation results of double-well (a, b) and direct cross-inhibition neuronal competition models with direct (c) and normalized inputs (d). The mean dominance durations for the two percepts and the alternation rates as a function of the fraction of dominance of percept B are shown in the left and right panels, respectively. The entropy as defined in Figure 8 is plotted as a function of f for each condition (black lines). The figures were obtained by varying the strengths of the percepts (input currents I; see Methods section), which led to changes of both the fractions of dominance and the mean dominance durations.
Rate-based models
We model the dynamics of two populations, A and B, whose states are described by their firing rates rA and rB, respectively. The firing rates obey coupled differential equations with input noise and firing rate adaptation, as described in the Supplementary material. Two models that work in different regimes are considered. In the first one, perceptual switches occur because of the presence of strong adaptation currents (competition neuronal model with direct cross-inhibition), while in the second one perceptual switches occur as a consequence of noise (attractor model with indirect cross-inhibition), as described in Moreno-Bote et al. (2007). For the two models, the state with large activity of population A and low activity of population B corresponds to dominance of the percept encoded by the population A. Percept B dominates if the reversed activity configuration occurs. We compare the dynamics of the model when the inputs to populations A and B are the stimulus strengths IA and IB, respectively, and when the inputs are normalized:
| (5) |
(i = A, B) where Ibg = 0.01 represents background activity present in the network irrespective of the external inputs and s is a scaling coefficient. This equation implements a normalization of the stimulus evidence (strengths) supporting each percept.
Results
Experiment 1: Binocular rivalry
In the binocular rivalry experiment (Figure 2), the contrast of the right-tilted drifting grating (labeled B) changed from trial to trial, while the contrast of the left-tilted drifting grating (labeled A) was fixed across trials. Each grating was independently projected to one of the two eyes. Prolonged viewing of the stimulus leads to periods of dominance of one grating followed by periods of dominance of the other grating. The fraction of dominance of grating B and the mean dominance durations of gratings A and B as a function on the contrast of grating B are shown in Figures 2a and 2b, respectively. Increasing the contrast of image (grating) B leads to an increase of the fraction of dominance of the same image, fB. The dependence is highly nonlinear, with a large effect of variations of the contrast at low values, and relatively smaller effect on variations of the contrast at higher values. As a function of the contrast, TA decreases and TB increases. There is a point in which the mean dominance duration curves meet (TA ≅ TB), which occurs approximately when the fraction of dominance of each percept is close to 0.5 (this point is defined as the equi-dominance point). When the contrast of grating B is lowered from the equi-dominance point, TA changes more abruptly than TB does (this result is consistent with Levelt’s second proposition). However, when the contrast is increased from the equi-dominance point, TA changes very little and TB largely increases, contrary to Levelt’s Proposition II (Brascamp et al., 2006).
Experiment 2: Coherent vs. transparent plaid motion
Prolonged viewing of moving plaids leads to perceptual bistability in the perception of motion of the display: either the two constituent gratings move coherently in a single direction (coherent percept), or the two gratings move in different directions (transparent percept). Figure 3 shows the mean dominance duration of coherent and transparent percepts (labeled Tcoh and Ttrans) as a function of the angle (α) between the directions of motion of the constituent drifting gratings. As a function of the angle, Tcoh decreases and Ttrans increases. For this experiment, the angle at which both dominance durations happen to have approximately the same value is around 120°. Figure 3a shows that the fraction of dominance of coherency decreases monotonically as a function of the angle. At a point around 120°, the fraction of dominance of coherency or transparency is close to 0.5, and therefore, it corresponds to the equi-dominance point. Thus, the mean dominance durations are close to each other at the equi-dominance point, as shown in Figure 3b. A reduction of the angle below the equi-dominance angle produces a large variation of Tcoh, while the variation of Ttrans is rather modest. If the angle is increased beyond its equi-dominant value, Tcoh barely changes, while Ttrans varies by a large amount, both in an absolute and a relative sense.
Experiment 3: Gratings’ depth reversals
When the angle between the directions of motion of the two gratings in a plaid stimulus is large, then subjects report most of the time perceiving transparent motion. Subjects become spontaneously aware, however, that the motion is still ambiguous as to the depth ordering of the two gratings (labeled 1 and 2): grating 1 can be seen as being behind grating 2, or the reversed ordering, and subjects spontaneously perceive alternations between the two possible percepts (i.e., two possible depth orderings; Moreno-Bote et al., 2008). Figure 4 shows the mean dominance durations of two possible depth orders in a display formed by the superposition of two drifting gratings as a function of the wavelength of one of them. Figure 4b shows that the mean dominance durations of those two possible percepts (labeled T1,beh and T2,beh; the index indicates which grating is perceived as being behind) strongly depend on the wavelength of the first grating, λ1, related to that in the second grating (Moreno-Bote et al., 2008). As a function of the wavelength, λ1, T2,beh increases and T1,beh decreases. When λ1 equals the value of the wavelength of the second grating, λ2 = 2.7°, the gratings are identical apart from its motion direction, and therefore, they expend equal amounts of time as being behind. At this point, both mean dominance durations are equal, and the fraction of dominance for each percept is near 0.5, as shown in Figure 4a. This point corresponds to the equi-dominance point of the stimulus. As the wavelength of grating 1 is reduced from the equi-dominance point, T1,beh changes largely, while T2,beh changes very little. If the wavelength is increased from the equi-dominance point, T2,beh is the mean duration more sensitive to the stimulus parameter variation.
