Pulmonary hypertension (PH) secondary to left-sided heart disease (Group 2 PH) is a frequent complication of heart failure (HF) that worsens exercise capacity, risk for hospitalization, and survival independent of left ventricular ejection fraction (LVEF) or stage of HF. Increased pulmonary artery pressure (PAP) in HF patients often represents a combination of increased left-sided filling pressures (“passive” component) and elevated pulmonary vascular resistance (PVR) due to functional and structural abnormalities of the pulmonary vascular bed (“reactive” component). The latter may be reversible with standard HF treatment in the earlier stages when remodeling of the pulmonary vasculature has not set in and abnormalities in pulmonary arterial tone are the major driver for elevated PVR. However, chronic exposure to elevated pulmonary capillary wedge pressure (PCWP) may lead to permanent changes in the pulmonary arterial bed (irreversible or “fixed” PH).1 Considering that a number of drug classes have successfully modified the natural history of pulmonary arterial hypertension (Group 1 PH) and demonstrated that intervention is possible even after pulmonary vascular remodeling has occurred,2 Group 2 PH is a natural target for screening and potential intervention in patients with HF.
In the second part of this two-part review, we discuss the prognostic impact of PH in HF, the contemporary diagnostic and evaluation approaches, the current evidence from clinical studies in Group 2 PH, the challenges of appropriate patient selection in clinical trials, and potential ways to overcome these challenges in trial designs.
Prognostic Significance of Pulmonary Hypertension in Heart Failure
Echocardiographic Studies
Studies using either right heart catheterization (RHC) or echocardiography for determination of PAP have consistently shown that PH considerably worsens prognosis in HF. Abramson et al. first reported that tricuspid regurgitation jet velocity >2.5 m/s was associated with 3.4 times higher mortality in 108 patients with dilated cardiomyopathy followed for up to 28 months3; hospitalization rate for HF was also three times higher in these patients.3 In more recent studies, estimated right ventricular systolic pressure (RVSP) has been used to evaluate the presence of PH. Although variable RVSP cut-off points have been used to define PH in these studies,4–9 higher RVSP has been consistently associated with higher mortality and hospitalization rates. Of note, in a study of cardiac resynchronization therapy (CRT) recipients, higher RVSP at baseline was associated with worse survival, but patients with reductions in RVSP on follow up had better outcomes.4 The importance of elevated PAP post-CRT has been also reported by other groups.10, 11
Invasive Studies
Studies with RHC in chronic HF have mostly included patients with severe systolic dysfunction and advanced HF.12–14 Among 377 patients referred for transplant evaluation, 51.3% of those with mean PAP (mPAP) >20mmHg died or were transplanted urgently compared to 13.5% of those with mPAP≤20mmHg.12 In that study, right ventricular dysfunction further increased risk in patients with PH.12 In a landmark study on 1134 patients with newly diagnosed cardiomyopathy, mortality sharply increased when PVR exceeded 3 Wood units (WU).13 In a study with serial RHC data, baseline PH predicted mortality and decompensation of HF, and worsening mPAP over time further increased risk.14 In a large series of HF patients without any specific LVEF inclusion criteria, mortality was two-fold higher in patients with PH vs. those without.15 When PH (mPAP ≥ 25mmHg) was further classified into passive (PVR ≤ 2.5 WU) vs. reactive (PVR >2.5 WU), reactive PH was associated with more pronounced risk.15 Regardless of method of PH determination (echocardiography or RHC), right ventricular dysfunction worsens outcomes further.12, 16
In contrast to studies in chronic HF, results from RHC data in acute HF are conflicting.17, 18. Using data from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, Khush et al. reported that reactive PH (mPAP ≥ 25mmHg, PCWP >15mmHg, and PVR ≥ 3 WU), present in 47% of patients at enrollment, was not associated with worse 6-month outcomes.17 In contrast, in the Vasodilation in the Management of Acute Congestive (VMAC) Heart Failure trial,18 a reactive PH profile (using the same definition as in ESCAPE) was associated with higher 6-month mortality (48.3%) compared to passive PH (21.8%) or no PH (8.6%) (Figure 1). However, in VMAC, the PH profile was determined using the post-treatment values, potentially reflecting a profile closer to steady state conditions. Of note, in that study, post-treatment values of hemodynamic parameters classified 50% of patients to a different PH category compared to pre-treatment values,18 highlighting the challenges of PH profile determination in HF patients during the decompensated phase.
Figure 1.
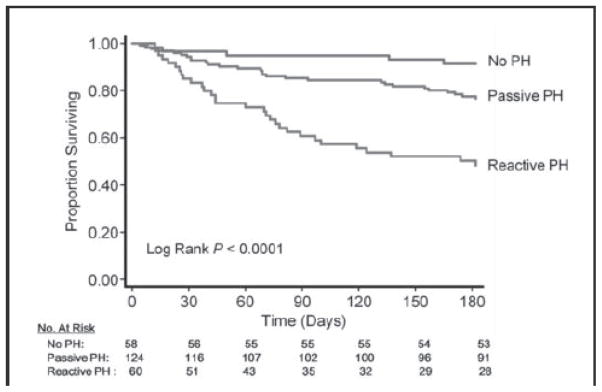
Six-month survival among patients hospitalized with acute heart failure according to their post-treatment pulmonary hypertension profile. Reproduced with permission from Aronson et al., Circ Heart Fail. 2011;4:644–650.
Table 1 summarizes the studies on prognostic significance of Group 2 PH.
Table 1.
Prognostic Significance of Pulmonary Hypertension in Patients with Heart Failure
| Study | N | Population | Method | Follow up | Threshold | Outcome | Effect |
|---|---|---|---|---|---|---|---|
| Heart Failure with Reduced Ejection Fraction | |||||||
| Abramson 19923 | 108 | Dilated CMP; mean LVEF 17.2% | Echo | 28 months (maximum) | Tricuspid regurgitation jet velocity >2.5m/s | Mortality HF admission Combined |
57% vs. 17%; RR 3.4 75% vs. 26%; RR 2.9 89% vs. 32%; RR 2.8 |
| Ghio 200112 | 377 | Tx evaluation; LVEF 21.8±6.7% | RHC | 17±9 months | Continuous mPAP | Mortality or urgent Tx | aHR 1.10 per 5 mmHg (95% CI 1.00 to 1.21) |
| Cappola 200213 | 1134 | New-onset CMP; LVEF: N/A | RHC | 4.4 years (average) | PVR >3.0 WU (vs. PVR <2.5 WU) | Mortality | aHR 1.86 to 2.04 (depending on PVR level) |
| Grigioni 200614 | 196 | NYHA class III–IV; LVEF 27%±9% | RHC | 24±20 months | mPAP >25 mmHg | Mortality plus acute HF | Adjusted RR 2.30 (95%CI 1.42 to 3.73) |
| Shalaby 20084 | 270 | CRT recipients; LVEF 22.6%±9.7% | Echo | 19.4±9.0 months | RVSP ≥ 45 mmHg (vs. RVSP <30 mmHg) | Mortality HF admission |
34% vs. 12%; aHR 2.62 (95% CI 1.07 to 6.41) 40% vs. 9%; aHR 6.35 (95% CI 2.55 to 15.8) |
| Szwejkowski 20128 | 1612 | LVSD, loop diuretics, and measured RVSP | Echo | 2.8±2.5 years | RVSP >52mmHg (vs. <33 mmHg) | Mortality | 60.7% vs. 47.4%; aHR 1.50 (95% CI 1.19 to 1.90) |
| Heart Failure with Preserved Ejection Fraction | |||||||
| Lam 20096 | 244 | In- and out-patients; LVEF≥ 50% | Echo | 2.4±1.2 years | Continuous RVSP | Mortality | aHR 1.20 per 10 mmHg |
| Heart Failure with Reduced or Preserved Ejection Fraction | |||||||
| Damy 2010 7 | 413 | 270 with LVEF≤ 45% 143 with LVEF >45% and measurable TR |
Echo | 66 (56–74) months* | Q4 vs. Q1–Q3 RVSP | Mortality | NT-proBNP-adjusted HR 1.46 (95% CI: 1.07 to 1.98) |
| Tatebe 201215 | 676 | HF patients NYHA II–IV referred for RHC | RHC | 2.6 years (median) | mPAP ≥ 25mmHg PVR ≤ 2.5 WU PVR >2.5 WU |
Mortality | 29.1% (PH) vs. 15.3% (no PH) 24.0% (passive PH) 37.9% (reactive PH) |
| Bursi 20129 | 1049 | Community in- and out-patients with HF | Echo | 2.7±1.9 years | Tertiles RVSP (<41, 41–54, >54 mmHg) | Mortality | T2 vs. T1: aHR 1.45 (95% CI 1.13 to 1.85) T3 vs. T1: aHR 2.07 (95% CI 1.62 to 2.64) |
| Acute Heart Failure | |||||||
| Kjaergaard et al. 20075 | 388 | Admitted for HF; LVEF 33 (23–50) % | Echo | 2.8 years | Continuous RVSP | Mortality | HR per 5mmHg 1.09 (95% CI 1.04 to 1.14) |
| Khush et al. 200917 | 171 | Clinical trial; SBP ≤ 125 mmHg; LVEF ≤ 30% | RHC | 6 months | mPAP ≥ 25mmHg; PVR ≥ 3 WU (Pre-treatment values) | Mortality Readmission |
No difference No difference |
| Aronson et al. 201118 | 242 | Clinical trial; LVEF 25%±13% | RHC | 6 months | mPAP >25 mmHg PVR ≤ 3 WU (“passive”) PVR >3 WU (“reactive”) (Post-treatment values) |
Mortality | Reactive PH: 48.3%; Passive PH: 21.8%; No PH: 8.6% Passive vs. No PH: aHR 1.7 (95% CI: 0.6 to 4.5) Reactive vs. No PH: aHR: 4.8 (95% CI: 2.1–17.5) |
aHR: adjusted hazard ratio; CMP: cardiomyopathy; CRT: cardiac resynchronization therapy; HF: heart failure; HR: hazard ratio; LVEF: left ventricular ejection fraction; LVSD: left ventricular systolic dysfunction; mPAP: mean pulmonary artery pressure; NYHA: New York Heart Association; PCWP; PVR: pulmonary vascular resistance; RHC: right heart catheterization; RR: risk ratio; RVSP: right ventricular systolic pressure; SBP: systolic blood pressure; TR: tricuspid regurgitation; Tx: transplantation; WU: Wood units
Follow-up for the entire cohort of 2100 patients (measured and unmeasured RVSP).
