Abstract
Lung epithelial cells can influence immune responses to airway allergens1,2. Airway epithelial cells also undergo apoptosis after encountering environmental allergens3; yet, relatively little is known about how these are cleared, and their effect on airway inflammation. Here we show that airway epithelial cells efficiently engulf apoptotic epithelial cells and secrete anti-inflammatory cytokines, dependent upon intracellular signalling by the small GTPase Rac1. Inducible deletion of Rac1 expression specifically in airway epithelial cells in a mouse model resulted in defective engulfment by epithelial cells and aberrant anti-inflammatory cytokine production. Intranasal priming and challenge of these mice with house dust mite extract or ovalbumin as allergens led to exacerbated inflammation, augmented Th2 cytokines and airway hyper-responsiveness, with decreased interleukin (IL)-10 in bronchial lavages. Rac1-deficient epithelial cells produced much higher IL-33 upon allergen or apoptotic cell encounter, with increased numbers of nuocyte-like cells1,4,5. Administration of exogenous IL-10 ‘rescued’ the airway inflammation phenotype in Rac1-deficient mice, with decreased IL-33. Collectively, these genetic and functional studies suggest a new role for Rac1-dependent engulfment by airway epithelial cells and in establishing the anti-inflammatory environment, and that defects in cell clearance in the airways could contribute to inflammatory responses towards common allergens.
Exposure to environmental pollutants, allergens and pathogens can induce apoptosis of airway epithelial cells3,6,4,7. In fact, clusters of uncleared epithelial cells referred to as ‘Creola bodies’ in the sputum have been seen in patients with asthma for decades8,9. Although the lung contains professional phagocytes such as macrophages and dendritic cells, many so-called ‘non-professional phagocytes’ can engulf apoptotic cells10,11. Because the bronchial epithelial cells come in contact with allergens first, and greatly outnumber the professional phagocytes in situ, we asked whether epithelial cells might contribute to cell clearance in the airways.
We tested phagocytosis by primary murine airway epithelial cells, the human bronchial epithelial cell line BEAS-2B and the murine alveolar epithelial cell line MLE-12. To score internalization, we labelled the apoptotic epithelial cells with a pH-dependent dye (CypHer5), whose fluorescence increased within acidic phagolysosomes12,13 (Fig. 1a, b). BEAS-2B, MLE-12 and the primary murine airway epithelial cells (CD45− EpCAM+) engulfed apoptotic targets; this was dependent on phosphatidylserine (PtdSer) recognition on the apoptotic cells (inhibitable by annexin V) (Fig. 1b). Apoptotic cell recognition by professional phagocytes is known to elicit anti-inflammatory mediators that dampen inflammation in the local tissue environment14,15. Airway epithelial cells also produced active transforming growth factor (TGF)-β and PGE2 after apoptotic cell recognition, and dependent on phosphatidylserine and Rac1 (Fig. 1c, d and Supplementary Fig. 1a, b). This suggests that airway epithelial cells can engulf apoptotic targets and produce anti-inflammatory mediators.
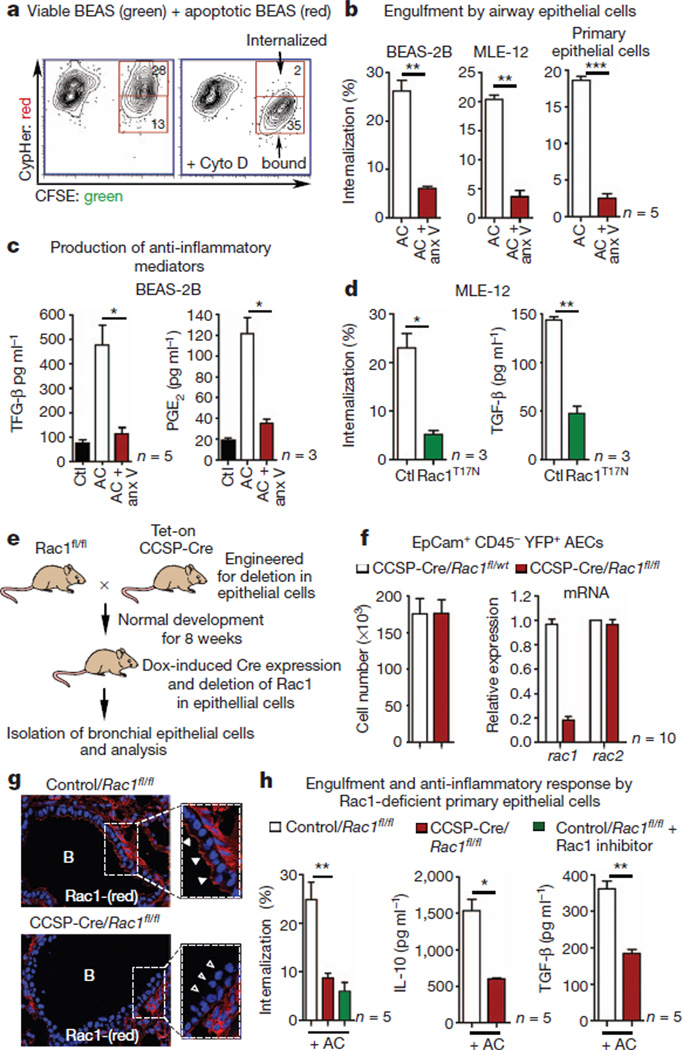
Figure 1. Rac1-dependent engulfment and anti-inflammatory cytokine production by airway epithelial cells.
a, Uptake of CypHer5-labelled apoptotic epithelial cells by viable epithelial cells (CFSE). b, Internalization is blocked by annexin (anx) V (n = 5). AC, airway epithelial cells. c, TGF-β (n = 5) and PGE2 (n = 3) production by BEAS-2B epithelial cells. d, Phagocytosis and TGF-β production in MLE-12 epithelial cells transfected with Rac1T17N (n = 3). e, Schematic for generating CCSP-Cre/Rac1fl/fl mice and Dox-induced (‘Tet-On’) Rac1 deletion in airway epithelial cells. f, Left, YFP+ epithelial cells from control and Rac1-deficient mice. Right, Rac1 mRNA expression in epithelial cells (n = 10). g, Loss of Rac1 in the airways of Dox-treated CCSP-Cre/Rac1fl/fl mice (open arrowheads). h, Left, engulfment by airway epithelial cells from control and Rac1-deficient mice (n = 5). **P < 0.01, representative of three experiments. Middle and right, IL-10 and TGF-β in BAL fluid of control and Rac1-deficient mice after intranasal administration of apoptotic cells (f, h, n = 5–10 mice from three experiments). *P < 0.05, **P < 0.01, unpaired Student’s t-test with Welch’s correction (b, c, d, h), Mann–Whitney test (h). Error bars, s.e.m.
