Abstract
The kinases Mnk1 and Mnk2 are activated downstream of the p38 MAPK and MEK/ERK signaling pathways. Extensive work over the years has shown that these kinases control phosphorylation of the eukaryotic initiation factor 4E (eIF4E) and regulate engagement of other effector elements, including hnRNPA1 and PSF. Mnk kinases are ubiquitously expressed and play critical roles in signaling for various cytokine receptors, while there is emerging evidence that they have important functions as mediators of pro-inflammatory cytokine production. In this review the mechanisms of activation of MNK pathways by cytokine receptors are addressed and their roles in diverse cytokine-dependent biological processes are reviewed. The clinical-translational implications of such work and the relevance of future development of specific MNK inhibitors for the treatment of malignancies and auto-immune disorders are discussed.
Introduction
Mnk1 and Mnk2 are serine/threonine kinases that were initially identified as Erk substrates in two independent studies [1,2]. In the study by Fukunaga et al (1997), a search was performed for Erk1 substrates by screening a cDNA library with a novel solid-phase phosphorylation method using purified Erk1 and labeled δ-ATP [1]. Mnk1 was identified by this screen and was shown to be activated by various stress inducing agents as well as cytokines in a p38 MAPK- or Erk-dependent manner, while it was established that such activation is JNK-independent [1]. In a separate study by Waskiewicz et al (1997), a yeast-two hybrid system search for Erk2 binding partners identified Mnk1 and Mnk2 as potential Erk targets [2]. These investigators demonstrated that Mnk1 can interact with and undergo phosphorylation by Erk2 or p38 MAPK on Thr197/Thr202 in mice and Thr209/Thr214 in humans, whereas Mnk2 could only interact with and be activated by Erk2 [2]. This study also demonstrated that Mnk1 is activated in response to mitogens and stress inducing agents and identified the eukaryotic initiation factor 4E (eIF4E) as a possible Mnk1 substrate [2]. Mnk1 regulates eIF4E phosphorylation in response to external stimuli, while basal Mnk2 activity is high in cells and accounts for the constitutive eIF4E phosphorylation levels [3,4]. Mnk1 and Mnk2 share 72% of their amino acid sequence and contain conserved MAP kinase phosphorylation sites in the T-loop of the kinase domain; a catalytic domain; and an Erk binding domain in the carboxyl terminus [2]. Mnk1 and Mnk2 are both alternatively spliced in humans (a and b isoforms). It should be noted that the b isoforms lack a MAPK binding domain and are therefore resistant to regulation by Erk or p38 MAPK [5,6].
Although most studies do not focus on differences in the functions of Mnk1 and Mnk2, there is emerging evidence that variances in the amino acid sequence of the catalytic domains and the C-terminal regions of the kinases contributes to differences in their activities [7]. Mnk1 has lower basal activity as compared to Mnk2, while Mnk2 activity is more resistant to upstream inhibition of either p38 MAPK or Erk [7]. An Asp residue in the catalytic domain of Mnk2 is necessary for its enhanced catalytic activity, while mutating the corresponding amino acid residue in the Mnk1 catalytic domain to Asp enhances the catalytic activity of Mnk1 [7]. Interestingly, swapping the Mnk1 and Mnk2 C terminal domains results in decreased activity in Mnk2 but fails to enhance Mnk1 activity [7]. Mnk1 can interact with both p38 MAPK and Erk whereas Mnk2 cannot bind p38 MAPK and, compared to Mnk1, exhibits enhanced binding ability for phosphorylated Erk [7]. Such enhanced binding of Mnk2 to Erk appears to be partially mediated by a glutamine (Gln) residue in the MAPK binding domain, but this is not sufficient to completely explain the observed differences and additional mechanisms may be involved [7].