Insights from Continuous Ambulatory Hemodynamic Monitoring
In patients with HF, even mild increases in chronic ambulatory PAP, monitored through wireless implantable devices, are associated with higher rates of hospitalization for HF regardless of LVEF.19–21 In the Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS-HF) Study, the likelihood of an HF event increased progressively with higher estimated diastolic PAP (an estimate of LV filling pressure).20 The estimated average diastolic PAP over 6 months among patients who presented with acute HF was 31±8 mmHg vs. 26±6 mmHg among those without events. However, the corresponding estimated RVSP was 55±15 and 45±13 mmHg, respectively, potentially indicating the presence of a reactive pulmonary vascular component among patients with events.20 In the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial, a 1-mmHg lower daily average mPAP in the device-guided treatment group compared to the control group over 6 months was accompanied by fewer hospitalizations for HF (32% vs. 44%).21
The Role of Echocardiography
Right heart catheterization is the gold standard for the definitive diagnosis of PH. However, RHC is an invasive procedure and therefore would be better reserved for patients with advanced (Stage D) HF, where decisions on advanced therapies are contingent upon the absence of fixed PH. From a clinical trial design perspective, RHC is appropriate for the detailed characterization of hemodynamic response in phase I/IIa trials with novel vasoactive agents. However, noninvasive alternatives for PH screening and evaluation of response to investigational agents are needed for Stage C HF patients in larger, phase IIb/III clinical trials. Echocardiography is being increasingly used for these purposes. Newer echocardiographic parameters have become available to supplement the traditional RVSP estimates, including estimates of left ventricular (LV) filling pressures and PVR to better phenotype these patients.
Feasibility of RVSP Determination
A comprehensive echocardiographic evaluation of the HF patient should include estimation of RVSP based on the tricuspid regurgitation jet velocity. Potentially, an estimate of right atrial pressure using the inferior vena cava diameter and its respiratory fluctuation can be added to the transtricuspid gradient to better approximate RVSP. In the absence of pulmonary stenosis, RVSP is an adequate approximation to systolic PAP (sPAP). The reported feasibility of RVSP determination in chronic HF patients is highly dependent on the setting. In prospective cohorts focusing on right-sided hemodynamics, feasibility ranges from 80%–90% in community HF6, 9, 22 to almost 100% in advanced HF.23, 24 Feasibility is much lower when RVSP determination is not mandated by protocol or is retrospectively assessed.5, 7, 8, 25 Recently, Nagueh et al. reported that RVSP determination is feasible in 80% of patients in the acute HF setting.26
Validity of Right Ventricular Systolic Pressure for Assessment of Pulmonary Hypertension
Several studies have investigated the validity of echocardiographic RVSP estimates in patients with HF using RHC values as the gold standard. As expected, most data come from patients with advanced systolic HF. In 70 patients with systolic HF, a concordance correlation coefficient of 0.88 between RHC and RVSP values was reported, with ±20 mmHg 95% limits of agreement and no bias.27 Narrower limits of agreement (<10mmHg) and clinically relevant correlation between RHC and RVSP (r=0.82–0.97) have been consistently reported in Stage D HF populations.23, 24, 28, 29 Nagueh et al. have reported that echocardiographic RVSP determinations correlate well (r=0.83) with invasive estimates in acute HF, with ±15mmHg limits of agreement.26 Importantly, echocardiography identified patients with invasive sPAP >35 mmHg with 94% sensitivity and 90% sensitivity.26 A recent analysis from the ESCAPE trial suggested that the accuracy of echocardiographic RVSP estimates in systolic HF might be compromised by right ventricular systolic dysfunction.30 However, it is important to note that echocardiography in ESCAPE was not protocol driven and the time differential between RHC and echocardiographic determinations was widely variable.
Although the need for noninvasive alternatives to invasive estimation of PH is higher in the community setting, most validation data for echocardiographic RVSP estimates come from referral populations, whereas data on community HF are lacking. However, the clinical validity of RVSP estimates in the community setting is supported by strong prognostic information.6,9,8 Therefore, it may be acceptable to select Stage C HF patients for clinical trials on Group 2 PH on the basis of echocardiographic evidence of PH, considering the projected risk associated with elevated echocardiographic RVSP.
Assessment of Pulmonary Vascular Resistance with Echocardiography
Direct transportation of the RHC formula (difference between mPAP and PCWP divided by the cardiac output) for echocardiographic estimation of PVR has been proposed,31,32 but application in practice has been limited, mainly because of the uncertainty introduced by PCWP and cardiac output estimation. Similarly, approaches based on Doppler-derived intervals have been proposed33 but adoption has been limited. Abbas et al. have introduced an estimate of PVR based on the ratio of tricuspid regurgitation jet velocity (TRV) to right ventricular outflow tract time-velocity integral (TVI) (Figure 2), with good correlation to invasive estimates (r=0.93) and ±0.8 WU 95% limits of agreement.34 A TRV/TVI ratio of ≥ 0.175 had 77% sensitivity and 81% specificity to determine PVR >2 WU. This approach and variations thereof have been validated in liver transplant candidates,35 in patients with pulmonary arterial hypertension,36, 37,38 in a mixed non-HF PH population 39, and in congenital heart disease.40 Encouraging results have also been obtained in pediatric41 and postoperative 42 populations. A common theme among these studies is that the TRV/TVI ratio has a high sensitivity and negative predictive value to detect elevated PVR (>1.5–2.0 WU), but actual correlation with invasive PVR worsens as PVR increases. Thus, noninvasive PVR estimates are best reserved as a screening tool to exclude high PVR. More recently, Dahiya et al. reported that correction of noninvasive PVR for LV filling pressures using the E/e’ ratio improved correlation with invasive PVR in a large non-HF PH population.38 In this study, corrected noninvasive PVR was a reliable surrogate of invasive PVR over 12 months of follow up after initiation of pulmonary vasoactive treatment.38 It is important to note, however, that noninvasive methods for PVR assessment have not been validated in HF populations.
Figure 2.