The choice of a common axis: Mean dominance durations vs. fraction of dominance plots
The primary motivation for our experiments was to study, in different bistable phenomena, how parametric variations affect the mean durations of the competing percepts, and in particular to examine the commonalities and differences between those dependencies. Although there are some rough qualitative similarities in the results shown in Figures 2b, 3b, and 4b, in detail they are quite different in the three cases, and therefore, at first glance we might conclude that the differences outweigh the commonalities. However, this comparison needs to be made with care, taking into account also the differences in the effect of each parameter on the relative strengths of the competing percepts (Figures 2a, 3a, and 4a). Consider the case of binocular rivalry: Figure 2a shows that the effect of changing the contrast of the image B projected to one eye is very nonlinear. When B’s contrast was raised above the point of equi-dominance (grating contrast close to 9% in our case), its predominance increased at a moderate rate, asymptoting at a contrast of only about 50%. However, when image B’s contrast was reduced below the point of equi-dominance, the fraction of time it was dominant plummeted rapidly, falling below 0.05 for a contrast of 3%. This behavior is not surprising in itself: contrast is known to have nonlinear effects in a host of sensory and perceptual phenomena, particularly for large variations (Carandini & Heeger, 1994; Ohzawa, Sclar, & Freeman, 1982, 1985). However, in the present context it poses a challenge: what is the appropriate scale to use in order to compare the effect of contrast in binocular rivalry with, say, the effect of the angle between the motion directions of the constituent gratings (α) on plaid transparent motion. Turning to examine the latter case (Figure 3a), we find a large range of values over which α has a gradual and near-linear effect on the fraction of time the transparent percept is dominant (roughly 90°–170°), but also a large range over which that linear trend breaks down. The question therefore arises whether it is possible to make any direct comparisons of the effects of these two parameters (contrast and α) on the mean dominance durations of the competing percepts of the corresponding bistable phenomena (binocular rivalry and plaid global motion), when these parameters have such divergent effects on the relative strength of the competing percepts. (Similar questions can be posed with regard to the third bistable phenomenon we studied, gratings’ depth reversals.)
We therefore reasoned that, in order to make the comparisons meaningful—i.e., to directly compare between the effects of parameters on TA and TB in the three experiments—we first needed to transform the scale the parameter used in each of the three experiments so as to put them on a common footing. We further reasoned that the most natural transformation to use is one in which constant changes of the transformed parameter would yield constant increments in the value of fB—in other words, to transform the scale of the parameter so that it has a linear effect on fB. Formally, this is equivalent to plotting TA and TB against fB itself.
Such a replotting of the mean dominance durations as a function of the fraction of dominance, instead of the actual parameter that has been experimentally manipulated (contrast in Experiment 1, angle in Experiment 2, and wavelength in Experiment 3), has the advantage that it allows to compare the three different experiments using an axis that is common to all of them (Figure 5). Therefore, the (nonlinear) transformation of the horizontal axis puts all three experiments into a common currency that can be used to compare all of them (see next section).
Figure 5.
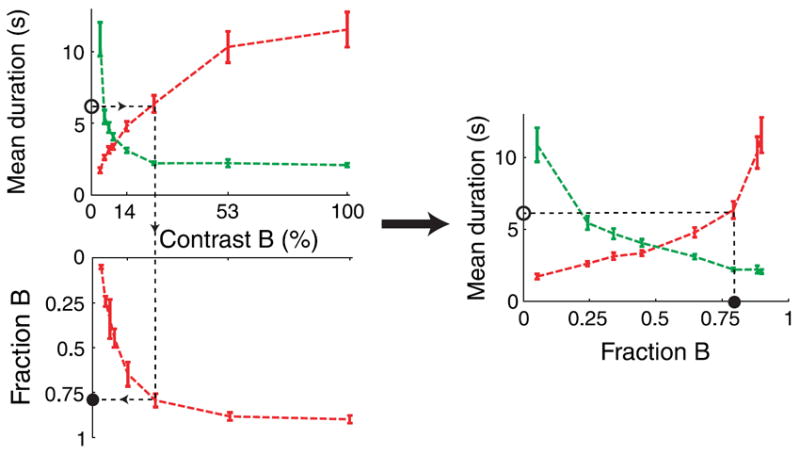
Transformation between stimulus parameter (contrast in this case, for binocular rivalry) and the fraction of dominance of one percept (here percept B). Upper left panel shows mean dominance durations as a function of the contrast of image B, and the lower panel shows the fraction of dominance of percept B as a function of the contrast of image B. These mean dominance durations data can be replotted as a function of the fraction of dominance (right).
The transformation between the parameter being manipulated and the fraction of dominance of one percept can be also thought of as a nonlinear transformation of coordinates. For the case of binocular rivalry, this corresponds to stretching the contrast of low values and compressing the contrast at high values, so as to produce a more symmetrical figure of the mean dominance durations (Figure 5, right panel, compared to upper left panel). Therefore, another advantage of this horizontal axis transformation is that it represented the effect of parameter manipulations in a scale that is perfectly linearly related to the fraction of dominance of one percept, rather than as a function of the arbitrarily defined stimulus parameter, being contrast, angle, or wavelength.
The mean dominance duration of the stronger percept is the most sensitive one to stimulus parameter manipulations
Figure 6 (top panels) summarizes and compares the experimental results in the three different experimental settings studied in a format that allows a comparison between them. One can see that the mean durations vs. fraction of dominance plots are almost symmetrical around the equi-dominance point (f = 0.5). Since the stronger percept is defined as that having the largest fraction of dominance, then we can summarize the dependence of the mean dominance duration of each percept on the stimulus manipulation as follows: “The mean dominance duration of the stronger percept changes more than that of the weaker percept under stimulus parameter manipulations.”
Figure 6.