The initiation factor eIF4E is the best characterized target of Mnk kinases, although the precise role of phosphorylation of eIF4E and its resulting effects on translation remain to be defined. Mice with targeted disruption of either Mnk1 or Mnk2 or both Mnk1 and Mnk2 are viable and are phenotypically similar to the wild type mice under unstressed conditions [8]. Interestingly, phosphorylation of eIF4E is not detected in Mnk1/2−/− mice, suggesting that such phosphorylation is not essential for survival [8]. Moreover, Mnk1/2 −/− mice do not have impaired rate of protein synthesis or cap dependent translation [8]. In another study, the expression of constitutively active Mnk1 and Mnk2 mutants or the activation of Mnk1 by stimulation of either p38 or Erk was found to diminish the rate of cap dependent translation relative to the internal ribosome entry site (IRES) mediated translation [9]. Given the reduced affinity of capped RNA for phosphorylated eIF4E it has been suggested that phosphorylation on S209 may result in the disassociation of eIF4E from the initiation complex allowing the 40S ribosome to scan for the initiation codon [10,11].
Role of Mnk kinases in cytokine production
The immune response to pathogens requires tight regulation of genes that mediate early, transient, and late immune responses. The innate immune response is launched on detection of pathogens by leukocytes, mainly circulating dendritic cells (DCs), neutrophils, natural killer (NK) cells, monocytes, eosinophils, and basophils, along with tissue-resident mast cells and macrophages) [12]. The initial phase results in the release of various cytokines such as tumor necrosis factor α (TNFα), interferons (IFNs), interleukins (IL)-1β, IL-4, IL-6, IL-10, IL-12, IL-18, RANTES, and transforming growth factor (TGF)-β [13]. The adaptive immunity is regulated by activated T lymphocytes that secrete a variety of cytokines such as IL-2, IL-4, IL-5, IFNγ, IL-13 and others that regulate differentiation of T cells into T helper cells, proliferation and activation of B lymphocytes as well as stimulation of antibody synthesis by B cells [14]. Notably, MAPK pathways play important roles in the production of cytokines involved in both innate and adaptive immunity ([reviewed in [15]). As Mnk kinases are downstream effectors of MAPK pathways, they are good candidates as putative mediators of MAPK mediated cytokine production. These observations have triggered extensive studies to address their potential involvement in such responses.
To assess the role of Mnk kinases in cytokine production, Rowlett et al. (2008) examined cytokine production in response to various stimuli in the presence or absence of the Mnk pharmacological inhibitor CGP57380 [16]. These investigators demonstrated that inhibition of Mnk kinase activity resulted in attenuated TNFα production by macrophages after treatment with multiple Toll like receptor (TLR) agonists such as ODN1826 (TLR9 agonist ), LPS (TLR4 agonist), imiquimod (TLR5 agonist), FSL (TLR6/2 agonist), and flagellin (TLR5 agonist) [16]. Additionally the Mnk inhibitor also suppressed the production of IL-6 by murine macrophages in response to TLR agonists [16]. In other studies, inhibition of Mnk kinase activity in bone marrow derived macrophages from a spontaneous murine model of Crohn’s-like ileitis attenuated production of TNFα, IL-6 and monocyte chemoattractant protein (MCP)-1 and transiently induced the expression of IL-10 [16]. Altogether these studies established that Mnk kinases mediate signals important for pro-inflamatory responses, raising the possibility that targeting Mnk kinases may provide a potential novel therapeutic approach for the treatment of Crohn’s disease.
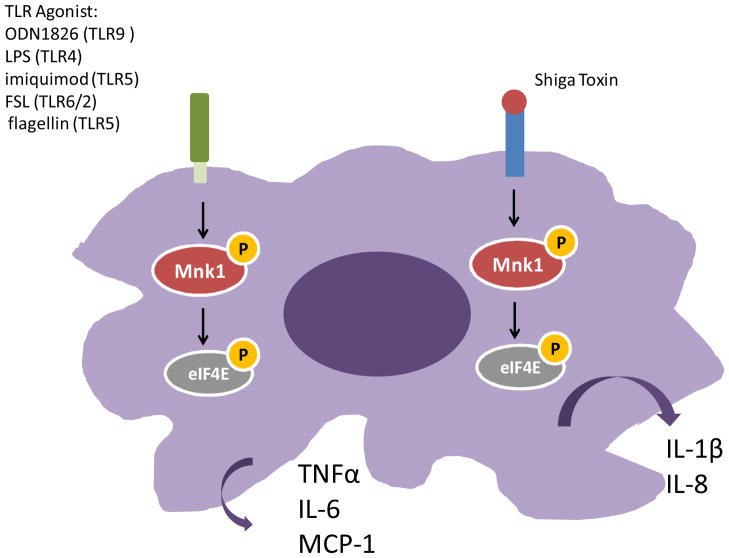
Kjellerup et al (2008) demonstrated an important role for the Mnks in the release of pro-inflammatory cytokines in keratinocytes [17]. In that study it was shown that inhibition of the Mnk kinases in cultured human keratinocytes attenuates TNFα, IL-6, or IL-1β release in response to stimulation with the p38 MAPK agonist anisomycin. Additionally the presence of the Mnk inhibitor also suppressed IL-1β-stimulated TNFα release [17]. Inhibition of Mnk kinases also negatively regulates post-transcriptional regulation of IFNγ and IL-4 in activated murine NK cells [18] and blocks Shiga toxin mediated release of IL-1β and IL-8 [19]. Thus Mnk kinases play important roles in the control of immune responses and elucidating the precise mechanisms of Mnk mediated post-transcriptional regulation of cytokine production will have important therapeutic implications. The role of the Mnk kinases in regulating cytokine production in macrophages is summarized in Figure 1. In the following sections we summarize the current studies that have examined the mechanism(s) of Mnk mediated production of different cytokines.