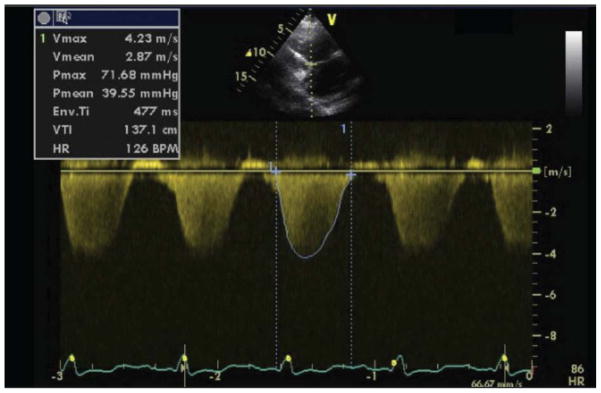
Echocardiographic estimation of pulmonary vascular resistance using the tricuspid regurgitation jet velocity (TRV) and the corresponding time-velocity integral (TVI). In this patient, despite a gradient of 72mmHg through the tricuspid valve, the estimated pulmonary vascular resistance (PVR) would be PVR=TRV(m/s)/TVI(cm)×10+0.16=(4.23/137.1)×10+0.16=0.47 Wood Units, suggesting the absence of a significant reactive component.
Treatment of Pulmonary Hypertension in Heart Failure
The epidemiological data suggest that PH could be a natural therapeutic target in patients with HF. However, no adequately powered trials of PH-specific treatment have demonstrated to date that decreasing PAP or PVR improves morbidity and mortality in HF patients. Despite promising results in acute hemodynamic studies,43–54 the experience with prostacyclin analogues and endothelin antagonists in outcome-driven trials in chronic and acute HF has been invariably neutral or negative to date.55–64 These results are in contrast to the favorable effects of these pulmonary vasoactive agents in populations with Group 1 PH.65 However, trials with prostacyclin analogues and endothelin antagonists in HF did not target patients with evidence of concomitant PH, but rather enrolled relatively unselected HF populations on the premise that a more comprehensive neurohormonal blockade would improve outcomes for all HF patients.66 Only recently the focus has shifted to HF patients with concomitant PH, investigating predominantly the effects of phosphodiesterase type 5 (PDE5) inhibitors67–70 (Table 2). Besides demonstrating favorable effects on physiologic endpoints in patients with systolic HF and PH,67, 69 long-term sildenafil treatment improved hemodynamics in a single-center trial of patients with preserved ejection fraction (HFpEF) and PH.69 It is important to note however, that the degree of right ventricular dysfunction and right heart congestion in this study was higher than that seen in typical HFpEF populations.71
Table 2.
Long-term, Placebo-Controlled Studies in Patients with Heart Failure and Pulmonary Hypertension
| Study | Drug | Duration | Population | N | Results |
|---|---|---|---|---|---|
| Lewis et al. 200767 | Sildenafil (25 to 75 mg t.i.d.) | 12 weeks | NYHA class II–IV, LVEF <40%, mPAP >25 mmHg | 34 | Sildenafil increased peak VO2 and cardiac output and reduced PVR with exercise; no effect on PCWP, blood pressure, or heart rate; improved 6-MWT distance and reduced HF admissions; higher incidence of headache |
| Kaluski et al. 200868 | Bosentan (8–125 mg b.i.d.) | 20 weeks | NYHA class IIIB-IV, LVEF <35%, RVSP ≥40 mmHg (echo), supine SBP ≥100 mmHg | 94 | No difference from baseline to week 20 in RVSP (0.1±11.5 mm Hg, p = 0.97) or other echocardiographic parameter; more SAEs in the bosentan arm |
| Guazzi et al. 201169 | Sildenafil (50 mg t.i.d.) | 1 year | LVEF ≥50%, RVSP ≥40 mmHg (echo) | 44 | Sildenafil reduced mean PAP by 42.0±13.0%, improved right ventricular function, and reduced right atrial pressure by 54.0±7.2% and PCWP by 15.7±3.1% |
| Guazzi et al. 201270 | Sildenafil (50 mg t.i.d.) | 1 year | LVEF <45%, mean PAP 25–35mmHg | 32 | Sildenafil increased peak VO2 and exercise ventilation efficiency, and decreased PCWP, mean PAP, and pulmonary vascular resistance |
LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PAP: pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; RVSP: right ventricular systolic pressure; SAE: serious adverse event; SBP: systolic blood pressure
Following the paradigm of other pulmonary vasoactive agents, the long-term effects of PDE5 inhibitors were initially tested in unselected HF populations,72–74 on the basis of early encouraging experience from short-term use in patients with HF and erectile dysfunction75–77 and the favorable effects of these agents on endothelial function.78–80 However, despite promising effects on exercise capacity69, 70, 72, 73, 81 and pulmonary hemodynamics,69, 70, 72, 73, 82 these phase II trials with PDE5 inhibitors were not powered to detect effects on clinical outcomes, both for unselected and selected HF populations. In fact, no outcome-driven trials have been conducted with PDE5 inhibitors in HF to date. The NIH-funded (1U01HL105562-01A1) Phosphodiesterase Type 5 Inhibition with Tadalafil Changes Outcomes in Heart Failure (PITCH-HF) trial will be the first clinical trial to (1) investigate the effect of pulmonary vasoactive treatment on mortality and hospitalizations in patients with HF and PH and (2) the effects of PDE5 inhibition on clinical outcomes in HF.
Thus, while appropriately powered trials are underway, agents that enhance the cyclic guanosine monophosphate (cGMP) signaling appear to hold promise in patients with HF and PH, as they may not share the profile seen with more pulmonary arterial selective drug classes such as prostacyclin analogues or endothelin receptor antagonists. The pulmonary selectivity of the latter, which omit parallel unloading of the LV while pulmonary venous flow is enhanced, could potentially underlie the failure of these agents in HF. High PVR may be a protective adaptation to LV failure,83 as selective pulmonary arterial vasodilation might worsen left heart congestion and trigger pulmonary edema. In contrast, agents that also unload the LV, such as nitroprusside, safely improve PAP and PVR without acute increase in left atrial pressures.84 Therefore, therapeutic interventions with “balanced” pulmonary arterial and systemic vasodilator effects could be more promising. Drugs with such desirable hemodynamic profiles include cGMP-enhancing agents such as nitrates,85 though this class is limited by tolerance and resultant oxidative stress induction86–88; PDE5 inhibitors89; and soluble guanylate cyclase (sGC) stimulators and activators hold promise in this respect.90
Pulmonary Hypertension in Heart Transplant Candidates
Fixed PH increases mortality both early and late after heart transplantation (HT), because the right ventricle may fail when a normal donor heart faces significantly elevated PVR in the post-HT period.66 Mortality increases continuously with increasing PVR and no threshold confidently precludes right ventricular failure, supporting the view that PVR should be considered a relative rather than an absolute contraindication to HT.91–93 However, a resting PVR >5 WU indicates that the patient may not be a good candidate for HT or, alternatively, that they should be offered heterotopic HT or heart-lung transplantation.94 On the other hand, if PVR can be reduced to <2.5 WU without hypotension, post-HT outcomes are comparable to patients without PH.95, 96 In a series of 410 HT recipients, reversible PH did not affect negatively short- or long-term (5-year) survival97; however, residual post-HT PH was associated with decreased long-term survival. In another series of 217 patients who received HT, 10-year survival among the 40 patients with reversible PH was comparable to those without PH (61% vs. 63%).98
Left ventricular assist device (LVAD) implantation improves pulmonary hemodynamics in patients not responding to vasodilatory treatment, suggesting that LVAD may be a strategy for HT candidates with fixed PH. 99 In several studies, PVR was significantly reduced and patients became eligible for HT with good post-HT outcomes.100–105 Both pulsatile104 and continuous-flow103 LVADs improve pulmonary hemodynamics and candidacy for HT. Long-term survival post-HT in these patients was similar to that of HT recipients without PH who either received105 or did not receive LVAD.102 However, there are no data directly comparing patients with PH who received vs. those who did not receive LVAD.106 Improvement in hemodynamics has been reported early after LVAD implantation even in severe PH, and this improvement lasts with longer support.104 A recent study reported that the timeframe in which significant reductions in mean PAP, PCWP, and PVR of patients with fixed PH occur is within 6 months after LVAD placement with no additional benefit after that period, giving thus reasonable time for HT candidacy decisions.107
Favorable outcomes were observed after HT in a small series of patients with initially unresponsive PH who regained “reversibility” of PH following a 12-week treatment with oral sildenafil in a small prospective uncontrolled trial.108 In a small retrospective study, HT candidates with severe PH on sildenafil who continued to receive sildenafil after HT had significant reduction in PVR/TPG, successful HT, and comparable post-transplant survival with those without PH.109 In another retrospective study of patients with severe Group 2 PH, pre- and post-HT survival was better in those receiving sildenafil.110 These preliminary findings suggest that pulmonary vasoactive treatment could precondition previously disqualified HT candidates for safe transplantation.
