Abstract
Re-expression of KISS1 in tumor cell lines allows all antecedent steps of metastasis, but prevents colonization of secondary sites. Because tumor cells have already disseminated by the time of cancer diagnosis, KISS1 may represent a new opportunity for therapeutic intervention. Moreover, numerous clinical reports demonstrate that a loss or reduction of KISS1 expression in different human cancers inversely correlates with tumor progression, metastasis, and survival. Taken together, these observations compel the hypothesis that KISS1 could be of tremendous utility in controlling metastasis in a therapeutic context. In this review, we highlight some key findings from preclinical and clinical studies and discuss strategies whereby KISS1 may be exploited clinically to treat metastases.
Keywords: KISS1, kisspeptin, GPR54, G-protein coupled receptor, paracrine
1. Introduction
Metastasis hinges upon a stringently orchestrated cascade of events; therefore, interruption of any step effectively halts the process. An attractive group of candidates to treat metastasis are the metastasis suppressors, defined by their abilities to inhibit metastasis without blocking orthotopic tumor growth. This growing family of functionally-defined molecules now exceeds 25 1, 2. This number continues to grow since several other inhibitors of the steps in metastasis have been identified, but effects on metastasis have not yet been assessed in vivo. Although our understanding of the mechanisms of action grows rapidly, collectively, much remains to be learned about these important molecules.
In this short review, we will focus on the KISS1 metastasis suppressor, which affects colonization, the last step of the metastatic cascade. We will take readers through its discovery, preclinical characterization, and theoretical utility and limitations. In short, we will provide data showing that KISS1 expression in neoplastic cells renders them dormant after they have disseminated, effectively giving them what we term a good night kiss. In addition, we refer readers throughout this article to several more comprehensive reviews on KISS1 that have been published previously.
1.1 Discovery of the KISS1 metastasis suppressor
The discovery of KISS1 began by taking note of early karyotypic analyses of melanomas. Although percentages vary on a case-by-case basis, deletions or rearrangements of chromosome 6, particularly involving the long arm, are involved in >80% of melanomas 3. For this reason, full-length chromosome 6 was introduced into the human metastatic melanoma cell line C8161 employing microcell-mediated transfer (the hybrids were designated neo6/C8161) 3. These and subsequent studies revealed that the introduction of a normal copy of chromosome 6 suppressed metastasis without affecting tumorigenicity or local invasion 3.
KISS1 was subsequently identified as a human melanoma metastasis suppressor gene using subtractive hybridization between highly metastatic and nonmetastatic cell lines and respective cell line variants 4-6. Transfection of full-length KISS1 cDNA into melanoma 4-6 and breast carcinoma 7 cell lines suppressed metastasis in athymic mice using both spontaneous and experimental metastasis assays.
1.2 KISS1 is regulated by genes residing on chromosome 6
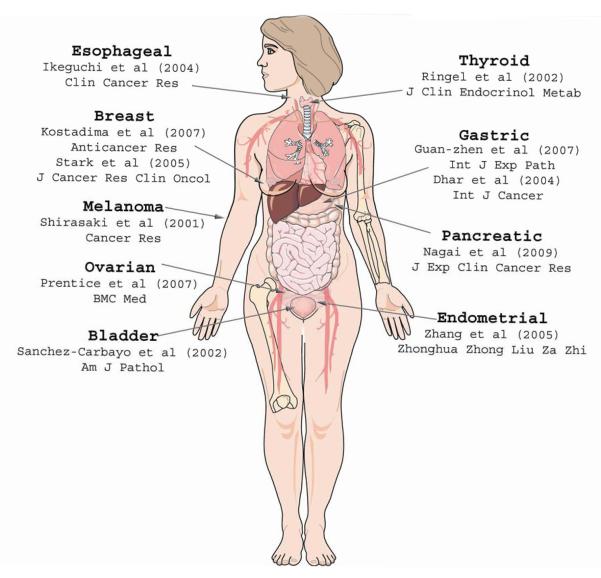
Unexpectedly, KISS1 mapped to chromosome 1q32. Those data were evidence for the existence of a regulatory gene on chromosome 6. Subsequent studies designed to explicitly identify the putative regulatory locus on chromosome 6 identified a 40-cM region between 6q16.3 and q23 as the principle regulatory region of KISS1 8. In addition, complementary studies by Shirasaki and colleagues 9 found that a loss of 6q16.3-q23 was observed in more than 50% of melanoma metastases and importantly, that a loss of heterozygosity (LOH) of this region was strongly associated with a loss of KISS1 (Figure 1).