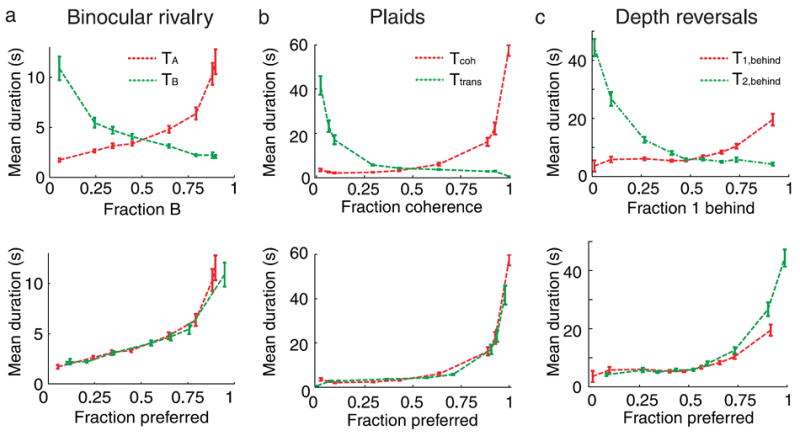
The stronger percept is more sensitive to stimulus manipulations than the weaker percept. (Top) Mean dominance durations for (a) binocular rivalry, (b) ambiguous plaid motion, and (c) gratings’ depth reversals as a function of the fraction of dominance time of a preselected percept (image B, Coherent percept, and grating 1 behind, respectively). When the fraction is larger than one half for one percept, its mean dominance duration is more sensitive to variations of the fraction of dominance; the mean dominance duration of the weaker percept is less sensitive or even remains constant. The absolute slopes are larger for the stronger percept in all cases. (Bottom) Same as before, but mean dominance durations for each percept are plotted as a function of its fraction of dominance (fpreferred).
It is easy to check that this proposition is true for the three cases, for example for ambiguous moving plaids, whose results are presented in Figure 6b. To the right of fcoh = 0.5, Coherency is the stronger percept, and Transparency is the weaker percept. The mean dominance duration of Coherency changes dramatically, while the mean dominance duration of Transparency changes very little. To the left of fcoh = 0.5, Transparency is the stronger percept, and the effects on the dominance durations is the reversed. Therefore, the proposition that the stronger percept has the most sensitive dominance durations is true. The same conclusion can be drawn from Figure 6a or 6c for binocular rivalry and gratings’ depth reversals.
Symmetry of the mean duration vs. fraction of dominance curves
Figure 6 (bottom panels) shows the mean dominance durations as a function of the fraction of dominance, but this time, of their preferred percept, that is, the one for which the mean dominance duration increases. This corresponds to plot the increasing (red) lines in the top panels unchanged and plot the decreasing (green) lines symmetrically reversed around the equi-dominance point. The figure shows that the dependence of the mean dominance durations of one percept is, strikingly, very similar across the two percepts, indicating an almost perfect symmetry of the curves around equi-dominance. This result is totally unexpected, since the physical manipulations made on the stimuli for the three experiments were not symmetrical (i.e., not interchangeable) in its two interpretations (e.g., in the binocular rivalry experiment, only the contrast of one image was manipulated, while the contrast for the competing image remained fixed, which is far from being a symmetrical manipulation on the physical properties of the two interpretations). This result indicates that neuronal networks underlying perceptual bistability treat the percepts in the same way when they have the same fraction of dominance, regardless of their identity or visual appearance.
The symmetry of those curves around equi-dominance can also be seen in plots where the mean dominance duration of one percept is plotted as a function of the mean dominance durations of the other percept, as shown in Figure 7. The curves are rather symmetric around the diagonal but notably differ across the three experimental conditions. Interestingly, the curve corresponding to the binocular rivalry data (blue) is close to a hyperbola, as indicated by the linearity of the data with slope close to −1 in a log–log scale (inset). In contrast, the data corresponding to the ambiguous plaid motion and gratings’ depth reversals substantially deviate from the hyperbolic shape (not shown).
Figure 7.
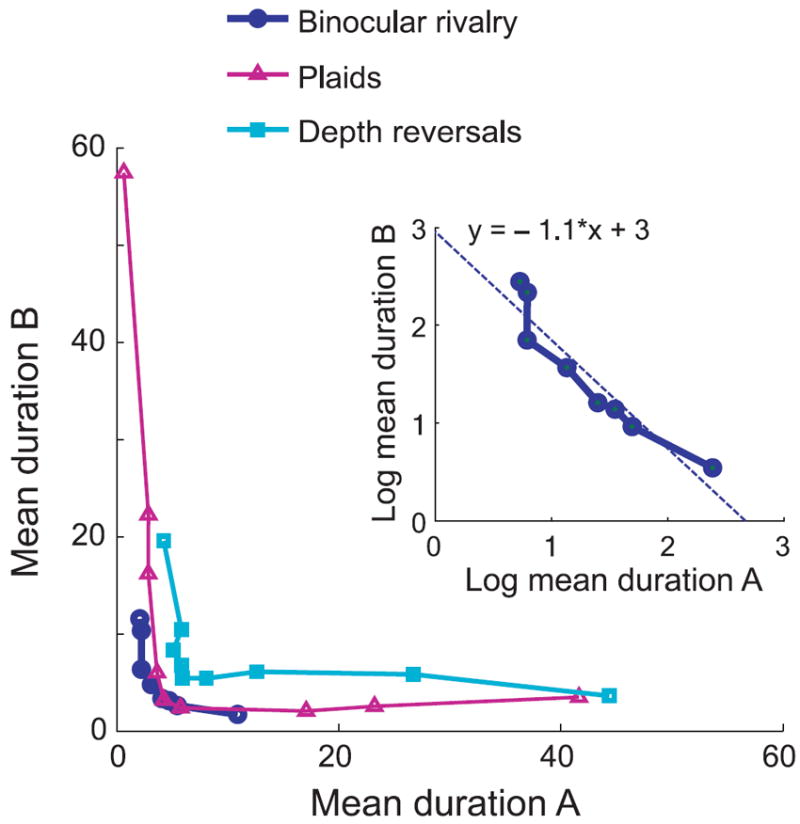
Mean dominance durations of percept A vs. mean dominance durations of percept B curves are symmetric around the diagonal. Percepts A and B correspond to images A and B in binocular rivalry, to Transparency and Coherency in ambiguous plaid motion, and to “grating 2 behind” and “grating 1 behind” for the depth reversal stimulus, respectively. (Inset) The binocular rivalry data are close to a straight line with slope −1 in a log–log scale, indicating its similarity to a hyperbola (TA × TB = constant). Dashed line corresponds to the linear fit.