To test the importance of cell clearance by airway epithelial cells in vivo, we wished to delete engulfment genes specifically within epithelial cells and assess airway inflammation. Because phagocytes often express several engulfment receptors, and the relative importance of particular engulfment receptors on the epithelial cells is not fully characterized, we targeted the small GTPase Rac1, which functions downstream of several engulfment signalling pathways15,16. Deletion of Rac1 in the airway epithelial cells was achieved by crossing Rac1fl/fl mice with clara cell secretory protein (CCSP)-rtTA/tetO-Cre transgenic mice, which express Cre under tissue-specific and temporal control (Fig. 1e and Supplementary Fig. 2a)16,17. Doxycycline (Dox) given through drinking water induced Cre expression in the epithelial cells of the trachea, bronchi and bronchioles under the promoter for the Clara cell-specific secretory protein (CCSP)18 (Supplementary Fig. 2b). Dox administration to CCSP-Cre/Rac1fl/fl mice led to Rac1 deletion specifically in the epithelial cells (Fig. 1f, g), but not in other cells in the bronchial tissue, or in CD45+ haematopoietic cells (Fig. 1f). Rac1 expression in CCSP-Cre/Rac1fl/fl mice was unaffected before doxycycline treatment, and Dox treatment of Rac1wt/wt mice did not affect Rac1 or Rac2 expression.
The doxycycline-inducible system allowed the airways to develop normally, until Rac1 deletion at 8 weeks. However, because Rac1 is a key cytoskeletal regulator in many cell types, we were concerned about potential unintended effects. Several independent lines of evidence alleviated this concern. First, the architecture and epithelial cell morphology of the airways were comparable at steady state in control and Rac1 deleted mice (Supplementary Fig. 2b). Second, numbers of yellow fluorescent protein (YFP+)-expressing epithelial cells in CCSP-Cre/Rac1fl/fl mice crossed to Rosa26STOP-EYFP reporter mice were comparable to controls (Fig. 1f); importantly, Rac1 expression was deleted in the YFP+ epithelial cells but not CD45+YFP− haematopoietic cells (Fig. 1f and Supplementary Fig. 3c). Third, primary epithelial cell cultures yielded similar total numbers of cells positive for nitrotetrazolium blue from control and CCSP-Cre/Rac1fl/fl mice (Supplementary Fig. 4a). Fourth, the epithelial cell integrity and tight junction formation was unaffected after Rac1 deletion, on the basis of staining for the tight-junction protein occludin (Supplementary Fig. 4c, d) and transmission electron microscopy (Supplementary Fig. 5a). Fifth, integrity of the alveolar-capillary membrane in CCSP-Cre/Rac1fl/fl was intact on the basis of intravenous injection of Evan’s blue and tracking the dye in the lung (Supplementary Fig. 4b). Sixth, the uptake of intranasally administered fluorescently labelled antigens by epithelial cells, dendritic cells, and alveolar macrophages was similar between control and Rac1-deficient mice (Supplementary Fig. 5b, c). Collectively, Dox-induced deletion of Rac1 in the airway epithelial cells in the CCSP-Cre/Rac1fl/fl mice did not affect the integrity of the epithelial cells.
Airway epithelial cells from CCSP-Cre/Rac1fl/fl mice showed significant reduction in phagocytosis of apoptotic cells in vitro (Fig. 1h), and after intranasal administration of apoptotic cells in vivo (Supplementary Fig. 6a); in contrast, alveolar macrophages from these mice showed no phagocytic defect (Supplementary Fig. 6b). Intranasal administration of apoptotic cells resulted in significantly decreased amounts of anti-inflammatory cytokines IL-10 and TGF-β in the CCSP-Cre/Rac1fl/fl mice (Fig. 1h). Because the CCSP-Cre/Rac1fl/fl mice have normal Rac1 in CD45+ cells (such as macrophages), the epithelial cells clearly influenced cytokine production in the bronchial spaces in response to apoptotic cells. Furthermore, in Rac1fl/fl mice crossed with LysM-Cre mice (Cre expression targeted to the myeloid lineage)19, intranasal administration of apoptotic cells did not show a decrease in IL-10 or TGF-β in the bronchoalveolar lavage (BAL) fluid (Supplementary Fig. 8b). Thus, mice lacking Rac1 in the airway epithelial cells showed defective ability to engulf apoptotic cells and altered inflammatory response in vivo.
We next tested these mice in airway inflammation models. One common approach to induce airway inflammation is to prime with antigen intraperitoneally and subsequently challenge with the same antigen by the intranasal route. We tested the development of allergic inflammation in CCSP-Cre/Rac1fl/fl mice to low-endotoxin house dust mite (HDM) extract, a common airway allergen. The control mice showed T-cell and eosinophil infiltration, along with immunoglobulin-E (IgE) response and Th2 cytokines IL-4, IL-5 and IL-13, but all these parameters were heightened in the CCSP-Cre/Rac1fl/fl mice (Supplementary Fig. 7), suggesting that losing Rac1 expression in airway epithelial cells exacerbates inflammation.
Because humans encounter environmental antigens through the airways before developing symptoms of airway inflammation, we established a system where both the primary allergen encounter and secondary challenge were through the intranasal route (Fig. 2a). After Dox-induced Rac1 deletion followed by intranasal priming and challenge with HDM, the CCSP-Cre/Rac1fl/fl mice showed a strong airway inflammation phenotype with significant accumulation of mucus (Fig. 2b). In contrast, controls showed minimal or undetectable airway inflammation after intranasal priming and challenge. These control conditions included Dox-treated, saline injected CCSP-Cre/Rac1fl/fl mice, Dox-treated Rac1fl/fl mice without Cre, CCSP-Cre/Rac1fl/wt mice and CCSP-Cre/Rac1fl/fl mice that did not receive doxycycline. Although we often observed more uncleared apoptotic nuclei in allergen challenged airways of CCSP-Cre/Rac1fl/fl mice (by TdT-mediated dUTP nick end labelling and cleaved caspase 3 staining), the staining was variable between samples, probably because of mucus and other secretions in inflamed tissues (data not shown).