Pulmonary Pressures from Implanted Devices as a Therapeutic Target
Recent trials with implanted devices for hemodynamic monitoring demonstrated that goal-directed therapies based upon real-time diastolic PAP assessments, as a surrogate of left-sided filling pressures, might reduce HF hospitalizations.20, 21 In the COMPASS-HF study,20 the risk for HF events was 1.10 per 6 months when daily median estimated diastolic PAP was ≥ 25mmHg at baseline and remained chronically ≥ 25mmHg vs. 0.47 when pressures declined to <25mmHg for more than half of the days. Patients with low baseline daily median estimated diastolic PAP (<25 mm Hg) who increased only after initiation of the ambulatory monitoring to ≥ 25 mm Hg for the majority of their days, had a high HF event rate during 6 months of 1.10, compared with a rate of only 0.23 in those who remained low at <25 mm Hg.20
However, the 21% reduction in HF events by the implanted device remained nonsignificant.111 Although the CHAMPION trial was not restricted to HF patients with PH,21 maintenance of less elevated filling pressures could also represent an appealing target for PAP-active drugs such as the cGMP-enhancing PDE5 inhibitors or sGC stimulators.
Ongoing Clinical Trials
Currently, a number of clinical trials in various planning and conduct stages are investigating the effects of PDE5 inhibitors and sGC activators in HF patients with our without PH. The recently completed, NIH-funded Evaluating the Effectiveness of Sildenafil at Improving Health Outcomes and Exercise Ability in People With Diastolic Heart Failure (RELAX) trial is a double-blind, placebo controlled phase III trial testing the hypothesis that the PDE% inhibitor sildenafil will improve exercise capacity after 24 weeks of therapy in patients with HFpEF.112 This trial will also assess the effects of sildenafil on a host of pathophysiological parameters postulated to impact clinical status and exercise performance in HFpEF. Results are awaited in Spring 2013. The Study to Test the Effects of Riociguat in Patients With Pulmonary Hypertension Associated With Left Ventricular Systolic Dysfunction (LEPHT) trial is a phase IIb, double-blind placebo-controlled trial enrolling patients with LVEF ≤ 40% and mPAP ≥ 25 mmHg at rest.113 Patients on optimized HF therapy received placebo or the sGC activator riociguat for 16 weeks with mPAP as the primary efficacy endpoint; secondary endpoints include LVEF, exercise capacity, quality of life, and other hemodynamic and echocardiographic measurements. Follow-up was completed in August 2012 and results were announced during the 2012 American Heart Association Scientific Sessions. Riociguat was well tolerated but no significant reduction in mPAP was observed with any of the three doses tested. However, (1) cardiac index increased without changes in heart rate or systemic blood pressure, (2) systemic and pulmonary vascular resistance decreased in parallel, and (3) quality of life improved in patients receiving 2 mg tid riociguat.114 As previously discussed, the phase III PITCH-HF will be the first clinical trial to investigate the effect of PDE5 inhibition on hard outcomes in patients with HF. In order to accomplish this goal, 2102 patients with HF and reduced LVEF (<40%), NYHA class II-IV symptoms, and either mPAP ≥ 25mmHg at rest or ≥ 30mmHg with exercise, or RVSP ≥ 40 mmHg at rest, will be assigned to receive either the PDE5 inhibitor tadalafil or placebo for an average of 2.5 years. The trial is currently in the planning stages.
Future Steps and Study Designs
There are two distinct uses of elevated PAP as a therapeutic target in HF. Acute fluctuations in PAP secondary to increased LV filling pressures and elevated PCWP are a signal of impending acute HF.19 Noninvasive monitoring of these fluctuations can potentially serve as a treatment goal to fine-tune HF therapy and eventually improve outcomes.21 Chronic PAP elevation despite optimal therapy, on the other hand, is more likely to signify a permanent remodeling component in the pulmonary vasculature and a plausible target for long-term treatment in patients with HF. Oral agents with combined pulmonary and systemic vasodilatory activity appear to be promising to this end and their therapeutic role is being evaluated in ongoing clinical trials.93, 113 However, two related challenges need to be addressed in the planning stage of such trials: (1) how to identify patients with the most promising benefit vs. risk profile; and (2) which surrogate markers to use, either as selection criteria to optimize the benefit-risk ratio or as phase II endpoints.
To address specificity in patient selection, careful characterization of responder profiles would be required as part of dose-finding phase II studies. Due to the complexity of the various hemodynamic profiles in HF with PH, this would require in-depth mechanistic phase II trials, including RHC, echocardiography, and cardiopulmonary exercise testing. These profiles could then identify the appropriate population for long-term, outcome-driven trials to establish clinical benefit. Subsequently, simplified phase III designs would be desirable to ensure feasibility and better representation of the HF population at large.
The issue of surrogate markers and endpoints poses a challenge for HF clinical trials in general115 and in HF with PH in specific. Hemodynamic (e.g. echocardiographic RVSP) and circulating (e.g. B-type natriuretic peptide) markers in HF are characterized by short-term dynamic changes under the influence of multiple confounders. Current evidence suggests that functional surrogates (improvement in B-type natriuretic peptide or exercise capacity) do not always match the results of outcome-driven trials.116 On the other hand, although improvement in structural characteristics of the failing heart appears to match long-term outcomes better,117 it is difficult to implement this approach in the case of HF with PH. Effects on LV function may not be apparent in the traditional remodeling time frame (6 to 12 months) because the target is primarily the pulmonary circulation; and the right ventricle, the primary chamber of interest in PH, is notoriously difficult to quantify echocardiographically outside the research arena. Hence, the optimal surrogates in this population remain an open question. In this direction, ancillary studies with novel biomarkers (e.g. markers of cGMP pathway activity) as part of the ongoing phase III trials might help bridge this gap.
Conclusion
Group 2 PH portends worse prognosis and is a plausible therapeutic target in HF. Evaluation of right-sided hemodynamics with RHC is the gold standard for stage D patients and for initial detailed characterization of responses to novel agents in phase I/IIa trials. Echocardiography is a reasonable alternative for screening, enrollment, and response monitoring among the large stage C population for phase IIb/III trials. The initial experience with selective pulmonary vasodilating agents in unselected HF populations has been disappointing. However, shifting the focus to cGMP-enhancing agents, which provide a more balanced vasodilation, and selecting patients on the basis of elevated PAP, has yielded promising results in phase II studies. Phase III clinical trials currently underway will answer the fundamental question: does reduction of PVR and/or PAP on a long-term basis improve outcomes in HF with PH?
Footnotes
Disclosures
None.