Maximum alternation rate in bistable perception occurs at equi-dominance
One of the implications of the previous results is that at the equi-dominance point, both dominance durations reach a low value, i.e., at equi-dominance the mean dominance durations of the percepts are very close to their absolute minimum values observed in each experiment. This indicates that the equi-dominance point is special in the sense that, at that point, the perceptual reversals occur fast in relation to other conditions. This is clearly seen when the number of stable percepts reported per unit time, or alternation rate, is plotted as a function of the fraction of dominance of a predefined percept, as shown in Figures 8a, 8b, and 8c for binocular rivalry, ambiguous plaid motion, and gratings’ depth reversals. The alternation rate plots have an inverted U-shape, with a maximum close to the equi-dominance point (f = 0.5) and symmetry around it. We will use these features to distinguish between possible models of perceptual bistability in the next section.
Figure 8.
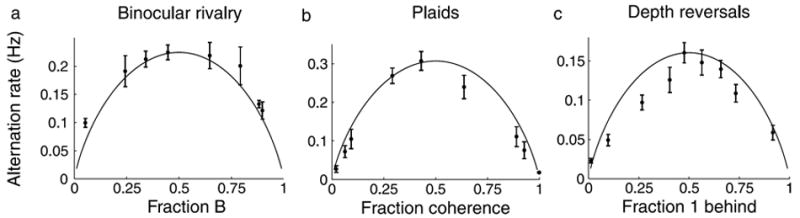
Maximum alternation rate occurs at equi-dominance. The alternation rate (number of perceptual switches per unit of time) is plotted as a function of the fraction of dominance of a preselected percept (data points) in (a) binocular rivalry, (b) ambiguous plaid motion, and (c) gratings’ depth reversals. The entropy of a system with two states having probabilities f and 1 − f is plotted as a function of f, the fraction of dominance of the preselected percept (solid curve; for comparison, the entropy has been vertically rescaled so that its range is similar to that of the experimental data in each condition).
The equi-dominance point is interesting also because it is the point at which the stimulus is the most ambiguous. To measure the ambiguity of the bistable stimulus, we speculate that the fraction of dominance f of a percept is an indication of the degree of belief that the brain should deposit on a particular interpretation of the stimulus. If f is close to one, the interpretation should be believed to be more likely, and if f is close to zero, the interpretation should be believed to be unlikely. Since perceptual switches are stochastic (Borsellino, De Marco, Allazetta, Rinesi, & Bartolini, 1972; Fox & Herrmann, 1967; Lehky, 1995), the probability of finding the brain having one percept will be close to f for that percept. As a crude approximation, the brain can be considered as a binary machine with two states, one with probability f and the other with probability 1 − f. The entropy of such a binary system is (Cover & Thomas, 2006)
| (6) |
and it can be used as a measure of the ambiguity of the bistable stimulus. The entropy is plotted, after scaling to match dynamical range of the data, in Figures 8a, 8b, and 8c as a function of the fraction of dominance for the three experimental setups tested. The entropy is the largest at the equi-dominance point and decreases symmetrically to both sides. It has an inverted U-shape that closely follows that of the alternation rate vs. fraction curves.
Models with input strength normalization reproduce experimental results
The experimental results that we have presented impose important constraints on neuronal models of perceptual bistability. In particular, the results that the mean dominance duration of the stronger percept is more sensitive to stimulus manipulation and that the alternation rate reaches a maximum close to equi-dominance are not satisfied trivially in neural competition models, as we see below.
To gain some intuition, we first consider a double-well energy model (Figure 9a), where the state of the system is described by a single variable, denoted r. This variable might correspond to the difference in the firing rates of two competing neuronal populations (see description of the rate-based model in Supplementary material). The energy has two minima, one close to r ~ 1, corresponding to dominance of percept A, and another at r ~ −1, corresponding to dominance of percept B. Double-well energy models have been shown before to reproduce Levelt’s propositions (Moreno-Bote et al., 2007), and here they are adapted to account for the new observed dependences of the mean dominance durations and alternation rate on stimulus parameter manipulations. When the two percepts are equi-dominant, the depth of the energy wells (energy barriers) for the two states are identical (black line). As described in the Methods section, when the strength of, e.g., percept B is made larger than the strength of percept A, then the energy landscape is tilted clockwise because of the addition of a straight line with positive slope (gray lines). This has the effect of increasing the energy barrier for percept B, while decreasing the energy barrier of percept A by a similar amount. As a result, the fraction of dominance of percept B (respectively, A) increases (respectively, decreases). Because of the symmetry of the double-well energy model, the model naturally leads to a symmetrical dependence of the mean dominance durations on the fraction of dominance of an arbitrarily chosen state (Figure 9b, left). Furthermore, because of the nonlinearity intrinsic to the system, the magnitude of the increase of the mean dominance durations of the stronger percept is larger than the magnitude of the reduction of the mean dominance durations of the weaker percept, and as a consequence, the alternation rate displays a maximum at equi-dominance (gray line, right panel). Interestingly, the alternation rate vs. f curve virtually matches the scaled entropy of a binary system with probabilities f and 1 − f, H(f) (black line).