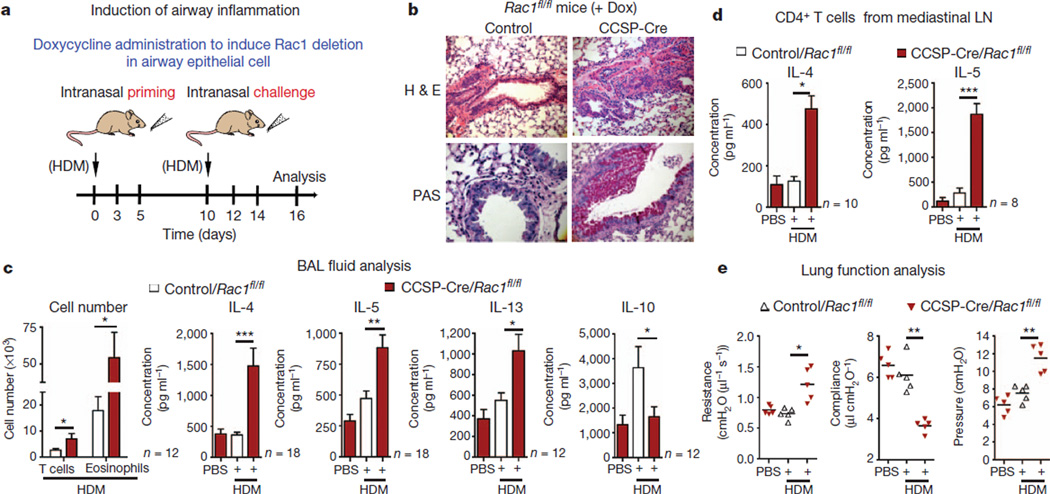
Figure 2. Mice lacking Rac1 in airway epithelial cells show increased allergic inflammation.
a, Protocol for inducing airway inflammation. b, Representative haematoxylin and eosin (H&E) and periodic acid Schiff (PAS) staining of lung sections from control or CCSP-Cre/Rac1fl/fl mice. c, Infiltrating CD4+ T cells, eosinophils and Th2-type cytokines in BAL fluid of control or Rac1-deficient mice (n = 12–18 mice per group from more than four experiments). d, Cytokine production by mediastinal lymphnode (LN) cells restimulated in vitro for 5 days with HDM (n = 8–10 mice per group from three experiments). e, Airway resistance, compliance and pleural pressure in control or in Rac1-deficient mice (n = 5 mice from two experiments). * P < 0.05, **P < 0.01, *** P < 0.001, unpaired Student’s t-test with Welch’s correction (c–e).
Allergic airway inflammation involves Th2 responses in the lungs5,20,21. The CCSP-Cre/Rac1fl/fl mice showed high concentrations of Th2 cytokines IL-4, IL-5, IL-13, eosinophil and T-cell infiltration in BAL fluid (Fig. 2c). IL-10 was significantly reduced in the Rac1- deficient mice (Fig. 2c), correlating with low IL-10 seen in airways of asthmatic patients22. When re-stimulated with HDM ex vivo, CD4+ T cells from the mediastinal lymph nodes of CCSP-Cre/Rac1fl/fl mice produced much higher amounts of IL-4, IL-5 and IL-13 than control mice (Fig. 2d). When Rac1 deletion in the airway epithelial cells was controlled (by Dox administration) at either the priming (sensitization) or challenge phases, keeping Rac1 expression normal at the priming phase and deleting at the challenge phase showed minimal airway inflammation to HDM (Supplementary Fig. 9). These data suggest that Rac1 expression in airway epithelial cells plays a critical role in preventing sensitization to common airway allergens encountered by the intranasal route.
When we analysed lung function parameters, HDM-sensitized CCSP-Cre/Rac1fl/fl mice showed physiological changes consistent with asthma including much greater airway resistance, decreased compliance and higher pulmonary arterial pressure (Fig. 2e). Loss of Rac1 led to a twofold increase in methacholine-induced airway hyper-responsiveness compared with wild-type littermates (Supplementary Fig. 10). Thus, Rac1 expression in airway epithelial cells critically controls allergen-induced airway inflammation and the physiological changes of asthma. Interestingly, intranasal priming and challenge of the LysM-Cre/Rac1fl/fl mice with HDM did not show enhanced airway inflammation (Supplementary Fig. 8c, d).
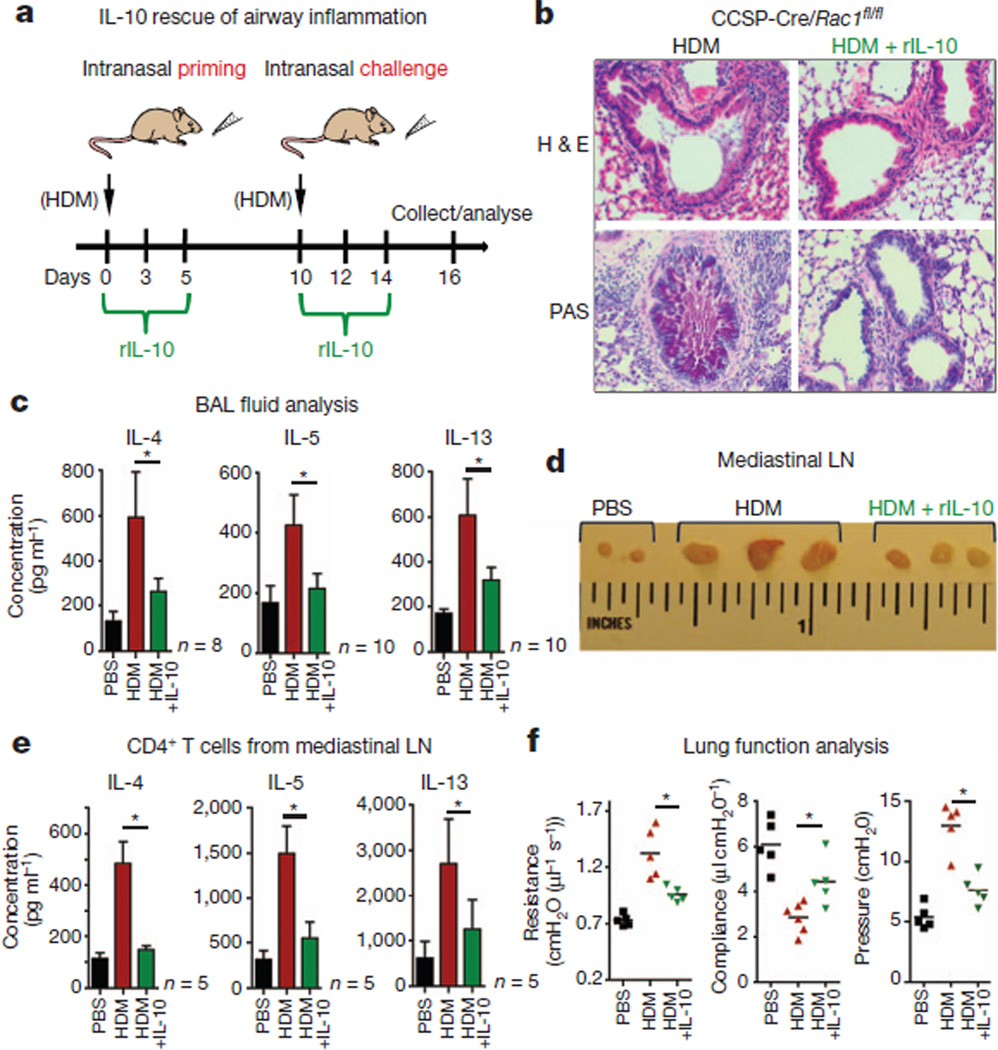