Fig. 1. Clinical studies examining KISS1, KISS1R, and KP in various cancers.
The majority of reports cited in this figure provide evidence in support of KISS1 as an anti-metastatic. Studies finding negative observations or correlations regarding KISS1 are designated negative associations. Numbers in brackets correspond to the reference.
Subsequent studies by Goldberg et al. showed that a thioredoxin interacting protein (TXNIP, also known as vitamin D up-regulated protein 1, VDUP1, or thioredoxin binding protein 2) was expressed more highly in nonmetastatic melanomas and in the neo6/C8161 melanoma cell line 10. Furthermore, increased TXNIP expression by transfection into C8161.9 melanoma cells inhibited metastasis and up-regulated KISS1. As unexpectedly, TXNIP also mapped to chromosome 1q. Subsequent PCR karyotyping revealed that CRSP3/DRIP130 (co-factor required for SP1 activity or vitamin D receptor interacting protein) mapped to chromosome 6. CRSP3 transfected cells up-regulate both KISS1 and TXNIP expression and were suppressed for metastasis 10. In addition, analyses of clinically derived melanoma samples indicated that a loss of CRSP3 expression correlates with decreased KISS1 expression and increased metastasis 10.
In summary, these pivotal studies concluded that CRSP3 is an upstream regulator of TXNIP, which, in turn, regulates KISS1 expression. As a result, a loss or structural abnormality of chromosome 6, as is frequent in late-stage melanoma, results in a loss of CRSP3 expression, consequently altering the appropriate regulation of downstream mediators (i.e., TXNIP and KISS1).
2. The KISS1 gene produces kisspeptins that bind to GPR54, a G-protein coupled receptor
The KISS1 gene was predicted to encode a 154-amino acid protein. Yet, despite numerous attempts, our laboratory was unsuccessful in identifying an intact KISS1 protein. The mystery was solved in 2001 when three laboratories independently determined that internal peptides of KISS1 (subsequently termed kisspeptins, KP) bound to a then-orphan G-protein coupled receptor GPR54 (also known as AXOR12 or hOT7T175, but now referred to as the KISS1 receptor, KISS1R; Figure 2). Systematic examination of KISS1R expression reveals high KISS1R expression in placenta, pituitary gland, pancreas, brain, and spinal cord 11, 12. KISS1 expression is slightly more restricted, located primarily in the placenta, pancreas, kidney, and the arcuate nucleus of the hypothalamus 4, 12, 13.
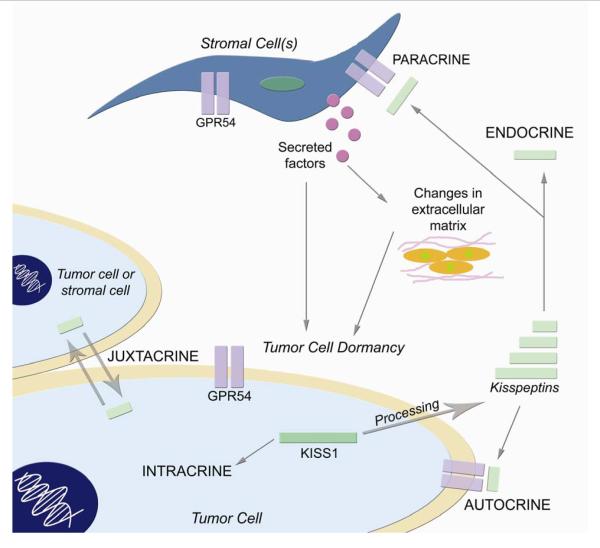
Fig. 2. Possible mechanisms by which KISS1 may cause dormancy in disseminated tumor cells at secondary sites.