Next, we address the question of whether it is possible to reproduce the experimental results with more realistic neuronal networks. Most models of perceptual bistability are based on a competition through mutual inhibition between two neuronal populations whose activities are described in terms of their population mean firing rates (Blake, 1989; Freeman, 2005; Haken, 1994; Kim, Grabowecky, & Suzuki, 2006; Lago-Fernandez & Deco, 2002; Laing & Chow, 2002; Moreno-Bote et al., 2007; Shpiro, Curtu et al., 2007; Wilson, 2003). Models of this sort have been used to describe the dynamics of perceptual switches in binocular rivalry, including Levelt’s second proposition (Brascamp et al., 2006; Laing & Chow, 2002; Lehky, 1988; Moreno-Bote et al., 2007; Shpiro, Curtu et al., 2007; Wilson, 2007). We consider two models that follow this principle but differ in details of the architecture: a neuronal network with direct cross-inhibition and an attractor neuronal network with indirect cross-inhibition (see Supplementary material). The models also contain large amounts of external noise; noise has such an important role in the temporal dynamics of perceptual switches (Haken, 1994; Kim et al., 2006; Moreno-Bote et al., 2007; Riani & Simonotto, 1994) and on the mean duration vs. stimulus parameters dependences (Moreno-Bote et al., 2007; Shpiro, Curtu et al., 2007; Shpiro et al., 2009) that it cannot be disregarded in a first approximation. For each model, we also study the effect of direct inputs versus normalized inputs onto the two competing neuronal populations (see Methods section and Supplementary material). Gain modulation is thought to be an important step in the processing of sensory inputs (see, e.g., Carandini & Heeger, 1994; Ohzawa et al., 1982, 1985) and here it is modeled as a normalization of the strengths of the competing inputs (Equation 5, Methods section).
Figures 9c and 9d show the mean dominance durations (left panels) and alternation rate (right panels) generated from the model with direct cross-inhibition as a function of the fraction of dominance of one designated state when the inputs to the populations are not normalized (Figure 9c) and when the inputs are normalized (Figure 9d). The attractor model with indirect cross-inhibition (see Supplementary material) displayed qualitatively the same behavior (results not shown). Without input normalization (direct inputs), the mean dominance durations and alternation rate vs. f curves are clearly asymmetrical, and the alternation rate has a maximum very far from the equi-dominance point. This result is typical of networks with direct inputs: when the stimulus strength to, e.g., population B is increased, its input is intensified, leading to an increase of its mean dominance duration and fraction of dominance, while leading to a very little change of the mean dominance duration of the competing population A (except when the stimulus strength is very large; see Moreno-Bote et al., 2007). Introducing gain normalization into the models produces a symmetrical mean dominance duration vs. fraction curve, and a rate vs. fraction curve with inverted U-shape, peaking at the equi-dominance point and being symmetrical around it, therefore reproducing the experimental results. The reason why the model with input normalization features this property can be understood as follows. With gain normalization, increasing the stimulus strength of one percept leads to both an increase in the inputs of its associated population and to a reduction in the inputs to the competing population (see Equation 5). This has a similar effect to that of tilting the energy landscape in the double-well energy model and leads to the symmetric effects of parameters on the mean dominance durations. Finally, it is noteworthy that the entropy (black lines) closely follows the alternation rate in the models with gain normalization, but not if the inputs are not normalized.
Discussion
Mean dominance durations as a function of the fraction of dominance
We have studied the effect of parameter manipulations on the mean dominance duration of the percepts and their alternation rate in three bistable visual stimuli. The three cases studied cover a broad range of visual phenomena: binocular rivalry, motion, and depth perception. We have argued that the fraction of dominance of the percepts represent a common currency that can be used to highlight the similarities of the mean dominance duration dependences on parameter manipulations across perceptually bistable phenomena. The parameters being manipulated (i.e., contrast, angle, or wavelength) are inherently different, and therefore, it is not clear how they relate to a hypothetical measure of strength of the bistable interpretations of the stimulus. On the contrary, the fraction of dominance might constitute the relevant measure of strength of the percepts since it is dimensionless and directly measures the predominance over time of the percepts. In general, there is a nonlinear monotonic mapping between the physical parameter manipulated and the fraction of dominance. When the mean dominance durations were plotted as a function of the fraction of dominance, the curves were nearly symmetrical around the equi-dominance point (f = 0.5), revealing that the two percepts are treated equally by the visual system, regardless of their identity. The definition of strength of the percept as its fraction of dominance f allowed us to define the stronger percept uniformly across paradigms (i.e., the stronger percept is the one having the largest f). Furthermore, we could summarize the results by the following proposition: “The mean dominance duration of the stronger percept changes more than that of the weaker percept under stimulus parameter manipulations.” This proposition is consistent to those suggested previously (Bossink et al., 1993; Brascamp et al., 2006; Levelt, 1968; Mueller & Blake, 1989; van Boxtel et al., 2007). While here we have defined the strength of the percept as its fraction of dominance, Klink et al. (2008) has recently formulated a very similar proposition where the strength of the percept was implicitly identified with the “stimulus strength” (for instance, the contrast of one grating in binocular rivalry). As we have explained above, the fraction of dominance constitutes a more natural measure of strength of a percept, as it is independent of the physical parameter that was manipulated in the experiment. More importantly, it allows describing the effects of parameter manipulations on the mean dominance durations in cases where the notion of stimulus strength is not well defined, as in our ambiguous plaid motion stimulus, where the angle between the gratings cannot be naturally associated with the stimulus strength of a particular percept.