Figure 1. Mnk pathway mediated cytokine production in macrophages.
Macrophage stimulation by various TLR agonists results in the release of pro-inflammatory cytokines, whose expression which can be blocked by the Mnk kinase inhibitor. Inhibition of the Mnk kinases also attenuates IL-1β and IL-8 secretion in response to treatment with Shiga toxin.
TNFα
TNFα was initially identified as a cytotoxic factor secreted by macrophages with antitumor activities [20]. Later studies demonstrated an important role for TNFα in mediating systemic inflammation based on its ability to mediate lethal endotoxin poisoning and lethal septic shock [21–23]. The production of this cytokine is deregulated in variety of human diseases including malignancies [24], Alzheimer’s disease [25], major depression [26] and inflammatory bowel disease (IBD) [27]. TNFα production is tightly controlled by both transcriptional and post-transcriptional mechanisms. The 3’untranslated region (UTR) of the TNFα mRNA has been found to contain AU-rich elements (AREs) that are important in the post-transcriptional regulation of TNFα mRNA [28] by controlling mRNA processing [29], mRNA stability [30] as well as translational inhibition [31]. The importance of the ARE in regulating TNFα production is underscored by studies demonstrating that mice lacking AREs in the 3’UTR exhibit enhanced TNFα production resulting in chronic inflammatory arthritis and Crohn’s-like inflammatory bowel disease [30]. The involvement of the MAPK pathways, specifically p38 MAPK and Erk in the control of TNFα production in response to various stimuli has been extensively established [32,33], and has suggested that common downstream targets of these distinct MAPK pathways may play a role in TNFα production.
Work by Buxade et al (2005) showed that TNFα production in activated T-cells is partially inhibited by inhibition of either p38 or Erk MAPK, while concurrent inhibition of p38 and Erk completely abolished TNFα production [34]. Pharmacological inhibition of Mnk or Mnk1 knockdown demonstrated similar effects, suggesting an important role for Mnk kinases in TNFα production [34]. Interestingly, this study demonstrated that although the Mnk inhibitor completely abolished the phosphorylation of eIF4E and suppressed TNFα production by 70%, global mRNA translation was blocked by only 20% suggesting that eIF4E mediated translation initiation does not account for all observed effects of the Mnk inhibitor on TNFα production [34]. This work also established that the heterogeneous nuclear ribonucleoprotein (hnRNP) A1, an ARE binding protein, can be phosphorylated by Mnk1 [34]. Mnk mediated phosphorylation of hnRNPA1 on residues 192 and 301/302/303 was found to reduce the affinity of hnRNPA1 for the ARE in the TNFα mRNA 3’UTR, facilitating the translation of the TNFα mRNA [34]. Altogether these results provided evidence for an important role for Mnk1 mediated phosphorylation of hnRNPA1 in promoting TNFα mRNA translation. Notably, a variety of mRNAs such as those encoding granulocyte-macrophage colony-stimulating factor (GM-CSF), IFNγ, interleukin-3, c-fos, and v-myc have been shown to contain ARE sequences [35], raising the possibility for regulatory effects for Mnk1 and hnRNPA1 in the mRNA translation of such mRNAs, although this remains to be directly determined.