Intracrine signaling: (un)processed KISS1 protein may interact with other intracellular proteins to initiate anti-proliferative signals. Juxtacrine signaling: unprocessed (KISS1) or processed KISS1 (KP) may be exchanged between adjacent tumor cells or stromal cells. Autocrine signaling: despite the lack of KISS1R expression on many tumor cell lines that are suppressed for metastasis by engineering them to express KISS1, KP could signal through KISS1R or an as-yet unidentified alternative receptor in an autocrine fashion to induce dormancy. Endocrine signaling: as potent mediators of various endocrine processes, KP may influence the endocrine system to produce dormancy-inducing factors or cause deviations from endocrine homeostasis that may bring about metastasis suppression. Paracrine signaling: KP may activate or induce stromal cells to produce secreted factors that may (in)directly elicit dormancy in metastatic cells or manipulate structural changes in the surrounding extracellular milieu that may also be capable of inducing dormancy. At the time of submission, all of these mechanisms are, for the most part, still speculative. However, experimental data support non-autocrine mechanisms 15.
Ohtaki and colleagues found that an amidated internal 54-amino acid peptide, which they termed metastin, binds and activates KISS1R 13. Kotani and colleagues reported the existence of multiple internal peptides, which they termed KP 11. Some KPs bind KISS1R, whereas others do not. Hereafter, we will retain the nomenclature of KPs (defined based upon the number of amino acids in the peptide) because it defines the gene that originally encoded the peptide.
A precise understanding of the mechanisms by which KPs are processed remains unidentified. Since processing is not the focus of this volume, we refer readers to more comprehensive reviews on the topic 14, 15. Briefly, however, proteolytic processing of the KISS1 protein is thought to occur by furins or prohormone convertases 11, 13 based upon the amino acids at the ends of the fragments. Specifically, cleavage at the dibasic sites R66-R and K123-R results in production of KP54. Shorter fragments of KP54 have been identified (e.g., KP10, KP13, and KP14). Each represents the C-terminal portion of KP54 and binds to and activates KISS1R 11, 16-19. The potency of the peptides is enhanced by amidation, although amidation is not required 13.
Transfection of the KISS1R into Chinese hamster ovary (CHO) cells followed by stimulation with KP results in PtdIns(4,5)P2 hydrolysis, Ca2+ mobilization, arachidonic acid release, stress fiber formation, and ERK1/2, p38, and MAP kinase phosphorylation; however, cell proliferation is inhibited 11. Ohtaki et al. also engineered CHO cells to express KISS1R and found that chemotaxis and invasion were inhibited in vitro 13. Moreover, administration of KP54 to C57BL/6 mice bearing B16 melanomas engineered to express KISS1R attenuated pulmonary metastases 13. As a result, most inferred that KISS1 exerted anti-metastatic effects via autocrine signaling. In yet another unexpected finding, we showed that none of the cell lines suppressed for metastasis by KISS1 re-expression express KISS1R, suggesting the existence of alternative signaling pathways 15.
We note that a preponderance of the primary literature describes the involvement of KISS1 and/or KP in numerous biological processes ranging from pubertal development to pregnancy (reviewed in Colledge17, Roa et al.20, Tena-Sempere21, and Gianetti et al. 22). For instance, remarkably, in pregnant women, plasma KP54 concentrations increase 900-fold over nonpregnant women in the first trimester of pregnancy, followed by a 7000-fold increase in KP54 during the third trimester 23. Furthermore, mutations in KISS1R are associated with autosomal recessive idiopathic hypogonadotropic hypogonadism in multiple animals and humans, suggesting that KISS1R is essential in the regulation of puberty 24.