We next asked whether exogenous IL-10 could reverse the inflammatory phenotype in CCSP-Cre/Rac1fl/fl mice. Recombinant mouse IL-10 (rIL-10) given at priming and challenge phases with HDM resulted in markedly reduced airway inflammation, manifested by reduced production of mucus (Fig. 3a, b), and lower Th2 cytokines IL-4, IL-5 and IL-13 in the airways. The mediastinal lymph nodes were substantially smaller in the rIL-10-treated CCSP-Cre/Rac1fl/fl mice, with a significant decrease in the HDM-induced production of Th2 cytokines (Fig. 3c–e). rIL-10-treated mice also showed improved pulmonary function (Fig. 3f). This suggests that Rac1 expression in epithelial cells and IL-10 amounts in the airways are complementary factors regulating airway inflammation.
Figure 3. Allergic airway inflammation in Rac1-deficient mice is rescued by rIL-10.
a, Schematic of inducing airway inflammation and IL-10 treatment. b, H&E and PAS staining of lung sections from CCSP-Cre/Rac1fl/fl mice treated with or without rIL-10 (1 µg), representative of several experiments. c, BAL fluid analysis showing IL-4, IL-5 and IL-13 production by Rac1-deficient mice treated with or without rIL-10 (n = 8–10 mice per group, from three experiments). d, Image of mediastinal lymph nodes (n = 3 mice per group). e, Cytokine production by lymph node cells re-stimulated in vitro for 5 days with HDM (n = 5 mice per group). f, Airway resistance, compliance and pleural pressure in Rac1-deficient mice treated with or without rIL-10 (n = 5) (d–f, representative of three experiments). *P < 0.05, unpaired Student’s t-test with Welch’s correction (c, e, f).
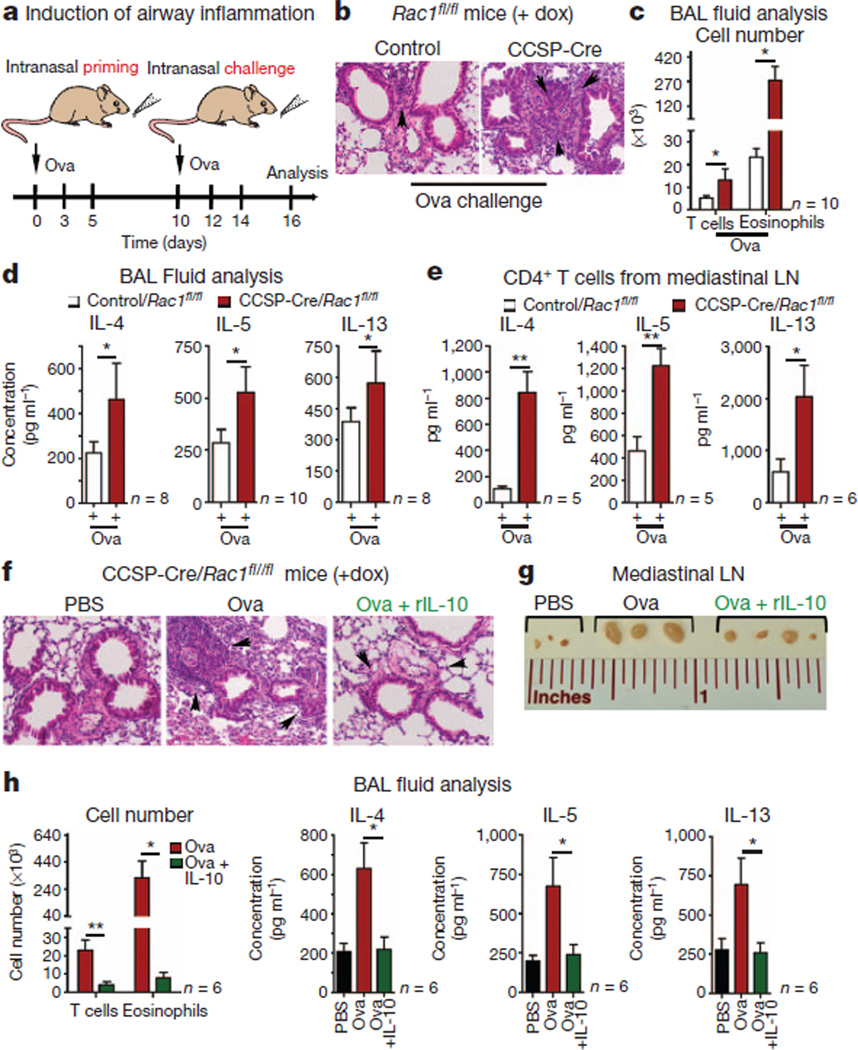
Preparations of HDM are enriched in the type-2-group allergen Der p2, an LPS-binding protein23,24. To test whether mice lacking Rac1 in the airway epithelial cells are inherently sensitive to allergen exposure in the absence of adjuvant activity, we first primed CCSP-Cre/Rac1fl/fl mice with recombinant Der p1, which showed augmented IgE and IgG1 antibody response and increased inflammation (Supplementary Fig. 7e, f). Second, although intranasal administration of chicken ovalbumin (Ova) without LPS often leads to tolerance induction25, in CCSP-Cre/Rac1fl/fl mice intranasal priming/challenge with Ova led to airway inflammation (Fig. 4a–d). Control mice showed minimal inflammation. Mediastinal lymph node cells from Rac1-deficient mice also produced higher IL-4, IL-5 and IL-13 (Fig. 4e). We confirmed that control mice primed with (Ova plus alum) by the intraperitoneal route and challenged with Ova by the intranasal route showed airway inflammation (Supplementary Fig. 11). In CCSP-Cre/Rac1fl/fl mice, the amounts of IL-10 in the BAL fluid were lower after Ova priming and challenge, and administering rIL-10 significantly reduced this inflammation (Fig. 4f–h). Thus, Rac1 loss in the bronchial epithelial cells and reduced IL-10 creates a state that is prone to inflammation in response to normally innocuous inhaled allergens.