A separate study systematically used a proteomic approach to identify novel Mnk1 substrates based on their abilities to interact with a 5’-7-methylguanylate cap bound resin [36]. These studies identified PSF [PTB (polypyrimidine tract binding protein) associated splicing factor] as a Mnk substrate that undergoes phosphorylation on Ser8 and Ser283 [36]. A series of immunoprecipitation experiments indicated that the retention of PSF on the cap bound resin reflected its ability to interact with mRNA and not its ability to associate with cap binding proteins [36]. As previous studies had shown that PSF along with its binding partner p54nrb can bind ARE containing mRNAs [37], this study established that Mnk1 mediated phosphorylation of PSF in response to T cell activation facilitates the interaction between the PSF/p54nrb and the ARE containing mRNAs such as TNFα and GM-CSF [36]. The authors also demonstrated that PSF/p54nrb binding to ARE does not affect the nuclear-cytoplasmic distribution or TNFα mRNA stability [36]. The importance of Mnk induced binding of PSF/p54nrb to ARE containing mRNAs and its effects on translation of the respective mRNAs remains to be determined. It should be noted that many recent studies have shown that although eIF4E interacts with all capped mRNA, its phosphorylation on Ser209 affects mRNA translation of only a specific subset of mRNAs by mechanisms that remain unclear at this time [38,39] . The contribution of eIF4E in mediating the translation of TNFα mRNA remains to be precisely defined. Future studies examining TNFα production in cells overexpresing wild-type eIF4E or constitutive phosphorylated mutants or a mutants that cannot be phosphorylated should provide more conclusive evidence for the role of Mnk-mediated eIF4E phopshorylation in TNFα mRNA translation.
The role of the Mnk kinases in mediating TNFα production in activated T cells is summarized in Figure 2. TNFα production is elevated in various auto-immune diseases such as rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease and psoriasi [40]. Thus, inhibition of the Mnk kinases may have potential therapeutic applications in the treatment of the above diseases.
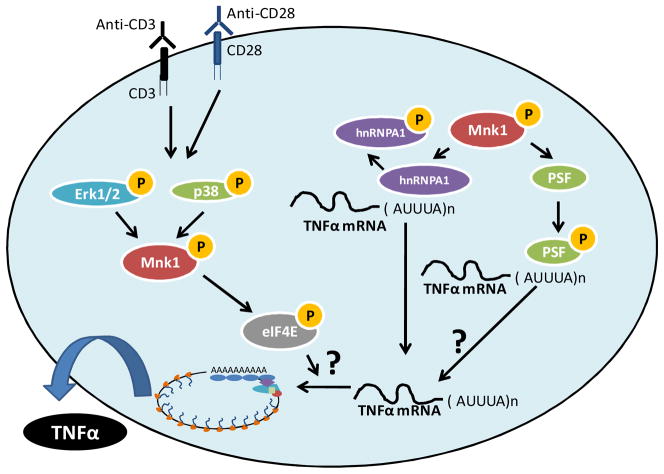
Figure 2. Role of Mnk Kinases in the Post-transcriptional regulation of TNFα mRNA.
Activation of T cells results in engagement of p38 MAPK and Erk, consequently resulting in phosphorylation/activation of Mnk1 and its downstream targets. Mnk1 mediated phosphorylation of hnRNPA1 results in its disassociation from the ARE of the TNFα mRNA, facilitating its translation. Engagement of Mnk1 also results in the phosphorylation of PSF, augmenting its binding to the ARE element in the TNFα mRNA, but its role in mediating TNFα translation remains to be explored. Activation of Mnk1 in activated T cells also results in phosphorylation of eIF4E and may play roles in mediating either translation or the nuclear export of the TNFα mRNA.