3. Preclinical/Clinical Evidence for KISS1 as a valid anti-metastatic
In animal models, KISS1 expression blocks the ability of melanoma, breast, and ovarian cancer cells to colonize and proliferate at secondary sites in cancer xenograft models 4-7, 25. Clinical reports compel the prediction that reduction of KISS1 expression would correlate with tumor progression, metastasis, and survival. These data are summarized below and in Figure 1. We emphasize that the majority of the clinical studies have measured mRNA expression by in situ hybridization or PCR-based methods. The former are less ambiguous than studies in which stromal cells contaminate the cell preparation, making it impossible to judge the origins of KISS1 or KISS1R. In part, measurement of mRNA was required because of difficulties in generating specific antibodies. Still, many of the commercially available antibodies used have not been validated (or the data are not provided in publications). Likewise, the processing of KISS1 to KP has not been evaluated in clinical samples. While the bulk of data from numerous pilot studies support the role of KISS1 as a metastasis suppressor in clinical settings, technical caveats to the experimental design and some conflicting data can be confusing.
From a patient’s perspective, diagnosis of cancer is accompanied by multiple fears. Patients who have undergone apparently successful surgical resection unfortunately experience recurrence locally or at distant sites months or years later. As a result of sometimes subjective pathological criteria coupled with information available to the oncologist, patients often receive therapies to eliminate residual cells or eliminate disseminated cells before bona fide metastases develop. Unfortunately, for many cancers, the histology of the primary tumor does not provide unambiguous predictions for whether the tumor has already spread (or not). As a result, a substantial proportion of patients undergo unnecessary treatments as a precaution. We and others hold out hope that biomarkers such as KISS1 could be coupled with traditional pathology to refine a prognosis so that toxic treatments to already cured patients would be minimized. In Figure 1, a comprehensive summary of clinical studies is presented. Selected histotypes are described below.
3.1 Melanoma
The first clinical study implicating KISS1 in a human cancer was performed in cutaneous melanomas. Shirasaki examined KISS1 mRNA expression at various stages of melanoma progression and found that KISS1 expression was found in all nevocellular nevi and eight primary melanomas (<4 mm thickness, i.e., early disease), while in large primary melanomas (>4 mm thickness) and in metastases, KISS1 expression was lost in nearly half of the samples 9.
3.2 Breast and Ovarian cancer
Animal studies examining KISS1 in breast cancer clearly demonstrated suppression of metastases in vivo. However, information is limited and somewhat contentious regarding KISS1 in the context of clinical breast cancer. Analyses of KISS1 mRNA from paraffin-embedded stage II or III lymph node positive breast adenocarcinomas shows that only 3% of samples yielded KISS1 mRNA, thus supporting the anti-metastatic role of KISS1 26. Likewise, another group showed that KISS1 mRNA expression was lower in breast cancer brain metastases in comparison to KISS1 expression in the primary tumor 27. In contrast to the studies supporting the anti-metastatic role of KISS1 in breast cancer, Martin et al. found KISS1 messenger elevated in node-positive breast tumors in comparison to node-negative samples, yet no differences were observed in KISS1R 28. Moreover, in another study, among estrogen receptor-alpha-positive tumor samples from patients treated with tamoxifen, Marot and others found that when both KISS1 and KISS1R expression were high, patients exhibited shorter disease-free survival 29. The data in breast are reported here with a cautionary note regarding the technical caveats listed above.
The role of KISS1 in ovarian cancer appears less ambiguous. Immunohistochemistry (IHC) of numerous ovarian cancer samples employing antibodies directed against the various KP and KISS1R found that positivity for both individually correlated with favorable prognosis and superior overall survival 30. Similarly, at approximately the same time, another study reported that high simultaneous KISS1R and KP54 expression was significantly associated with improved survival 31.