Maximum alternation rate at equi-dominance and perceptual bistability as foraging
By plotting the alternation rate as a function of the fraction of dominance of one arbitrarily chosen percept, we have found, consistent with previous work (Klink et al., 2008; Shpiro, Moreno-Bote, Bloomberg, Rubin, & Rinzel, 2007), that maximum alternation rate occurs at equi-dominance. Moreover, the alternation rate vs. fraction of dominance curve is symmetric around the equi-dominance point. These results might have important consequences for the understanding of the role of perceptual bistability in visual processing. Rather than being a mere curiosity, we have recently proposed (Moreno-Bote et al., 2008) that perceptual bistability plays a functional role in vision by allowing a faster matching between correct interpretation and stimulus compared to a hypothetical case without alternations. Under conditions of high ambiguity or noise in the stimulus, retaining the most likely percept forever would be harmful, because it is still possible that one of the other, less probable, percepts is the correct interpretation of the stimulus. For instance, missing a predator in the rain by confusing it with a mate could have devastating consequences. It is then tempting to think that perceptual switches have evolved as a necessary consequence for perceptual exploratory behavior that allows a correct and faster matching between interpretation and stimulus under conditions of highly ambiguous stimulation or noise.
Although perceptual switches might have a functional role in engaging perceptual exploration, why should the alternation rate have a maximum at equi-dominance, or even why the alternation rate should change at all? Our working hypothesis is that perceptual bistability is akin to animal foraging. Animals that forage for food allocated in distinct sources need to spend energy while traveling from one source of food to another, and this crucially affects their choice behavior (Brownstein & Pliskoff, 1968; Herrnstein, 1961, 1970, 1997). In analogy with this, the perceptual switches can be thought of as perceptual deflections that allow “traveling” from one percept to the other, allowing the exploration of the interpretations in conjunction with the stimulus. Following the proposed analogy with animal foraging, perceptual switches should come with a cost, the cost being higher the larger the number of perceptual switches per unit of time. Therefore, perceptual switches should be only reserved for conditions in which perceptual exploration can lead to substantially larger rewards compared to the case of no exploration. Then, we should expect that the alternation rate reaches a maximum when the stimulus is maximally ambiguous, as observed experimentally.
A simple model that exemplifies the above ideas and reproduces qualitatively the main experimental results is given as follows. Let f be the probability that one percept is correct; hence 1 − f is the probability that the competing percept is correct. Assume that each percept is “chosen” with the same probability that it is expected to be correct (i.e., either f or 1 − f), and that the cost of switching is proportional to the square of the alternation rate r. If the initial state is the one with probability f, then the probability of transitioning to the other percept and that it is the correct one is (1 − f)r. This happens with probability f(1 − f)r. If the initial state is the one with probability 1 − f, then the probability of transitioning to the other percept and that it is the correct one is fr. This situation happens with probability (1 − f)fr. For the sake of simplicity, we assume that exploration occurs mainly during the initial periods after the transitions, rather than continuously throughout the whole dominance epochs. Therefore, the expected gain because of exploration is, on average, 2f(1 − f)r. After subtracting from it the cost of exploration, the total expected gain per unit time after each transition is
| (7) |
where a is a constant. For the cost of transitions, we chose the squared rate instead of linear or other nonlinear dependences because it is the simplest case that leads to nontrivial results (i.e., not choosing always either the most likely or less likely percept). Note that when the alternation rate is zero, the reward obtained from exploration is zero. Exploring with a large alternation rate is very costly, because of the square in the cost term, and it is not optimal either. There is a value of alternation rate for which the expected reward per unit time reaches a maximum, and this is attained when
| (8) |
This optimal alternation rate is in turn a function of f, it is very similar to the entropy, and it has a maximum at f = 1/2 (not shown). This simplistic model illustrates, consistently with experimental results, that perceptual alternations can lead to maximization of reward, and that the brain can pay the higher cost of increasing the alternation rate if the sensory input is highly ambiguous. Although the hypothesis that perceptual bistability is a form of exploration is consistent with the experimental observations, further research is required to determine its adequacy.
Generalization of the results to multistable phenomena
Using perceptually bistable stimuli, we have shown that the mean dominance duration of the stronger percept changes more than that of the weaker, and that the alternation rate reaches a maximum at equi-dominance. It remains to be seen whether these results hold for multistable phenomena with more than two possible interpretations (N > 2). With multiple stable interpretations, the strongest percept does not need to have a fraction of dominance f larger than one half, as in perceptual bistability, but rather it just need to have the largest f. Similarly, equi-dominance is defined as the point at which the fs for all percepts are equal to 1/N, rather than one half. A default hypothesis is that the above propositions will hold true even for the more general case of multistable perception, but its confirmation awaits further research.
Input gain normalization and the sense of absolute and relative stimulus strengths
We have shown that the symmetry of the mean dominance durations vs. f curves and the maximum of alternation rate at equi-dominance point are not easily obtained in neuronal network models of perceptual bistability. In fact, most common models based on direct inputs (i.e., each interpretation strength is modeled as input strength to a particular neuronal population) typically show rather asymmetrical mean dominance durations and alternation rate vs. f curves (Figure 9). We have shown that if inputs are normalized, then the curves become symmetrical. Input normalization can be interpreted as producing a normalization of the evidence supporting each percept. In fact, by normalizing the input, each of the competing populations no longer represents the absolute value of evidence supporting it, but rather the relative evidence in relation to any other source of information in the stimulus that supports other interpretations.
Since our results imply that input strengths are normalized in some stage of neuronal processing, it is important to ask whether there is any sense remaining to the notion of an “absolute strength” of the competing percepts. In binocular rivalry, it seems perfectly sensible to think of an increase of the contrasts of both eye’s images as a (simultaneous) increase in the absolute strengths of the competing stimuli, even without changing their relative strengths. It is therefore interesting to note that, in his Proposition IV, Levelt (1968) indeed asserted that increasing the stimulus strength of both eyes increases the alternation rate. This finding would seem to indicate that, indeed, the system retains information not only about the relative strengths of the competing stimuli but also about their absolute strengths. It remains to be determined whether this is a remnant of imperfect contrast normalization or has actual function (e.g., related to the system’s need to keep some information about absolute, not just relative contrasts). In either case, it is noteworthy that our preliminary results in binocular rivalry indicate that the effect on the mean dominance durations of simultaneously increasing the contrast to both eyes is very modest compared to the effect of changing the contrast to just one of the eyes’ images.