References
- 1.Delgado JF, Conde E, Sanchez V, Lopez-Rios F, Gomez-Sanchez MA, Escribano P, Sotelo T, Gomez de la Camara A, Cortina J, de la Calzada CS. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. European journal of heart failure. 2005;7:1011–1016. doi: 10.1016/j.ejheart.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Gomberg-Maitland M, Dufton C, Oudiz R, Benza R. Compelling evidence of long-term outcomes in pulmonary arterial hypertension? A clinical perspective. J Am Coll Cardiol. 2011;57:1053–1061. doi: 10.1016/j.jacc.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Abramson SV, Burke JF, Kelly JJ, Jr, Kitchen JG, 3rd, Dougherty MJ, Yih DF, McGeehin FC, 3rd, Shuck JW, Phiambolis TP. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Ann Intern Med. 1992;116:888–895. doi: 10.7326/0003-4819-116-11-888. [DOI] [PubMed] [Google Scholar]
- 4.Shalaby A, Voigt A, El-Saed A, Saba S. Usefulness of pulmonary artery pressure by echocardiography to predict outcome in patients receiving cardiac resynchronization therapy heart failure. Am J Cardiol. 2008;101:238–241. doi: 10.1016/j.amjcard.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 5.Kjaergaard J, Akkan D, Iversen KK, Kjoller E, Køber L, Torp-Pedersen C, Hassager C. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol. 2007;99:1146–1150. doi: 10.1016/j.amjcard.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 6.Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damy T, Goode KM, Kallvikbacka-Bennett A, Lewinter C, Hobkirk J, Nikitin NP, Dubois-Rande JL, Hittinger L, Clark AL, Cleland JG. Determinants and prognostic value of pulmonary arterial pressure in patients with chronic heart failure. Eur Heart J. 2010;31:2280–2290. doi: 10.1093/eurheartj/ehq245. [DOI] [PubMed] [Google Scholar]
- 8.Szwejkowski BR, Elder DHJ, Shearer F, Jack D, Choy AMJ, Pringle SD, Struthers AD, George J, Lang CC. Pulmonary hypertension predicts all-cause mortality in patients with heart failure: a retrospective cohort study. European journal of heart failure. 2012;14:162–167. doi: 10.1093/eurjhf/hfr159. [DOI] [PubMed] [Google Scholar]
- 9.Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CSP, Weston SA, Jiang R, Roger VL. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern J, Heist EK, Murray L, Alabiad C, Chung J, Picard MH, Semigran MJ, Ruskin JN, Singh JP. Elevated estimated pulmonary artery systolic pressure is associated with an adverse clinical outcome in patients receiving cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2007;30:603–607. doi: 10.1111/j.1540-8159.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Han Y, Zang H, Yu H, Wang S, Wang Z, Jing Q. Prognostic effects of pulmonary hypertension in patients undergoing cardiac resynchronization therapy. J Thorac Dis. 2010;2:71–75. [PMC free article] [PubMed] [Google Scholar]
- 12.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 13.Cappola TP, Felker GM, Kao WHL, Hare JM, Baughman KL, Kasper EK. Pulmonary hypertension and risk of death in cardiomyopathy: patients with myocarditis are at higher risk. Circulation. 2002;105:1663–1668. doi: 10.1161/01.cir.0000013771.30198.82. [DOI] [PubMed] [Google Scholar]
- 14.Grigioni F, Potena L, Galiè N, Fallani F, Bigliardi M, Coccolo F, Magnani G, Manes A, Barbieri A, Fucili A, Magelli C, Branzi A. Prognostic implications of serial assessments of pulmonary hypertension in severe chronic heart failure. J Heart Lung Transplant. 2006;25:1241–1246. doi: 10.1016/j.healun.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Tatebe S, Fukumoto Y, Sugimura K, Miyamichi-Yamamoto S, Aoki T, Miura Y, Nochioka K, Satoh K, Shimokawa H. Clinical significance of reactive post-capillary pulmonary hypertension in patients with left heart disease. Circ J. 2012;76:1235–1244. doi: 10.1253/circj.cj-11-1288. [DOI] [PubMed] [Google Scholar]
- 16.Adhyapak SM. Effect of right ventricular function and pulmonary pressures on heart failure prognosis. Prev Cardiol. 2010;13:72–77. doi: 10.1111/j.1751-7141.2009.00053.x. [DOI] [PubMed] [Google Scholar]
- 17.Khush KK, Tasissa G, Butler J, McGlothlin D, De Marco T ESCAPE Investigators. Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) database. Am Heart J. 2009;157:1026–1034. doi: 10.1016/j.ahj.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Aronson D, Eitan A, Dragu R, Burger AJ. Relationship between reactive pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circ Heart Fail. 2011;4:644–650. doi: 10.1161/CIRCHEARTFAILURE.110.960864. [DOI] [PubMed] [Google Scholar]
- 19.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson LW, Zile M, Bennett TD, Kueffer FJ, Jessup ML, Adamson P, Abraham WT, Manda V, Bourge RC. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail. 2010;3:580–587. doi: 10.1161/CIRCHEARTFAILURE.109.923300. [DOI] [PubMed] [Google Scholar]
- 21.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 22.Ristow B, Ali S, Ren X, Whooley MA, Schiller NB. Elevated pulmonary artery pressure by Doppler echocardiography predicts hospitalization for heart failure and mortality in ambulatory stable coronary artery disease: the Heart and Soul Study. J Am Coll Cardiol. 2007;49:43–49. doi: 10.1016/j.jacc.2006.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temporelli PL, Scapellato F, Eleuteri E, Imparato A, Giannuzzi P. Doppler echocardiography in advanced systolic heart failure: a noninvasive alternative to Swan-Ganz catheter. Circ Heart Fail. 2010;3:387–394. doi: 10.1161/CIRCHEARTFAILURE.108.809590. [DOI] [PubMed] [Google Scholar]
- 24.Kuppahally SS, Michaels AD, Tandar A, Gilbert EM, Litwin SE, Bader FM. Can echocardiographic evaluation of cardiopulmonary hemodynamics decrease right heart catheterizations in end-stage heart failure patients awaiting transplantation? Am J Cardiol. 2010;106:1657–1662. doi: 10.1016/j.amjcard.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Oh JK, Pellikka PA, Panza JA, Biernat J, Attisano T, Manahan BG, Wiste HJ, Lin G, Lee K, Miller FA, Stevens S, Sopko G, She L, Velazquez EJ STICH Trial Investigators. Core lab analysis of baseline echocardiographic studies in the STICH trial and recommendation for use of echocardiography in future clinical trials. J Am Soc Echocardiogr. 2012;25:327–336. doi: 10.1016/j.echo.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagueh SF, Bhatt R, Vivo RP, Krim SR, Sarvari SI, Russell K, Edvardsen T, Smiseth OA, Estep JD. Echocardiographic evaluation of hemodynamics in patients with decompensated systolic heart failure. Circ Cardiovasc Imaging. 2011;4:220–227. doi: 10.1161/CIRCIMAGING.111.963496. [DOI] [PubMed] [Google Scholar]
- 27.Lanzarini L, Fontana A, Lucca E, Campana C, Klersy C. Noninvasive estimation of both systolic and diastolic pulmonary artery pressure from Doppler analysis of tricuspid regurgitant velocity spectrum in patients with chronic heart failure. Am Heart J. 2002;144:1087–1094. doi: 10.1067/mhj.2002.126350. [DOI] [PubMed] [Google Scholar]
- 28.Stein JH, Neumann A, Preston LM, Costanzo MR, Parrillo JE, Johnson MR, Marcus RH. Echocardiography for hemodynamic assessment of patients with advanced heart failure and potential heart transplant recipients. J Am Coll Cardiol. 1997;30:1765–1772. doi: 10.1016/s0735-1097(97)00384-7. [DOI] [PubMed] [Google Scholar]
- 29.Mansencal N, d’Allonnes LR, Beauchet A, Fabre S, Digne F, Farcot J-C, Joseph T, Dubourg O. Reliability of echocardiography for hemodynamic assessment of end-stage heart failure. Am J Cardiol. 2007;100:998–1001. doi: 10.1016/j.amjcard.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 30.McClanahan A, Guglin M. Right ventricular dysfunction compromises accuracy of echocardiographic diagnosis of pulmonary hypertension in heart failure. J Card Fail. 2011;17:1023–1027. doi: 10.1016/j.cardfail.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Selimovic N, Rundqvist B, Bergh C-H, Andersson B, Petersson S, Johansson L, Bech-Hanssen O. Assessment of pulmonary vascular resistance by Doppler echocardiography in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2007;26:927–934. doi: 10.1016/j.healun.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Lindqvist P, Söderberg S, Gonzalez MC, Tossavainen E, Henein MY. Echocardiography based estimation of pulmonary vascular resistance in patients with pulmonary hypertension: a simultaneous Doppler echocardiography and cardiac catheterization study. Eur J Echocardiogr. 2011;12:961–966. doi: 10.1093/ejechocard/jer222. [DOI] [PubMed] [Google Scholar]