3.3 Gastrointestinal cancers
Several studies have implicated KISS1 in various gastrointestinal cancers. In gastric cancer, Dhar et al. found low KISS1 expression in patients with distant metastases and worsened overall and disease-free survival 32. In another gastric cancer study, analysis of KISS1 protein expression in tissue microarrays found that KISS1 was reduced in lymph node and liver metastases compared with primary gastric tumors 33.
Pancreatic cancer is especially difficult to manage as early detection is rare and metastatic disease is common. Consequently, five-year survival is typically only 5%. Pancreaticoduodenectomy (Whipple procedure) is the only potentially curative approach, yet the majority of patients have incurable disease at diagnosis, and few patients are candidates for surgery. Normal pancreas expresses detectable KISS1 and pancreatic tumor tissues show significantly lower expression of KISS1 mRNA, yet curiously exhibit heightened KISS1R expression 34. Furthermore, exogenous KP54 does not affect proliferation of the pancreatic cancer cell line PANC-1, but reduces in vitro migration 34. IHC analysis showed KP54neg and KISS1Rneg tumors were significantly larger than tumors that expressed either KP54 or KISS1R 35. Strong expression of KP54 or KISS1R in samples was significantly correlated with improved survival. In fact, KP54 expression alone was found to be an independent prognostic factor for the survival of pancreatic cancer patients 35. In some patients, before surgical resection, plasma KP54 levels were measured by ELISA and patients were classified as having either high or low plasma KP54 35. While survival was not significantly different, unlike those patients with low plasma KP54, none of the patients with high KP54 levels died following surgery (up to 22.1 months) 35. In another study comparing plasma KP54 levels, the most significant relationship identified was that pancreatic cancer patients simply had higher plasma KP54 levels 36. Collectively, these findings are intriguing in that they establish KISS1 as an important prognostic indicator in pancreatic cancer, and for the first time, demonstrate the potential significance of plasma KP levels within patients.
The responsiveness of pancreatic tumors to KISS1 and/or KP suggested that KP could perhaps be used in a therapeutic setting. While not tested as a therapeutic, re-expression of KISS1 in a xenograft model of pancreatic cancer resulted in significantly reduced orthotopic tumor growth and metastases to lung and liver (L. McNally, D.R. Welch, D. Buchsbaum, submitted for publication).
The loss of KISS1 and KISS1R gene expression was not associated with tumor size or invasion in esophageal squamous cell carcinoma, but was found to be a significant predictor of lymph node metastasis 37. The same group, using RT-PCR, reported that over-expression of both KISS1 and KISS1R was positively correlated with HCC progression 38. These findings were corroborated by another study using immunohistochemistry 39.
4. Can KISS1 be exploited clinically?
Perhaps the most intriguing property of KISS1 derives from data in which re-expression of KISS1 still allows all antecedent steps of metastasis, including seeding at a secondary site without colonization of ectopic tissues. Briefly, KISS1 maintains disseminated tumor cells in a dormant state after they have seeded other tissues. While it has long been recognized that blocking any step of the metastatic cascade effectively prevents metastasis, it had been under-appreciated how critical the last step (colonization) in the process is. Since tumor cells have already disseminated by the time most cancers are diagnosed, even for a portion of the smallest tumors, interventions that target colonization afford new opportunities for cancer therapy 40. While dormancy per se would not be a cure, maintenance of tumors in an asymptomatic state would represent a significant advance.
How could properties of KISS1 be exploited in clinical settings? Lost KISS1 or KISS1R expression in tumor cells could be replaced using gene therapy strategies. Indeed, both non-viral and viral-mediated transfer of metastasis suppressor genes has shown promise in animal models of various cancers (reviewed in Smith and Theodorescu 41). At this time, a stand-alone therapy employing KISS1 is not especially feasible with current technologies. Nonetheless, it remains theoretically possible.