In contrast, in other bistable phenomena the relative strength of the two competing interpretations (or stimuli) is clearly a more relevant concept than that of “absolute” strength, and possibly the only relevant one. For example, in the apparent motion quartets, reducing the vertical distance between the moving dots increases the relative strength of the vertical-motion percept, but reducing both vertical and horizontal distances merely results in a spatial rescaling of the stimulus, lending little sense to the notion that “both the vertical- and the horizontal-motion percepts were strengthened.”
Gaining further insight about the questions raised above could have consequences that go beyond understanding bistability. This is because, for some bistable phenomena, it is not known how certain parameters that have a strong effect on the relative strength of the competing percepts exert their influence. A pertinent example is the effect of the angle between the motion directions of the constituent gratings (α) in plaid perception: we know that increasing α increases the frequency of the transparent-motion percept. However, does this come about via a strengthening of inputs to the neural population(s) encoding motion transparency? Or via a weakening of inputs to the population(s) representing the coherent interpretation? Or perhaps both? In addition, are these questions even valid, in terms of the neural organization of the system? This example therefore illustrates how insights from the general mechanisms of bistability could contribute to our understanding of visual processing.
Supplementary Material
Acknowledgments
We thank Carson Chow, David Leopold, Bob Shapley, and Ed Vessel for useful discussions. This work was supported by the Swartz Foundation Program for Theoretical Neuroscience and the National Eye Institute Grants T32EY071580 and R01EY014030.
Commercial relationships: none.
Contributor Information
Rubén Moreno-Bote, Center for Neural Science, New York University, New York, USA; Department of Brain and Cognitive Sciences, University of Rochester, Rochester, NY, USA.
Asya Shpiro, Department of Mathematics, Medgar Evers College, City University of New York, New York, USA.
John Rinzel, Center for Neural Science, New York University, New York, USA; Courant Institute of Mathematical Sciences, New York University, New York, USA.
Nava Rubin, Center for Neural Science, New York University, New York, USA.
References
- Blake R. A neural theory of binocular rivalry. Psychological Review. 1989;96:145–167. doi: 10.1037/0033-295x.96.1.145. [DOI] [PubMed] [Google Scholar]
- Blake R. A primer on binocular rivalry. Brain and Mind. 2001;2:5–38. [Google Scholar]
- Blake R, Logothetis NK. Visual competition. Nature Reviews Neuroscience. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Bonneh YS, Cooperman A, Sagi D. Motion-induced blindness in normal observers. Nature. 2001;411:798–801. doi: 10.1038/35081073. [DOI] [PubMed] [Google Scholar]
- Borsellino A, De Marco A, Allazetta A, Rinesi S, Bartolini B. Reversal time distributions in the perception of visual ambiguous stimuli. Kybernetic. 1972;10:139–144. doi: 10.1007/BF00290512. [DOI] [PubMed] [Google Scholar]
- Bossink CJ, Stalmeier PF, De Weert CM. A test of Levelt’s second proposition for binocular rivalry. Vision Research. 1993;33:1413–1419. doi: 10.1016/0042-6989(93)90047-z. [DOI] [PubMed] [Google Scholar]
- Brascamp JW, Ee RV, Noest AJ, Jacobs R, Berg AVD. The time course of binocular rivalry reveals a fundamental role of noise. Journal of Vision. 2006;6(11):8, 1244–1256. doi: 10.1167/6.11.8. http://www.journalofvision. [DOI] [PubMed] [Google Scholar]
- Brownstein AJ, Pliskoff SS. Some effects of relative reinforcement rate and changeover delay in response-independent concurrent schedules of reinforcement. Journal of the Experimental Analysis of Behavior. 1968;11:683–688. doi: 10.1901/jeab.1968.11-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Summation and division by neurons in primate visual cortex. Science. 1994;264:1333–1336. doi: 10.1126/science.8191289. [DOI] [PubMed] [Google Scholar]
- Cover TH, Thomas JA. Elements of information theory. 2. New Jersey: Wiley-Interscience; 2006. [Google Scholar]
- Fox R, Herrmann J. Stochastic properties of binocular rivalry alternations. Perception & Psychophysics. 1967;2:432–436. [Google Scholar]
- Freeman AW. Multistage model for binocular rivalry. Journal of Neurophysiology. 2005;94:4412–4420. doi: 10.1152/jn.00557.2005. [DOI] [PubMed] [Google Scholar]
- Haken H. A brain model for vision in terms of synergetics. Journal of Theoretical Biology. 1994;171:75–85. [Google Scholar]
- Herrnstein RJ. Relative and absolute strength of response as a function of frequency of reinforcement. Journal of the Experimental Analysis of Behavior. 1961;4:267–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ. On the law of effect. Journal of the Experimental Analysis of Behavior. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ. The matching law. Cambridge, MA: Harvard University Press; 1997. [Google Scholar]
- Hupé JM, Rubin N. The dynamics of bistable alternation in ambiguous motion displays: A fresh look at plaids. Vision Research. 2003;43:531–548. doi: 10.1016/s0042-6989(02)00593-x. [DOI] [PubMed] [Google Scholar]
- Kang MS. Size matters: A study of binocular rivalry dynamics. Journal of Vision. 2009;9(1):17, 1–11. doi: 10.1167/9.1.17. http://www.journalofvision.org/content/9/1/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Grabowecky M, Suzuki S. Stochastic resonance in binocular rivalry. Vision Research. 2006;46:392–406. doi: 10.1016/j.visres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Klink PC, van Ee R, van Wezel RJ. General validity of Levelt’s propositions reveals common computational mechanisms for visual rivalry. PloS One. 2008;3:e3473. doi: 10.1371/journal.pone.0003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago-Fernandez LF, Deco G. A model of binocular rivalry based on competition in IT. Neuro-computing. 2002;44:503–507. [Google Scholar]