Figure 4. Airway inflammation with Ova after Rac1 deletion.
a, Schematic of intranasal priming and challenge with Ova. b, H&E staining of lung sections from Dox-treated control and CCSP-Cre/Rac1fl/fl mice (×20 magnification). c, Infiltrating CD4+ T cells and eosinophils in BAL fluid of indicated mice. d, Th2-cytokines in the BAL fluid. (c, d, n = 10 mice per group). e, Cytokine production by mediastinal lymph node T cells re-stimulated in vitro for 5 days with Ova (n = 6 mice per group). (c–e, Representative of three experiments). f–h, Rac1-deficient mice primed and challenged with Ova with IL-10. f, H&E staining of lung sections. Arrowheads indicate leukocyte infiltration (×20 magnification). g, Image of mediastinal lymph nodes (f, g, representative of three experiments). h, BAL fluid analysis of infiltrating T-cells, eosinophils and Th2 cytokines (n = 6 mice combined from two experiments). *P < 0.05, **P < 0.01, unpaired Student’s t-test with Welch’s correction.
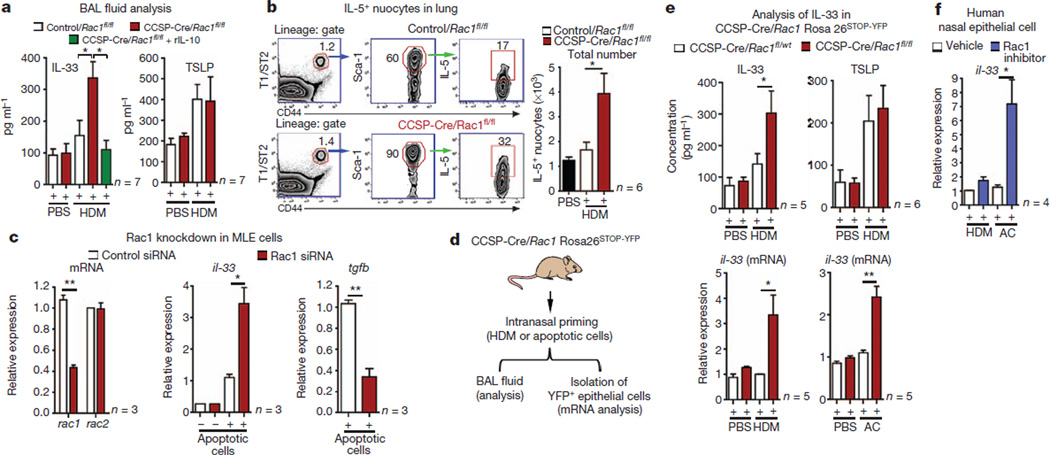
Thymic stromal lymphopoietin (TSLP) and IL-33 represent two cytokines linked to promoting airway inflammation1,26,27. Although TSLP did increase in the BAL fluid after HDM challenge, this was comparable between control and CCSP-Cre/Rac1fl/fl mice. However, IL-33 was significantly higher in the CCSP-Cre/Rac1fl/fl mice after allergen challenge (although basal IL-33 without HDM challenge was not increased) (Fig. 5a). Treatment with rIL-10 diminished the IL-33 amounts in the BAL fluid (Fig. 5a). IL-33 has been implicated in the pathogenesis of many diseases including asthma28. Recently, IL-33 has been shown to activate and expand a non-T/non-B population of innate lymphoid cells (‘nuocytes’), linked to promoting airway inflammation27,29. After HDM priming, lungs from CCSP-Cre/Rac1fl/fl mice contained significantly higher IL-5-expressing cell populations resembling nuocytes (lineage−, T1/ST2+ CD44+, Sca-1+, ICOS+) (Fig. 5b). Interestingly, after HDM priming of CCSP-Cre/Rac1fl/fl mice (less than 6 days), the lung nuocytes (but not CD4+ T cells) were positive for IL-5 (Supplementary Fig. 12a, b), suggesting inducible nuocyte activation. A neutralizing IL-33 antibody reduced the inflammation in the CCSP-Cre/Rac1fl/fl mice, with reduction in lymph node cellularity, infiltrating eosinophils and activated CD4+ T cells (Supplementary Fig. 12c, d). Although the function of this lung nuocyte-like population needs further testing, these data suggest a link between enhanced IL-33 production in the CCSP-Cre/Rac1fl/fl mice after allergen challenge, the expansion of an IL-5 producing nuocyte-like population and airway inflammation.
Figure 5. IL-33 upregulation in Rac1-deficient epithelial cells correlates with airway inflammation.
a, IL-33 and TSLP in BAL fluid of control or Rac1-deficient mice. b, Strategy for gating nuocyte-like cells and their numbers in lungs after HDM priming (a, b, n = 6 or 7 mice from two experiments). c, IL-33 or TGF-β expression in Rac1-siRNA-treated MLE-12 cells with apoptotic cells (n = 3) (c, representative of three experiments). d, e, In vivo IL-33 induction after intranasal HDM or apoptotic cells in CCSP-Cre/Rosa26YFP mice (d), or in purified YFP+ EpCam+ epithelial cells (n = 5 or 6 mice, from three experiments) (e). f, IL-33 mRNA in human nasal epithelial cells stimulated with HDM or apoptotic cells (n = 3); *P < 0.05, **P < 0.01, unpaired Student’s t-test with Welch’s correction. Error bars, s.e.m.