Defects in metastasis suppressor expression appear to be caused by lack of expression rather than loss-of-function mutations in the coding regions 40, 41. In a paradigm first advanced by Patricia Steeg and colleagues for Nm23 42, strategies to re-express KISS1 could represent a viable strategy. In the case of KISS1, upstream mediators were found to be responsible for reduced KISS1 expression. Replacement of the upstream regulators or mimetics (i.e., a peptidic or non-peptidic agonist that is not identical to the natural ligand) could be used. This strategy is complicated because systemic administration of agents that could up-regulate KISS1 or KISS1R could affect the biology of other tissues. Given its important endocrine roles and pleiotropic distribution in vivo, generalized re-expression may introduce unexpected off-target effects.
The third, and in our opinion most attractive, therapeutic option is treatment with KP (mimetics). This strategy is feasible because KPs are secreted. Moreover, systemic administration of KPs could access tumor cells throughout the body, unless they are in pharmacological sanctuaries. With our previous data showing that metastases to all sites were inhibited by KISS1 expression, the utilization of KPs would be predicted to block metastases to all tissues. Fortunately, the safety of KISS1/KP administration is already proven in humans! Subcutaneous administration of KP54 did not cause any reportable side effects; however, these treatments result in a robust release of gonadotropins 43, 44, as expected based upon the endocrine roles of these molecules. Further supporting a potential therapeutic window are data showing the extremely high KP levels present in plasma during pregnancy. The levels achieved during late pregnancy are higher than the doses currently considered. As above, however, manipulation of either KISS1 or KISS1R may affect normal physiological processes.
KISS1-based treatments would be theoretically straightforward as long as the metastatic tumor cells express the KISS1R. Unfortunately, data from our laboratory introduced another challenge to the KP therapy strategy — many of the tumor cells do not express KISS1R 15. We previously postulated that stromal cells, which differ in each tissue, might express KISS1R and that paracrine feedback to the tumor cell might be involved in the differential growth of tumor cells in various sites 14. Like many other labs, we found KISS1R to be expressed in selected stromal populations. However, a paracrine feedback loop has yet to be established, but is the subject of ongoing studies. If the paracrine hypothesis were to prove correct, it is possible that the relative genetic stability in stromal cells, compared with tumor cells, could be taken advantage of, since development of resistance to KISS1-based therapies would be less likely.
Although the biology and mechanism of action of KISS1-mediated metastasis suppression has been a challenge, this metastasis suppressor function offers great hope for future roles in predicting cancer outcome and, perhaps the effective treatment of cancer’s most deadly attribute.
Acknowledgments
Work from the Welch laboratory has been supported by grants from the National Foundation for Cancer Research and the U.S. Public Health Service (CA062168, CA087728 and CA134981). We are grateful to members of the lab for helpful comments and suggestions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bodenstine TM, Welch DR. Metastasis suppressors and the tumor microenvironment. Cancer Microenviron. 2008;1:1–11. doi: 10.1007/s12307-008-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaidya KS, Welch DR. Metastasis suppressors and their roles in breast carcinoma. J Mamm Gland Biol Neopl. 2007;12:175–90. doi: 10.1007/s10911-007-9049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welch DR, Chen P, Miele ME, et al. Microcell-mediated transfer of chromosome 6 into metastatic human C8161 melanoma cells suppresses metastasis but does not inhibit tumorigenicity. Oncogene. 1994;9:255–62. [PubMed] [Google Scholar]