- Laing CR, Chow CC. A spiking neuron model for binocular rivalry. Journal of Computational Neuroscience. 2002;12:39–53. doi: 10.1023/a:1014942129705. [DOI] [PubMed] [Google Scholar]
- Lehky SR. An astable multivibrator model of binocular rivalry. Perception. 1988;17:215–228. doi: 10.1068/p170215. [DOI] [PubMed] [Google Scholar]
- Lehky SR. Binocular rivalry is not chaotic. Proceedings of the Royal Society of London B: Biological Sciences. 1995;259:71–76. doi: 10.1098/rspb.1995.0011. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry. Nature. 1996;379:549–553. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- Levelt WJ. Note on the distribution of dominance times in binocular rivalry. British Journal of Psychology. 1967;58:143–145. doi: 10.1111/j.2044-8295.1967.tb01068.x. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. On binocular rivalry. The Hague/Paris; Mouton: 1968. [Google Scholar]
- Logothetis NK. A primer on binocular rivalry, including current controversies. Philosophical Transactions of the Royal Society of London B. 1998;353:1801–1818. doi: 10.1098/rstb.1998.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamassian P, Goutcher R. Temporal dynamics in bistable perception. Journal of Vision. 2005;5(4):7, 361–375. doi: 10.1167/5.4.7. http://www.journalofvision.org/content/5/4/7. [DOI] [PubMed] [Google Scholar]
- Merk I, Schnakenberg J. A stochastic model of multistable visual perception. Biological Cybernetics. 2002;86:111–116. doi: 10.1007/s004220100274. [DOI] [PubMed] [Google Scholar]
- Moreno-Bote R, Rinzel J, Rubin N. Noise-induced alternations in an attractor network model of perceptual bistability. Journal of Neurophysiology. 2007;98:1125–1139. doi: 10.1152/jn.00116.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Bote R, Shpiro A, Rinzel J, Rubin N. Bi-stable depth ordering of superimposed moving gratings. Journal of Vision. 2008;8(7):20, 1–13. doi: 10.1167/8.7.20. http://www.journalofvision.org/content/8/7/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TJ, Blake R. A fresh look at the temporal dynamics of binocular rivalry. Biological Cybernetics. 1989;61:223–232. doi: 10.1007/BF00198769. [DOI] [PubMed] [Google Scholar]
- Ohzawa I, Sclar G, Freeman RD. Contrast gain control in the cat visual cortex. Nature. 1982;298:266–268. doi: 10.1038/298266a0. [DOI] [PubMed] [Google Scholar]
- Ohzawa I, Sclar G, Freeman RD. Contrast gain control in the cat’s visual system. Journal of Neurophysiology. 1985;54:651–667. doi: 10.1152/jn.1985.54.3.651. [DOI] [PubMed] [Google Scholar]
- Riani M, Simonotto E. Stochastic resonance in the perceptual interpretation of ambiguous figures: A neural network model. Physical Review Letters. 1994;72:3120–3123. doi: 10.1103/PhysRevLett.72.3120. [DOI] [PubMed] [Google Scholar]
- Rubin N, Hupé JM. Dynamics of perceptual bistability: Plaids and binocular rivalry compared. In: Alais D, Blake R, editors. Binocular Rivalry. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- Shpiro A, Curtu R, Rinzel J, Rubin N. Dynamical characteristics common to neuronal competition models. Journal of Neurophysiology. 2007;97:462–473. doi: 10.1152/jn.00604.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpiro A, Moreno-Bote R, Bloomberg S, Rubin N, Rinzel J. Maximum alternation rate in bi-stable perception occurs at equidominance: Experiments and modeling. BMC Neuroscience. 2007;8:P78. [Google Scholar]
- Shpiro A, Moreno-Bote R, Rubin N, Rinzel J. Balance between noise and adaptation in competition models of perceptual bistability. Journal of Computational Neuroscience. 2009;27:462–473. doi: 10.1007/s10827-008-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner GR, Albright TD, Ramachandran VS. Transparency and coherence in human motion perception. Nature. 1990;344:153–155. doi: 10.1038/344153a0. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Bressan P. Occlusion and the perception of coherent motion. Vision Research. 1991;31:1967–1978. doi: 10.1016/0042-6989(91)90191-7. [DOI] [PubMed] [Google Scholar]
- van Boxtel JJ, van Ee R, Erkelens CJ. Dichoptic masking and binocular rivalry share common perceptual dynamics. Journal of Vision. 2007;7(14):3, 1–11. doi: 10.1167/7.14.3. http://www.journalofvision.org/content/7/14/3. [DOI] [PubMed] [Google Scholar]
- Wallach H. On Perception. New York: Quadrangle; 1976. [Google Scholar]
- Wheatstone C. Contributions to the physiology of vision Part I: On some remarkable, and hitherto unobserved, phenomena of binocular vision. London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, Series 4. 1838;3:241–267. [Google Scholar]
- Wilson HR. Computational evidence for a rivalry hierarchy in vision. Proceedings of the National Academy Sciences of United States of America. 2003;100:14499–14503. doi: 10.1073/pnas.2333622100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR. Minimal physiological conditions for binocular rivalry and rivalry memory. Vision Research. 2007;47:2741–2750. doi: 10.1016/j.visres.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Wilson HR, Kim J. A model for motion coherence and transparency. Visual Neuroscience. 1994;11:1205–1220. doi: 10.1017/s0952523800007008. [DOI] [PubMed] [Google Scholar]
- Wuerger S, Shapley R, Rubin N. “On the visually perceived direction of motion” by Hans Wallach: 60 years later. Perception. 1996;25:1317–1367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