Because epithelial cells are a main source of IL-33, we asked how Rac1 affects IL-33 expression. When MLE-12 epithelial cells were stimulated with HDM or apoptotic cells, IL-33 messenger RNA (mRNA) was upregulated; drug-mediated inhibition of Rac1 (ref. 30) or short interfering RNA (siRNA) knockdown of Rac1 led to a three- to sixfold increase in IL-33 (Supplementary Fig. 13 and Fig. 5c). This IL-33 mRNA upregulation due to Rac inhibition was not a generic effect, as the inhibitor greatly diminished Rac1-dependent TGF-β expression (Supplementary Fig. 13b).
To test whether Rac1 deletion affects IL-33 expression in primary airway epithelial cells during allergen challenge, we used the CCSP-Cre/Rac1fl/fl mice crossed to the Rosa26STOP-EYFP reporter mice. Although the YFP+ EpCAM+ airway epithelial cells from control and CCSP-Cre/Rac1fl/fl mice had comparable amounts of basal IL-33 message, this was significantly increased after HDM stimulation. Second, in the BAL fluid of Rac1-deficient mice, IL-33 cytokine was increased three- to fivefold after intranasal priming with HDM, whereas the TSLP amounts were comparable (Fig. 5d, e). Third, after intranasal injection of apoptotic epithelial cells, purified YFP+ EpCAM+ epithelial cells from Rac1-deficient mice had much higher IL-33 mRNA (Fig. 5e). This link between Rac1 and il-33 expression was also seen in human nasal epithelial cells, as production of IL-33 by HDM or apoptotic cells was strongly enhanced by Rac inhibition (Fig. 5f). Collectively, these data suggest a critical role for Rac1 expression in epithelial cells in regulating IL-33 expression and, in turn, the sensitization to inhaled allergens.
Although airway epithelial cells are the first to come in contact with the allergens, their contribution to tolerance towards common airway allergens has been less clear. The data presented here suggest that airway epithelial cells can be phagocytic, and that their ability to secrete/modulate cytokines can critically influence tolerance to common airway allergens. Thus, apart from a physical barrier, phagocytosis in the airways may be part of an extra line of immune protection against innocuous antigens. These data also identify a newRac-1 dependent IL-33:IL-10 axis in controlling airway inflammation. The amounts of the pro-inflammatory cytokine IL-33 and the anti-inflammatory cytokine IL-10 seem linked, with Rac1 expression in airway epithelial cells controlling both steps. Although asthmatic patients have deficits in IL-10 (ref. 22), the contribution of IL-10 to allergen sensitization and its potential therapeutic use remains unexplored. Our studies suggest IL-10 as a critical regulator of airway inflammation, and IL-10 might prove a new therapeutic approach to limiting airway inflammation. Lastly, although cell clearance and anti-inflammatory cytokine production by professional phagocytes have long been studied, the relative importance of cell clearance by non-professional phagocytes under physiological settings is still limited. These data suggest that airway epithelial cells can be both phagocytic and modulate the cytokine milieu to limit airway inflammation.
METHODS
Mice
Mice carrying floxed alleles for rac1 were crossed with mice carrying the Cre recombinase driven by the rat CCSP promoter (CCSP-rtTA/tetO-Cre)18. Rac1 deletion in CCSP-Cre/Rac1fl/fl mice was achieved by administering doxycycline (1 mg ml−1) in drinking water containing 0.4% sucrose. The various control mice included CCSP-Cre/Rac1fl/fl mice that received no doxycycline, Rac1fl/fl mice (no Cre) that received doxycycline, doxycycline-treated CCSP-Cre/Rac1fl/wt heterozygous mice or CCSP-Cre/Rac1wt/wt mice. To facilitate flow cytometric isolation of those airway epithelial cells with Rac1 deletion, the CCSP-Cre/Rac1fl/fl were further crossed with the Rosa26STOP-EYFP reporter mice (The Jackson Laboratory). The Cre/Rac1fl/fl mice were also crossed with either LysM-Cre strain to achieve deletion of Rac1 in the myeloid lineage, or the CD11c-Cre mice to achieve deletion in the dendritic cell lineage. The University of Virginia Animal Care and Use Committee approved all animal experiments.
Induction of airway inflammation
Mice were primed intranasally with 50 µl of low-endotoxin preparation of the HDM extract (10 µg, Indoor Biotechnologies) or with chicken Ova (20 µg, Sigma-Aldrich) on days 0, 3, 5. Control mice received saline during the priming and challenge phases. Mice were then challenged intranasally on days 10, 12 and 14. On day 16, the mice were analysed for Th2 cell-dependent eosinophilic airway inflammation. Alternatively, mice were primed with HDM, Der p1 or Ova by intraperitoneal injection with Alum (2.25 mg, Pierce) on days 0, 3, 5, followed by intranasal challenge on days 10, 12 and 14 and analysed for inflammation.
Collection of BAL fluid and total cell numbers
BAL was performed by injecting 1 ml of phosphate-buffered saline (PBS) containing 0.01 mM EDTA through a cannula, flushing twice and collecting 500–700 µl. The BAL fluid was centrifuged and the cells were washed with PBS containing 1% FBS. The cells were stained for monocytes/macrophages, neutrophils, T cells, alveolar macrophages and eosinophils using the following antibodies: anti-mouse Ly6G, Ly6C, F4/80, CD11b (for macrophages and neutrophils), CD4, CD25, CD44, CD69 (for T cells), CD11c (for dendritic cells) and Siglec F (BD Biosciences) (for eosinophils and alveolar macrophages). The absolute cell numbers of cells were determined by adding reference particles (Spherotec) during flow cytometry (Supplementary Fig. 14). Data were collected on FACS Canto (Becton Dickinson) and analysed with Flowjo software (Treestar). The BAL fluid was stored at −80 °C and further used in analysis of cytokines.
Cytokine analysis
IL-4, IL-5, IL-13, IL-10, IL-33 and TSLP were measured by ELISA (using antibodies from R&D Systems) in the BAL fluid by ELISA 48 h after the last challenge. HDM-specific IgE and IgG1 were measured in the sera and BAL fluid of mice sensitized and challenged with HDM by ELISA (antibodies from BD Biosciences). HDM or Ova-specific CD4+ T-cell responses were measured by re-stimulating 5 × 106 cells from mediastinal lymph nodes for 5 days with 10 µg of HDM or Ova in the presence of 5 × 106 mitomycin C-treated splenocytes.