- 4.Lee J-H, Miele ME, Hicks DJ, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–7. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 5.Lee J-H, Welch DR. Identification of highly expressed genes in metastasis-suppressed chromosome 6/human malignant melanoma hybrid cells using subtractive hybridization and differential display. Int J Cancer. 1997;71:1035–44. doi: 10.1002/(sici)1097-0215(19970611)71:6<1035::aid-ijc20>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Lee J-H, Miele ME, Hicks DJ, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene [erratum] J Natl Cancer Inst. 1997;89:1549. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 7.Lee J-H, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–7. [PubMed] [Google Scholar]
- 8.Miele ME, Jewett MD, Goldberg SF, et al. A human melanoma metastasis-suppressor locus maps to 6q16.3-q23. Int J Cancer. 2000;86:524–8. doi: 10.1002/(sici)1097-0215(20000515)86:4<524::aid-ijc13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Shirasaki F, Takata M, Hatta N, Takehara K. Loss of expression of the metastasis suppressor gene KiSS1 during melanoma progression and its association with LOH of chromosome 6q16.3-q23. Cancer Res. 2001;61:7422–5. [PubMed] [Google Scholar]
- 10.Goldberg SF, Miele ME, Hatta N, et al. Melanoma metastasis suppression by chromosome 6: Evidence for a pathway regulated by CRSP3 and TXNIP. Cancer Res. 2003;63:432–40. [PubMed] [Google Scholar]
- 11.Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–6. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 12.Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR12: A novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–75. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 13.Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–7. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 14.Nash KT, Welch DR. The KISS1 metastasis suppressor: mechanistic insights and clinical utility. Front Biosci. 2006;11:647–59. doi: 10.2741/1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nash KT, Phadke PA, Navenot J-M, et al. KISS1 metastasis suppressor secretion, multiple organ metastasis suppression, and maintenance of tumor dormancy. J Natl Cancer Inst. 2007;99:309–21. doi: 10.1093/jnci/djk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayasena CN, Dhillo WS. Kisspeptin offers a novel therapeutic target in reproduction. Curr Opin Invest Drugs. 2009;10:311–8. [PubMed] [Google Scholar]
- 17.Colledge WH. Kisspeptins and GnRH neuronal signalling. Trends Endocrinol Metab. 2009;20:115–21. doi: 10.1016/j.tem.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Tomita K, Oishi S, Ohno H, Peiper SC, Fujii N. Development of novel G-protein-coupled receptor 54 agonists with resistance to degradation by matrix metalloproteinase. J Med Chem. 2008;51:7645–9. doi: 10.1021/jm800930w. [DOI] [PubMed] [Google Scholar]
- 19.Tomita K, Oishi S, Cluzeau J, et al. SAR and QSAR studies on the n-terminally acylated pentapeptide agonists for GPR54. J Med Chem. 2007;50:3222–8. doi: 10.1021/jm070064l. [DOI] [PubMed] [Google Scholar]
- 20.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Tena-Sempere M. Timeline: the role of kisspeptins in reproductive biology. Nat Med. 2008;14:1196. doi: 10.1038/nm1108-1196. [DOI] [PubMed] [Google Scholar]
- 22.Gianetti E, Seminara S. Kisspeptin and KISS1R: a critical pathway in the reproductive system. Reproduction. 2008;136:295–301. doi: 10.1530/REP-08-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horikoshi Y, Matsumoto H, Takatsu Y, et al. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrin Metab. 2003;88:914–9. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- 24.Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, Berk M, Singh LS, et al. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis. 2005;22:369–76. doi: 10.1007/s10585-005-8186-4. [DOI] [PubMed] [Google Scholar]