- 33.Scapellato F, Temporelli PL, Eleuteri E, Corrà U, Imparato A, Giannuzzi P. Accurate noninvasive estimation of pulmonary vascular resistance by Doppler echocardiography in patients with chronic failure heart failure. J Am Coll Cardiol. 2001;37:1813–1819. doi: 10.1016/s0735-1097(01)01271-2. [DOI] [PubMed] [Google Scholar]
- 34.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–1027. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 35.Farzaneh-Far R, McKeown BH, Dang D, Roberts J, Schiller NB, Foster E. Accuracy of Doppler-estimated pulmonary vascular resistance in patients before liver transplantation. Am J Cardiol. 2008;101:259–262. doi: 10.1016/j.amjcard.2007.07.086. [DOI] [PubMed] [Google Scholar]
- 36.Haddad F, Zamanian R, Beraud A-S, Schnittger I, Feinstein J, Peterson T, Yang P, Doyle R, Rosenthal D. A novel non-invasive method of estimating pulmonary vascular resistance in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr. 2009;22:523–529. doi: 10.1016/j.echo.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Roule V, Labombarda F, Pellissier A, Sabatier R, Lognoné T, Gomes S, Bergot E, Milliez P, Grollier G, Saloux E. Echocardiographic assessment of pulmonary vascular resistance in pulmonary arterial hypertension. Cardiovasc Ultrasound. 2010;8:21. doi: 10.1186/1476-7120-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahiya A, Vollbon W, Jellis C, Prior D, Wahi S, Marwick T. Echocardiographic assessment of raised pulmonary vascular resistance: application to diagnosis and follow-up of pulmonary hypertension. Heart. 2010;96:2005–2009. doi: 10.1136/hrt.2010.204834. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopalan N, Simon MA, Suffoletto MS, Shah H, Edelman K, Mathier MA, López-Candales A. Noninvasive estimation of pulmonary vascular resistance in pulmonary hypertension. Echocardiography. 2009;26:489–494. doi: 10.1111/j.1540-8175.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 40.Bhatt DD, Manoj R, Mahajan R. Estimation of pulmonary vascular resistance: correlation between echocardiography and catheterization data in patients with congenital heart disease. Echocardiography. 2012;29:478–483. doi: 10.1111/j.1540-8175.2011.01609.x. [DOI] [PubMed] [Google Scholar]
- 41.Ajami GH, Cheriki S, Amoozgar H, Borzouee M, Soltani M. Accuracy of Doppler-derived estimation of pulmonary vascular resistance in congenital heart disease: an index of operability. Pediatr Cardiol. 2011;32:1168–1174. doi: 10.1007/s00246-011-0035-4. [DOI] [PubMed] [Google Scholar]
- 42.Albers J, Ister D, Kayhan N, Vahl CF. Postoperative non-invasive assessment of pulmonary vascular resistance using Doppler echocardiography. Interact Cardiovasc Thorac Surg. 2011;13:579–584. doi: 10.1510/icvts.2011.271619. [DOI] [PubMed] [Google Scholar]
- 43.Results from late-breaking clinical trials sessions at ACC. J Am Coll Cardiol. 2001;38:595–612. doi: 10.1016/s0735-1097(98)00204-6. [DOI] [PubMed] [Google Scholar]
- 44.Pacher R, Globits S, Wutte M, Rödler S, Heinz G, Kreiner G, Radosztics S, Berger R, Presch I, Weber H. Beneficial hemodynamic effects of prostaglandin E1 infusion in catecholamine-dependent heart failure: results of a prospective, randomized, controlled study. Crit Care Med. 1994;22:1084–1090. doi: 10.1097/00003246-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Kiowski W, Sütsch G, Hunziker P, Müller P, Kim J, Oechslin E, Schmitt R, Jones R, Bertel O. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346:732–736. doi: 10.1016/s0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- 46.Pacher R, Stanek B, Hülsmann M, Bojic A, Berger R, Frey B, Siegel A, Kos T, Ogris E, Grimm M, Laufer G. Prostaglandin E1 infusion compared with prostacyclin infusion in patients with refractory heart failure: effects on hemodynamics and neurohumoral variables. J Heart Lung Transplant. 1997;16:878–881. [PubMed] [Google Scholar]
- 47.Sütsch G, Kiowski W, Yan XW, Hunziker P, Christen S, Strobel W, Kim JH, Rickenbacher P, Bertel O. Short-term oral endothelin-receptor antagonist therapy in conventionally treated patients with symptomatic severe chronic heart failure. Circulation. 1998;98:2262–2268. doi: 10.1161/01.cir.98.21.2262. [DOI] [PubMed] [Google Scholar]
- 48.Wimmer A, Stanek B, Kubecova L, Vitovec J, Spinar J, Yilmaz N, Kos T, Hartter E, Frey B, Pacher R. Effects of prostaglandin E1, dobutamine and placebo on hemodynamic, renal and neurohumoral variables in patients with advanced heart failure. Jpn Heart J. 1999;40:321–334. doi: 10.1536/jhj.40.321. [DOI] [PubMed] [Google Scholar]
- 49.Givertz MM, Colucci WS, LeJemtel TH, Gottlieb SS, Hare JM, Slawsky MT, Leier CV, Loh E, Nicklas JM, Lewis BE. Acute endothelin A receptor blockade causes selective pulmonary vasodilation in patients with chronic heart failure. Circulation. 2000;101:2922–2927. doi: 10.1161/01.cir.101.25.2922. [DOI] [PubMed] [Google Scholar]
- 50.Torre-Amione G, Young JB, Durand J, Bozkurt B, Mann DL, Kobrin I, Pratt CM. Hemodynamic effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients with class III to IV congestive heart failure. Circulation. 2001;103:973–980. doi: 10.1161/01.cir.103.7.973. [DOI] [PubMed] [Google Scholar]
- 51.Schalcher C, Cotter G, Reisin L, Bertel O, Kobrin I, Guyene TT, Kiowski W. The dual endothelin receptor antagonist tezosentan acutely improves hemodynamic parameters in patients with advanced heart failure. Am Heart J. 2001;142:340–349. doi: 10.1067/mhj.2001.116760. [DOI] [PubMed] [Google Scholar]
- 52.Torre-Amione G, Young JB, Colucci WS, Lewis BS, Pratt C, Cotter G, Stangl K, Elkayam U, Teerlink JR, Frey A, Rainisio M, Kobrin I. Hemodynamic and clinical effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2003;42:140–147. doi: 10.1016/s0735-1097(03)00556-4. [DOI] [PubMed] [Google Scholar]
- 53.Cotter G, Kaluski E, Stangl K, Pacher R, Richter C, Milo-Cotter O, Perchenet L, Kobrin I, Kaplan S, Rainisio M, Frey A, Neuhart E, Vered Z, Dingemanse J, Torre-Amione G. The hemodynamic and neurohormonal effects of low doses of tezosentan (an endothelin A/B receptor antagonist) in patients with acute heart failure. European journal of heart failure. 2004;6:601–609. doi: 10.1016/j.ejheart.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Bergler-Klein J, Pacher R, Berger R, Bojic A, Stanek B. Neurohumoral and hemodynamic effects of the selective endothelin antagonist darusentan in advanced chronic heart failure. J Heart Lung Transplant. 2004;23:20–27. doi: 10.1016/s1053-2498(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 55.Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, Schulman K, Zannad F, Handberg-Thurmond E, Harrell FE, Jr, Wheeler W, Soler-Soler J, Swedberg K. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1997;134:44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 56.Mylona P, Cleland JG. Update of REACH-1 and MERIT-HF clinical trials in heart failure. Cardio. net Editorial Team. European journal of heart failure. 1999;1:197–200. doi: 10.1016/s1388-9842(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 57.Kalra PR, Moon JCC, Coats AJS. Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol. 2002;85:195–197. doi: 10.1016/s0167-5273(02)00182-1. [DOI] [PubMed] [Google Scholar]
- 58.Lüscher TF, Enseleit F, Pacher R, Mitrovic V, Schulze MR, Willenbrock R, Dietz R, Rousson V, Hürlimann D, Philipp S, Notter T, Noll G, Ruschitzka F Heart Failure ET(A) Receptor Blockade Trial. Hemodynamic and neurohumoral effects of selective endothelin A (ET(A)) receptor blockade in chronic heart failure: the Heart Failure ET(A) Receptor Blockade Trial (HEAT) Circulation. 2002;106:2666–2672. doi: 10.1161/01.cir.0000038497.80095.e1. [DOI] [PubMed] [Google Scholar]