RANTES
RANTES also known as CCL5 (Chemokine (C-C motif) ligand 5) was initially identified as a cytokine secreted by cytotoxic T lymphocytes [41]. RANTES is also secreted by platelets, macrophages, eosinophils, and fibroblasts, as well as endothelial, epithelial, and endometrial cells [42]. This chemokine functions as a chemoattractant for monocytes [43], NK cells [44], memory T cells [43], eosinophils [45] and DCs [46] and along with macrophage inhibitory protein (MIP)-1a and MIP-1b acts as an HIV suppressive factor [47]. Transcription of RANTES in activated T-cells is regulated by RANTES factor of late-activated T lymphocytes-1 (RFLAT-1), a transcription factor which binds to the RANTES promoter [48]. While RFLAT-1 mRNA is present at all stages of T cell differentiation, RFLAT-1 protein expression is observed only 3 days post T-cell stimulation [49]. The RFLAT-1 mRNA is characterized by a GC rich 5’UTR and multiple upstream open reading frames which contribute to its translational repression [49]. Overexpression of eIF4E or constitutively active Mnk1 enhanced translation of RANTES mRNA, while a kinase dead Mnk1 attenuated RFLAT-1 expression [49]. Additionally IL-2 stimulation of T cells appears to upregulate RFLAT-1 and RANTES protein levels with a corresponding increase in eIF4E phosphorylation [49]. As both Mnk1 and Mnk2 can phosphorylate eIF4E, Mnk2 may also be involved in RFLAT-1 translation and the consequent secretion of RANTES.
Various studies have suggested a role for MAPK pathways in controlling RANTES production [50]. Respiratory syncytial virus mediated post-trascriptional regulation of RANTES in airway epithelial cells in dependent on both p38 MAPK and Erk [51], suggesting that Mnk kinases may also be involved in the post-transcriptional regulation of RANTES mRNA. It should be noted that RANTES knockout mice are characterized by impaired T cell proliferation and an attenuated expression of IL-2 and IFNγ [52], while RANTES overexpression has been associated with various auto-immune diseases such as asthma [53], rheumatoid arthritis [54] and multiple sclerosis [55]. Although RANTES can promote immune responses against malignant cells, it also appears to play an important role in tumor progression and metastasis [56]. Thus a better understanding of the role of Mnk kinases as mediators and regulators of RANTES expression may establish a potential role for Mnk inhibitors as therapy for various auto-immune diseases as well as in cancer prevention and therapeutics.
Interleukin 17 (IL- 17)
IL-17 was identified as an ARE containing rodent cDNA transcript isolated from an activated T cell hybridoma [57]. This cytokine is mainly produced by activated CD4+ and CD8+ T cells [58] and can regulate T cell responses [59]. It can induce the production of cytokines or chemokines such as IL-8 [60], MCP-1 [61], MIP-2 [62], IL-6 [63] and Groα [64] resulting in an inflammatory response and recruitment of monocytes and neutrophiles [65,66]. IL-17 production by CD4+ T (Th17) cells is mediated by MAPK pathways [67]. Noubade et al (2011) examined the role of the p38 MAPK in mediating IL-17 production and in regulating experimental allergic encephalomyelitis (EAE), the experimental murine model for multiple sclerosis [68]. These studies established that IL-17 production is attenuated in primary murine CD4+ Th17 cells in the presence of a p38 MAPK inhibitor [68]. Such regulation was controlled at the post-transcriptional level as there were no significant differences in IL-17 mRNA levels in the presence or absence of the p38 MAPK inhibitor [68]. In vitro induced differentiation of CD4+ T cells into Th17 cells was found to result in the phosphorylation of eIF4E in a p38 MAPK and Mnk kinase dependent manner [68]. Importantly, inhibition of Mnk kinases using a pharmacological Mnk inhibitor significantly decreased IL-17 production by Th17 cells, while IL-2 secretion was unaffected [68]. Altogether, these results raise the possibility that inhibition of the Mnk kinases may prove to be a useful therapeutic for the treatment of auto-immune diseases such as multiple sclerosis.