- 26.Kostadima L, Pentheroudakis G, Pavlidis N. The missing kiss of life: Transcriptional activity of the metastasis suppressor gene KiSS1 in early breast cancer. Anticancer Res. 2007;27:2499–504. [PubMed] [Google Scholar]
- 27.Stark AM, Tongers K, Maass N, Mehdorn HM, Held-Feindt J. Reduced metastasis-suppressor gene mRNA-expression in breast cancer brain metastases. J Cancer Res Clin Oncol. 2004;131:191–8. doi: 10.1007/s00432-004-0629-9. [DOI] [PubMed] [Google Scholar]
- 28.Martin TA, Watkins G, Jiang WG. KiSS-1 expression in human breast cancer. Clin Exp Metastasis. 2005;22:503–11. doi: 10.1007/s10585-005-4180-0. [DOI] [PubMed] [Google Scholar]
- 29.Marot D, Bieche I, Aumas C, et al. High tumoral levels of Kiss1 and G-protein-coupled receptor 54 expression are correlated with poor prognosis of estrogen receptor-positive breast tumors. Endocr Relat Cancer. 2007;14:691–702. doi: 10.1677/ERC-07-0012. [DOI] [PubMed] [Google Scholar]
- 30.Prentice LM, Klausen C, Kalloger S, et al. Kisspeptin and GPR54 immunoreactivity in a cohort of 518 patients defines favourable prognosis and clear cell subtype in ovarian carcinoma. BMC Med. 2007;5:33. doi: 10.1186/1741-7015-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hata K, Dhar DK, Watanabe Y, Nakai H, Hoshiai H. Expression of metastin and a G-protein-coupled receptor (AXOR12) in epithelial ovarian cancer. Eur J Cancer. 2007;43:1452–9. doi: 10.1016/j.ejca.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Dhar DK, Naora H, Kubota H, et al. Downregulation of KiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. Int J Cancer. 2004;111:868–72. doi: 10.1002/ijc.20357. [DOI] [PubMed] [Google Scholar]
- 33.Guan-Zhen Y, Ying C, Can-Rong N, Guo-Dong W, Jian-Xin Q, Jie-Jun W. Reduced protein expression of metastasis-related genes (nm23, KISS1, KAI1 and p53) in lymph node and liver metastases of gastric cancer. Int J Exp Pathol. 2007;88:175–83. doi: 10.1111/j.1365-2613.2006.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masui T, Doi R, Mori T, et al. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Comm. 2004;315:85–92. doi: 10.1016/j.bbrc.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Nagai K, Doi R, Katagiri F, et al. Prognostic value of metastin expression in human pancreatic cancer. J Exp Clin Cancer Res. 2009;28:28–9. doi: 10.1186/1756-9966-28-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katagiri F, Nagai K, Kida A, et al. Clinical significance of plasma metastin level in pancreatic cancer patients. Oncol Rep. 2009;21:815–9. [PubMed] [Google Scholar]
- 37.Ikeguchi M, Yamaguchi K, Kaibara N. Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:1379–83. doi: 10.1158/1078-0432.ccr-1519-02. [DOI] [PubMed] [Google Scholar]
- 38.Ikeguchi M, Hirooka Y, Kaibara N. Quantitative reverse transcriptase polymerase chain reaction analysis for KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2003;129:531–5. doi: 10.1007/s00432-003-0469-z. [DOI] [PubMed] [Google Scholar]
- 39.Schmid K, Wang XW, Haitel A, et al. KiSS-1 overexpression as an independent prognostic marker in hepatocellular carcinoma: an immunohistochemical study. Virchows Arch. 2007;450:143–9. doi: 10.1007/s00428-006-0352-9. [DOI] [PubMed] [Google Scholar]
- 40.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–57. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith SC, Theodorescu D. Learning therapeutic lessons from metastasis suppressor proteins. Nat Rev Cancer. 2009;9:253–64. doi: 10.1038/nrc2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmieri D, Halverson DO, Ouatas T, et al. Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J Natl Cancer Inst. 2005;97:632–42. doi: 10.1093/jnci/dji111. [DOI] [PubMed] [Google Scholar]
- 43.Dhillo WS, Chaudhri OB, Thompson EL, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrin Metab. 2007;92:3958–66. doi: 10.1210/jc.2007-1116. [DOI] [PubMed] [Google Scholar]
- 44.Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrin Metab. 2005;90:6609–15. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]