- 59.Kaluski E, Kobrin I, Zimlichman R, Marmor A, Krakov O, Milo O, Frey A, Kaplan S, Krakover R, Caspi A, Vered Z, Cotter G. RITZ-5: randomized intravenous TeZosentan (an endothelin-A/B antagonist) for the treatment of pulmonary edema: a prospective, multicenter, double-blind, placebo-controlled study. J Am Coll Cardiol. 2003;41:204–210. doi: 10.1016/s0735-1097(02)02708-0. [DOI] [PubMed] [Google Scholar]
- 60.O’Connor CM, Gattis WA, Adams KF, Hasselblad V, Chandler B, Frey A, Kobrin I, Rainisio M, Shah MR, Teerlink J, Gheorghiade M Randomized Intravenous TeZosentan Study-4 Investigators. Tezosentan in patients with acute heart failure and acute coronary syndromes: results of the Randomized Intravenous TeZosentan Study (RITZ-4) J Am Coll Cardiol. 2003;41:1452–1457. doi: 10.1016/s0735-1097(03)00194-3. [DOI] [PubMed] [Google Scholar]
- 61.Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Lüscher TF EARTH investigators. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–354. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- 62.Packer M, McMurray J, Massie BM, Caspi A, Charlon V, Cohen-Solal A, Kiowski W, Kostuk W, Krum H, Levine B, Rizzon P, Soler J, Swedberg K, Anderson S, Demets DL. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail. 2005;11:12–20. doi: 10.1016/j.cardfail.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Prasad SK, Dargie HJ, Smith GC, Barlow MM, Grothues F, Groenning BA, Cleland JGF, Pennell DJ. Comparison of the dual receptor endothelin antagonist enrasentan with enalapril in asymptomatic left ventricular systolic dysfunction: a cardiovascular magnetic resonance study. Heart. 2006;92:798–803. doi: 10.1136/hrt.2004.049734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMurray JJV, Teerlink JR, Cotter G, Bourge RC, Cleland JGF, Jondeau G, Krum H, Metra M, O’Connor CM, Parker JD, Torre-Amione G, van Veldhuisen DJ, Lewsey J, Frey A, Rainisio M, Kobrin I VERITAS Investigators. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA. 2007;298:2009–2019. doi: 10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 65.Liu C, Chen J, Gao Y, Deng B, Liu K. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database Syst Rev. 2009:CD004434. doi: 10.1002/14651858.CD004434.pub4. [DOI] [PubMed] [Google Scholar]
- 66.Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, Gomberg-Maitland M, Murali S, Frantz RP, McGlothlin D, Horn EM, Benza RL. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult--a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2012;31:913–933. doi: 10.1016/j.healun.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 68.Kaluski E, Cotter G, Leitman M, Milo-Cotter O, Krakover R, Kobrin I, Moriconi T, Rainisio M, Caspi A, Reizin L, Zimlichman R, Vered Z. Clinical and hemodynamic effects of bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension--a multi-center randomized study. Cardiology. 2008;109:273–280. doi: 10.1159/000107791. [DOI] [PubMed] [Google Scholar]
- 69.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 70.Guazzi M, Vicenzi M, Arena R. Phosphodiesterase 5 inhibition with sildenafil reverses exercise oscillatory breathing in chronic heart failure: a long-term cardiopulmonary exercise testing placebo-controlled study. European journal of heart failure. 2012;14:82–90. doi: 10.1093/eurjhf/hfr147. [DOI] [PubMed] [Google Scholar]
- 71.Forfia PR, Borlaug BA. Letter by Forfia and Borlaug regarding article, “Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study”. Circulation. 2012;125:e408. doi: 10.1161/CIRCULATIONAHA.111.064584. author reply e409–410. [DOI] [PubMed] [Google Scholar]
- 72.Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–2144. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 73.Behling A, Rohde LE, Colombo FC, Goldraich LA, Stein R, Clausell N. Effects of 5′-phosphodiesterase four-week long inhibition with sildenafil in patients with chronic heart failure: a double-blind, placebo-controlled clinical trial. J Card Fail. 2008;14:189–197. doi: 10.1016/j.cardfail.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Guazzi M, Casali M, Berti F, Rossoni G, Colonna VDG, Guazzi MD. Endothelium-mediated modulation of ergoreflex and improvement in exercise ventilation by acute sildenafil in heart failure patients. Clin Pharmacol Ther. 2008;83:336–341. doi: 10.1038/sj.clpt.6100306. [DOI] [PubMed] [Google Scholar]
- 75.Bocchi EA, Guimaräes G, Mocelin A, Bacal F, Bellotti G, Ramires JF. Sildenafil effects on exercise, neurohormonal activation, and erectile dysfunction in congestive heart failure: a double-blind, placebo-controlled, randomized study followed by a prospective treatment for erectile dysfunction. Circulation. 2002;106:1097–1103. doi: 10.1161/01.cir.0000027149.83473.b6. [DOI] [PubMed] [Google Scholar]
- 76.Webster LJ, Michelakis ED, Davis T, Archer SL. Use of sildenafil for safe improvement of erectile function and quality of life in men with New York Heart Association classes II and III congestive heart failure: a prospective, placebo-controlled, double-blind crossover trial. Arch Intern Med. 2004;164:514–520. doi: 10.1001/archinte.164.5.514. [DOI] [PubMed] [Google Scholar]
- 77.Katz SD, Parker JD, Glasser DB, Bank AJ, Sherman N, Wang H, Sweeney M. Efficacy and safety of sildenafil citrate in men with erectile dysfunction and chronic heart failure. Am J Cardiol. 2005;95:36–42. doi: 10.1016/j.amjcard.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 78.Katz SD, Balidemaj K, Homma S, Wu H, Wang J, Maybaum S. Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow-mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 2000;36:845–851. doi: 10.1016/s0735-1097(00)00790-7. [DOI] [PubMed] [Google Scholar]
- 79.Guazzi M, Tumminello G, Di Marco F, Fiorentini C, Guazzi MD. The effects of phosphodiesterase-5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J Am Coll Cardiol. 2004;44:2339–2348. doi: 10.1016/j.jacc.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 80.Hirata K, Adji A, Vlachopoulos C, O’Rourke MF. Effect of sildenafil on cardiac performance in patients with heart failure. Am J Cardiol. 2005;96:1436–1440. doi: 10.1016/j.amjcard.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 81.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 82.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 83.Loh E, Stamler JS, Hare JM, Loscalzo J, Colucci WS. Cardiovascular effects of inhaled nitric oxide in patients with left ventricular dysfunction. Circulation. 1994;90:2780–2785. doi: 10.1161/01.cir.90.6.2780. [DOI] [PubMed] [Google Scholar]
- 84.Boilson BA, Schirger JA, Borlaug BA. Caveat medicus! Pulmonary hypertension in the elderly: a word of caution. European journal of heart failure. 2010;12:89–93. doi: 10.1093/eurjhf/hfp171. [DOI] [PubMed] [Google Scholar]
- 85.Cole RT, Kalogeropoulos AP, Georgiopoulou VV, Gheorghiade M, Quyyumi A, Yancy C, Butler J. Hydralazine and isosorbide dinitrate in heart failure: historical perspective, mechanisms, and future directions. Circulation. 2011;123:2414–2422. doi: 10.1161/CIRCULATIONAHA.110.012781. [DOI] [PubMed] [Google Scholar]
- 86.Horowitz JD. Tolerance induction during therapy with long-acting nitrates: how extensive is the “collateral damage”? Cardiovasc Drugs Ther. 2004;18:11–12. doi: 10.1023/b:card.0000025923.41144.ec. [DOI] [PubMed] [Google Scholar]
- 87.Kosmicki MA, Szwed H, Sadowski Z. Anti-ischaemic response to sublingual nitroglycerin during oral administration of isosorbide dinitrate in patients with stable angina pectoris: when does cross-tolerance occur? Cardiovasc Drugs Ther. 2004;18:47–55. doi: 10.1023/B:CARD.0000025755.94861.dc. [DOI] [PubMed] [Google Scholar]
- 88.Munzel T, Sayegh H, Freeman BA, Tarpey MM, Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kass DA. Cardiac role of cyclic-GMP hydrolyzing phosphodiesterase type 5: from experimental models to clinical trials. Curr Heart Fail Rep. 2012;9:192–199. doi: 10.1007/s11897-012-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gheorghiade M, Marti CN, Sabbah HN, Roessig L, Greene SJ, Bohm M, Burnett JC, Campia U, Cleland JG, Collins SP, Fonarow GC, Levy PD, Metra M, Pitt B, Ponikowski P, Sato N, Voors AA, Stasch JP, Butler J. Soluble guanylate cyclase: a potential therapeutic target for heart failure. Heart Fail Rev. 2012 May 24; doi: 10.1007/s10741-012-9323-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 91.Costanzo MR, Augustine S, Bourge R, Bristow M, O’Connell JB, Driscoll D, Rose E. Selection and treatment of candidates for heart transplantation. A statement for health professionals from the Committee on Heart Failure and Cardiac Transplantation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1995;92:3593–3612. doi: 10.1161/01.cir.92.12.3593. [DOI] [PubMed] [Google Scholar]