Microscopy and histology
The expression of CCSP, Rac1 and the Cre recombinase was determined by immunofluorescence staining. An optimal cutting temperature compound (O.C.T., Sakura Finetek) was injected into the lungs through a tracheal cannula. The lungs were excised and ‘snap frozen’ with liquid nitrogen and stored in −80 °C. Frozen sections (0.8 µm) were cut and stained with primary antibody to CCSP (1:50, Santa Cruz Biotechnology), Rac1 (1:20, Cytoskeleton) and Cre, using a FITC- (fluorescein isothiocyanate) labelled antibody (1:500, BioLegend) for 30 min at room temperature. The sections were washed three times with PBS and stained for 30 min at room temperature with anti-goat Alexa Fluor® 647 and anti-rabbit Alexa Fluor® 594 secondary antibodies (1:500, Invitrogen). The nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen). The samples were analysed using a Zeiss AxioImager Z2 equipped with Apotome for optical sectioning (Zeiss).
To assess the amount of inflammation and mucus secretion, lung sections were paraffin-embedded, fixed in 10% neutral buffered-formalin and 0.8 µm sections were stained with H&E and PAS, respectively. All staining procedures were performed by the histology core facility at the University of Virginia.
Airway epithelial cell isolation and culture
For primary airway epithelial cell purification and culture, lungs were perfused through the right ventricle with buffered saline and carefully excised. Two millilitres of dispase (BD bioscience) were instilled into the lungs, followed by 1% low-melting agrose (Invitrogen) and incubated for 1 h at 37 °C. After incubation, the lungs were minced and mechanically disrupted by passing them through 100 and 70 µm nylon strainers. Single-cell suspensions were washed with PBS containing 1% FBS and 100 units of DNase1 and treated with red blood lysis buffer (Sigma-Aldrich) for 5 min. The cells were then washed, centrifuged, and CD45+ cells were depleted from the single-cell suspensions by incubating with anti-CD45 microbeads and passed through a MACS® Cell Separation Column (Miltenyi Biotech). The cells were re-suspended in DMEM/F-12 media containing 10% FBS and plated in a 10cm culture dish at 37 °C for 2 h. After incubation, the non-adherent cells were collected, centrifuged and seeded on a 10 cm culture plate coated with calfskin collagen type I (Sigma-Aldrich).
Lung function analysis
Lung function was measured using an in situ buffer-perfused mouse lung system. Animals were anaesthetized and positive-pressure (2 cmH2O) ventilation (120 breaths per minute, tidal volume) was initiated. The lungs were perfused with Krebs–Henseleit buffer, containing 2% albumin, 0.1% glucose and 0.3% HEPES. The lungs were allowed to equilibrate for 5 min and pulmonary compliance, resistance and pulmonary arterial pressure were measured and recorded using Pulmodyn data-acquisition software (Hugo Sachs Electronik).
Airway hyper-responsiveness
Airway hyper-responsiveness was measured by exposing mice to increasing doses of aerosolized methacholine (0–10 mg ml−1). A tracheotomy tube was inserted and then connected to the inspiratory and expiratory ports of a volume-cycled ventilator (flexiVent; SCIREQ Scientific). The data from normal ventilation were collected to establish the baseline values for each animal. The change in airway resistance (ΔR) from baseline was calculated after each dose.
Transmission electron microscopy
Lungs from wild-type or Rac1-deficient mice were perfused with buffered saline and fixed in 4% paraformaldehyde solution containing 2% glutaraldehyde for 24 h. Lung sections were prepared and analysed by the University of Virginia advance microscopy facility.
In vitro engulfment assay
MLE-12, BEAS-2B cells or primary airway epithelial cell cultures were plated at a density of 3 × 105 in a 12-well plate and incubated with 3 × 106 CypHer5 or TAMRA-labelled apoptotic Jurkat T-cells or etoposide-(100 µM) treated apoptotic BEAS-2B for 4 h. In some experiments, viable BEAS-2B cells were stained with CFSE (2.5 µM) before incubation with apoptotic cells. Phagocytosis was analysed using a BD FACS Canto (Becton Dickinson). To block phagocytosis, 1 µM of cytochalasin D (Sigma-Aldrich) or 10 µgml−1 of purified human annexin-V (Enzo Life Sciences) were used. In some experiments the specific Rac1 inhibitor EHT1864 (30 µM, Sigma-Aldrich) was used.
In vivo engulfment assay
CCSP-Cre/Rac1fl/fl mice and control/Rac1fl/fl mice crossed with Rosa26STOP-EYFP reporter mice (and therefore having Cre-marked airway epithelial cells) were injected intranasally with 10 × 106 CypHer-labelled apoptotic thymocytes for 3 h. The lungs were then minced, digested with collagenase type II (183 U ml−1) for 1 h and passed through a 70 µm strainer. Single-cell suspensions were gated on YFPhi cells and engulfment of apoptotic cells was analysed. Alveolar macrophages were collected from BAL fluid and stained with anti-CD11b, anti-F4/80 antibodies and analysed for uptake of CypHer-labelled apoptotic cells.
In vivo treatment with HDM or apoptotic cells
Control or CCSP-Cre/Rac1fl/fl mice were treated intranasally for 24 h with 10 µg of HDM or 5 million to 10 million apoptotic ulraviolet-treated Jurkat or etoposide-treated (100 µM) BEAS-2B. After 24 h, BAL fluid was collected and the cytokine production was analysed by ELISA.
Vascular permeability
Vascular permeability in the lungs was determined using the Evans blue dye extravasation method. Evans blue (20 mg kg−1, Sigma-Aldrich) was injected intravenously for 1 h before the mice were killed. The mice were then perfused with 10 ml PBS to remove intravascular dye. The lungs were then homogenized in PBS to extract the dye and centrifuged. The absorption of Evans blue was measured in the supernatants at 620nm, and corrected for the presence of haem pigments as follows: A620 (corrected) = A620 − (1.426A740 + 0.030).
Antigen uptake assay
Uptake of soluble antigen by dendritic cells, alveolar macrophages, or epithelial cells, 10 µg of fluorescently labelled HDM or Ova (Alexa Fluor® 594) was injected intranasally for 1 h. Total lungs were minced and treated with collagenase or dispase, single-cell suspensions were prepared and stained for dendritic cells, alveolar macrophages and epithelial cells, and analysed for the percentage of antigen uptake.
Identification of nuocyte-like cells in the lung
Total lung suspensions were prepared and gated on lineage-negative populations by staining with the following cocktail of antibodies: mouse anti-CD3, CD4, CD8, B220, CD11c, CD11b, CD49b, Ly6C/Ly6G. These cells were further gated on the basis of T1/ST2 expression (mouse biotin-conjugated anti-T1/ST2 antibody (clone DJ8 1:50 dilution), BioLegend), CD44 and Sca-1 (1:100 dilution, eBioscience). These nuocyte-like cells were further confirmed by ICOS surface expression (anti-ICOS antibody, eBioscience). Intracellular IL-5 expression was analysed by intracellular staining using anti-mouse IL-5 antibody (eBioscience).
Treatment with anti-IL-33 neutralizing antibody
CCSP-Cre/Rac1fl/fl or control mice were intranasally primed and challenged with HDM (10 µg) in the presence of anti-IgG (isotype control, 6 µg per dose) or anti-IL-33 (6 µg per dose) antibody (R&DSystems). After the last challenge, the mediastinal lymph nodes were excised and total cellularity was analysed. BAL fluid was collected and stained for activated T cells and eosinophils.
Transfection with siRNA
MLE-12 cells were transfected with a nucleofector, with 250nM of siRNA targeting Rac1 or a control non-targeting SMARTpool (Thermo Scientific, catalogue number L-041170-00-0005), using the recommended protocol for primary mammalian epithelial cells (Lonza).
Quantitative PCR analysis
Total RNA was extracted from epithelial cells using the Qiagen Qiashredder and RNeasy kit, and cDNA was synthesized using SuperScript III kit (Invitrogen). Quantitative PCR for Rac1, Rac2, TGF-β and IL-33 was performed using the TagMan Gene Expression Assay (Applied Biosystems), and were run on a STEP One plus instrument (ABI). It is noteworthy that the higher IL-33 measured was not derived from the apoptotic cells as species-specific primers were used (Supplementary Fig. 13b).
Statistical analysis
Statistical significance for individual data points was determined by an unpaired Student’s two-tailed t-test, unless noted otherwise. A P value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We thank the members of the Ravichandran laboratory for their suggestions, especially J. Kinchen and P. Trampont. We thank J. Whitsett for the rtTA-CCSP/Cre mice, X. Liu for the TGF-β responsive cell line PE25, and J. Steinke and J. Kennedy for providing human nasal epithelial cells. This work was supported by an Immunology Training Grant (I.J.J.), a F32 postdoctoral fellowship from the NHLBI (I.J.J.), and grants from the American Asthma Foundation and the National Institutes of Health (K.S.R.). K.S.R. has been a William Benter Senior Fellow of the American Asthma Foundation.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions I.J.J. designed, performed and analysed most of the experiments in this study with input from K.S.R. A.K. optimized and performed the isolations and ex vivo cultures of primary epithelial cells and the nitrotetrazoleum staining. A.K.S. performed the lung function analysis. Y.M.S. performed the airway hyper-responsiveness experiments to determine the degree of airway resistance. L.B. provided intellectual input on specific experiments and helped with the human tissue studies. I.J.J. and K.S.R. wrote the manuscript with comments from co-authors.
The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
References
- 1.Fahy JV, Locksley RM. The airway epithelium as a regulator of Th2 responses in asthma. Am. J. Respir. Crit. Care Med. 2011;184:390–392. doi: 10.1164/rccm.201107-1258ED. [DOI] [PubMed] [Google Scholar]
- 2.Lambrecht BN, Hammad H. The airway epitheliumin asthma. Nature Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 3.Jyonouchi H. Airway epithelium and apoptosis. Apoptosis. 1999;4:407–417. doi: 10.1023/a:1009607607603. [DOI] [PubMed] [Google Scholar]
- 4.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammad H, et al. House dust mite allergen induces asthmavia Toll-like receptor 4 triggering of airway structural cells. Nature Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White SR. Apoptosis and the airway epithelium. J. Allergy. 2011;2011:948406. doi: 10.1155/2011/948406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daidoji T, et al. H5N1 avian influenza virus induces apoptotic cell death in mammalian airway epithelial cells. J. Virol. 2008;82:11294–11307. doi: 10.1128/JVI.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naylor B. The shedding of the mucosa of the bronchial tree in asthma. Thorax. 1962;17:69–72. doi: 10.1136/thx.17.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada Y, Yoshihara S, Arisaka O. Creola bodies in wheezing infants predict the development of asthma. Pediatr. Allergy Immunol. 2004;15:159–162. doi: 10.1111/j.1399-3038.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 10.Monks J, et al. Epithelial cells asphagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005;12:107–114. doi: 10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch T, et al. Engulfment of apoptotic cells by microvascular endothelial cells induces proinflammatory responses. Blood. 2007;109:2854–2862. doi: 10.1182/blood-2006-06-026187. [DOI] [PubMed] [Google Scholar]
- 12.Park D, et al. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. 2011;477:220–224. doi: 10.1038/nature10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park D, Hochreiter-Hufford A, Ravichandran KS. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr. Biol. 2009;19:346–351. doi: 10.1016/j.cub.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nature Rev. Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 16.Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott MR, Ravichandran KS. ELMO1 signaling in apoptotic germ cell clearance and spermatogenesis. Ann. NY Acad. Sci. 2010;1209:30–36. doi: 10.1111/j.1749-6632.2010.05764.x. [DOI] [PubMed] [Google Scholar]
- 18.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002;11:21–29. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- 19.Glogauer M, et al. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J. Immunol. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- 20.Cates EC, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J. Immunol. 2004;173:6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 21.Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol. Med. 2010;16:321–328. doi: 10.1016/j.molmed.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Borish L, et al. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 23.Thomas WR. Molecular mimicry as the key to the dominance of the house dust mite allergen Der p 2. Expert Rev. Clin. Immunol. 2009;5:233–237. doi: 10.1586/eci.09.5. [DOI] [PubMed] [Google Scholar]
- 24.Trompette A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd CM. IL-33 family members and asthma - bridging innate and adaptive immune responses. Curr. Opin. Immunol. 2010;22:800–806. doi: 10.1016/j.coi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nature Rev. Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 29.Barlow JL, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 2012;129:191–198. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 30.Shutes A, et al. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J. Biol. Chem. 2007;282:35666–35678. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.