- 92.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant. 2006;25:1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 93.Longenecker CT, Mondo C, Le VV, Jensen TP, Foster E. HIV infection is not associated with echocardiographic signs of cardiomyopathy or pulmonary hypertension among pregnant Ugandan women. Int J Cardiol. 2011;147:300–302. doi: 10.1016/j.ijcard.2010.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kittleson MM, Kobashigawa JA. Management of advanced heart failure: the role of heart transplantation. Circulation. 2011;123:1569–1574. doi: 10.1161/CIRCULATIONAHA.110.972810. [DOI] [PubMed] [Google Scholar]
- 95.Costard-Jackle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19:48–54. doi: 10.1016/0735-1097(92)90050-w. [DOI] [PubMed] [Google Scholar]
- 96.Drakos SG, Kfoury AG, Gilbert EM, Horne BD, Long JW, Stringham JC, Campbell BA, Renlund DG. Effect of reversible pulmonary hypertension on outcomes after heart transplantation. J Heart Lung Transplant. 2007;26:319–323. doi: 10.1016/j.healun.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 97.Goland S, Czer LS, Kass RM, De Robertis MA, Mirocha J, Coleman B, Capelli C, Raissi S, Cheng W, Fontana G, Trento A. Pre-existing pulmonary hypertension in patients with end-stage heart failure: impact on clinical outcome and hemodynamic follow-up after orthotopic heart transplantation. J Heart Lung Transplant. 2007;26:312–318. doi: 10.1016/j.healun.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 98.Klotz S, Wenzelburger F, Stypmann J, Welp H, Drees G, Schmid C, Scheld HH. Reversible pulmonary hypertension in heart transplant candidates: to transplant or not to transplant. The Annals of thoracic surgery. 2006;82:1770–1773. doi: 10.1016/j.athoracsur.2006.05.114. [DOI] [PubMed] [Google Scholar]
- 99.Haddad H, Elabbassi W, Moustafa S, Davies R, Mesana T, Hendry P, Masters R, Mussivand T. Left ventricular assist devices as bridge to heart transplantation in congestive heart failure with pulmonary hypertension. ASAIO J. 2005;51:456–460. doi: 10.1097/01.mat.0000169125.21268.d7. [DOI] [PubMed] [Google Scholar]
- 100.Martin J, Siegenthaler MP, Friesewinkel O, Fader T, van de Loo A, Trummer G, Berchtold-Herz M, Beyersdorf F. Implantable left ventricular assist device for treatment of pulmonary hypertension in candidates for orthotopic heart transplantation-a preliminary study. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2004;25:971–977. doi: 10.1016/j.ejcts.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 101.Salzberg SP, Lachat ML, von Harbou K, Zund G, Turina MI. Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2005;27:222–225. doi: 10.1016/j.ejcts.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 102.Zimpfer D, Zrunek P, Sandner S, Schima H, Grimm M, Zuckermann A, Wolner E, Wieselthaler G. Post-transplant survival after lowering fixed pulmonary hypertension using left ventricular assist devices. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2007;31:698–702. doi: 10.1016/j.ejcts.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 103.John R, Liao K, Kamdar F, Eckman P, Boyle A, Colvin-Adams M. Effects on pre- and posttransplant pulmonary hemodynamics in patients with continuous-flow left ventricular assist devices. The Journal of thoracic and cardiovascular surgery. 2010;140:447–452. doi: 10.1016/j.jtcvs.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 104.Nair PK, Kormos RL, Teuteberg JJ, Mathier MA, Bermudez CA, Toyoda Y, Dew MA, Simon MA. Pulsatile left ventricular assist device support as a bridge to decision in patients with end-stage heart failure complicated by pulmonary hypertension. J Heart Lung Transplant. 2010;29:201–208. doi: 10.1016/j.healun.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Torre-Amione G, Southard RE, Loebe MM, Youker KA, Bruckner B, Estep JD, Tierney M, Noon GP. Reversal of secondary pulmonary hypertension by axial and pulsatile mechanical circulatory support. J Heart Lung Transplant. 2010;29:195–200. doi: 10.1016/j.healun.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 106.Liden H, Haraldsson A, Ricksten SE, Kjellman U, Wiklund L. Does pretransplant left ventricular assist device therapy improve results after heart transplantation in patients with elevated pulmonary vascular resistance? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2009;35:1029–1034. doi: 10.1016/j.ejcts.2008.12.024. discussion 1034–1025. [DOI] [PubMed] [Google Scholar]
- 107.Mikus E, Stepanenko A, Krabatsch T, Loforte A, Dandel M, Lehmkuhl HB, Hetzer R, Potapov EV. Reversibility of fixed pulmonary hypertension in left ventricular assist device support recipients. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011;40:971–977. doi: 10.1016/j.ejcts.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 108.De Santo LS, Romano G, Maiello C, Buonocore M, Cefarelli M, Galdieri N, Nappi G, Amarelli C. Pulmonary artery hypertension in heart transplant recipients: how much is too much? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012;42:864–869. doi: 10.1093/ejcts/ezs102. discussion 869–870. [DOI] [PubMed] [Google Scholar]
- 109.Pons J, Leblanc MH, Bernier M, Cantin B, Bourgault C, Bergeron S, Proulx G, Morin J, Nalli C, O’Connor K, Chateauvert N, Senechal M. Effects of chronic sildenafil use on pulmonary hemodynamics and clinical outcomes in heart transplantation. J Heart Lung Transplant. 2012;31:1281–1287. doi: 10.1016/j.healun.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 110.Reichenbach A, Al-Hiti H, Malek I, Pirk J, Goncalvesova E, Kautzner J, Melenovsky V. The effects of phosphodiesterase 5 inhibition on hemodynamics, functional status and survival in advanced heart failure and pulmonary hypertension: A case-control study. Int J Cardiol. 2012 Oct 8; doi: 10.1016/j.ijcard.2012.09.074. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 111.Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM, Jr, Magalski A, Zile MR, Smith AL, Smart FW, O’Shaughnessy MA, Jessup ML, Sparks B, Naftel DL, Stevenson LW. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073–1079. doi: 10.1016/j.jacc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 112.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E. PhosphdiesteRasE-5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) trial: rationale and design. Circ Heart Fail. 2012;5:653–659. doi: 10.1161/CIRCHEARTFAILURE.112.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ghio S, Bonderman D, Felix SB, Ghofrani HA, Michelakis ED, Mitrovic V, Oudiz RJ, Frey R, Roessig L, Semigran MJ. Left ventricular systolic dysfunction associated with pulmonary hypertension riociguat trial (LEPHT): rationale and design. European journal of heart failure. 2012;14:946–953. doi: 10.1093/eurjhf/hfs071. [DOI] [PubMed] [Google Scholar]
- 114.Late-Breaking Clinical Trial Abstracts. Circulation. 2012;126:2776–2799. doi: 10.1161/CIR.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 115.Kalogeropoulos AP, Georgiopoulou VV, Butler J. Clinical adoption of prognostic biomarkers: the case for heart failure. Progress in cardiovascular diseases. 2012;55:3–13. doi: 10.1016/j.pcad.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wessler B, Kramer D, Kelly J, Trikalinos T, Kent D, Konstam M, Udelson J. Drug and device effects on peak oxygen consumption, 6-minute walk distance, and natriuretic peptides as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction. Circ Heart Fail. 2011;4:578–588. doi: 10.1161/CIRCHEARTFAILURE.111.961573. [DOI] [PubMed] [Google Scholar]
- 117.Kramer D, Trikalinos T, Kent D, Antonopoulos G, Konstam M, Udelson J. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 2010;56:392–406. doi: 10.1016/j.jacc.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