In addition to regulating IL-17 expression, there are many reports indicating that Erk and p38 MAPK are involved in IL-17 induced production of other cytokines [62,63,69], suggesting a role for Mnk1 in mediating IL-17-dependent biological responses. IL-17-mediated engagement of the p38 MAPK pathway enhances TNFα mediated IL-8 mRNA stability in human airway smooth muscle cells [69]. Additionally, IL-17-mediated activation of both p38 MAPK and Erk regulates IL-17-induced release of IL-6 and IL-8 from human bronchial epithelial cells [63], leading to clinical instability in patients with coronary artery disease [70]; and it can also synergize with TNFα and IL-1β in modulating MCP-1 and MIP-2 production in cultured mesangial cells [62]. Moreover, IL-17-dependent MAPK activation plays an important role in MMP-1 production by human cardiac fibroblasts [71] and modulates IL-17-induced C-reactive protein expression in hepatocytes, as well as in coronary artery smooth muscle cells [72]. The role of the Mnk kinases in IL-17 production and signaling is summarized in Figure 3. Thus, there is emerging evidence that IL-17 mediated activation of Erk and p38 MAPKs are important in the pathophysiology of chronic inflammation, asthma, atherosclerosis, and thrombosis.
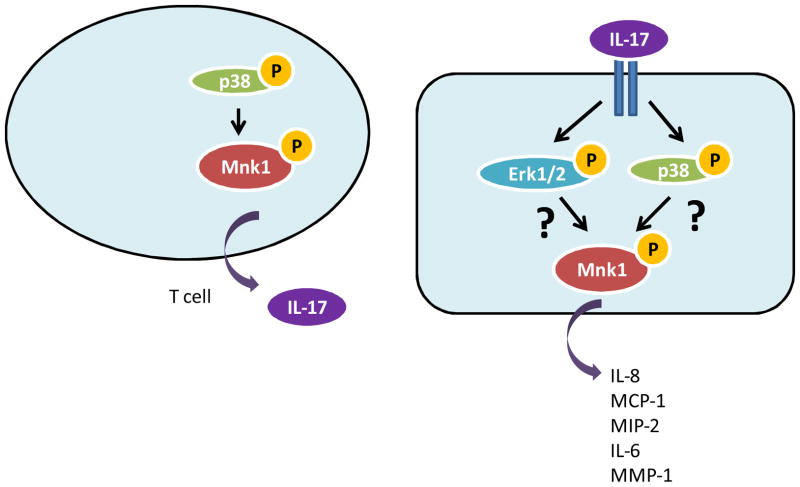
Figure 3. Role of Mnk kinases in IL-17 production and IL-17 signaling.
The differentiation of CD4+ T cells into Th17 helper cells results in the activation of p38 MAPK and its downstream targets Mnk1 and eIF4E, and facilitates post transcriptional regulation of IL-17. IL-17 binding to its receptor results in the engagement of the Erk and p38 MAPK pathways which are essential for the production of IL-8, MCP-1, MIP-2, IL-6 and MMP-1. As Mnk kinases lie downstream of both Erk and p38 and are known to regulate production of IL-8, IL-6, MCP-1 in response to other external stimuli, the role of Mnk kinases in regulating IL-17 mediated cytokine expression needs to be further explored.
Mnk kinases are known to mediate the production of cytokines such as IL-6 [16], IL-8[19], IL-1β [19], MCP-1 [16] which are also induced by IL-17 signaling. Therefore, Mnk kinases may play a role in mediating the biological responses to IL-17 signaling. Considering the important role of enhanced IL-17 production or enhanced IL-17 signaling in various diseases such as asthma [73], psoriasis [74], atopic dermatitis [75], inflammatory bowel disease, rheumatoid arthritis, autoimmune iritis, and central nervous system autoimmune syndromes [76]; it will be important and clinically relevant to further explore the role of Mnk kinases in IL-17 production and IL-17 signaling.
Mnk kinases in cytokine signaling
Various cytokines such as TNFα, IL-1β and others engage the Mnk1/eIF4E pathway [77] indicating that the Mnk kinases may be important mediators of cytokine signaling and promote generation of biological responses by these cytokines. Below, we summarize the role of Mnk kinases in mediating cellular responses for type I and type II IFNs, IL-2, IL-15 and TGF-β.
Mnk kinases in Type I and II IFN signaling
Interferons (IFNs) exhibit antineoplastic and antiviral properties and modulate immune responses [78,79]. Type I IFNs include IFNα, IFNβ, IFNω, IFNδ, IFNε IFNκ, IFNτ and other IFNs characterized by their selective binding to a common cell surface receptor, the Type I IFN receptor (IFNR) [78,79]. Receptor-associated JAK kinases are then activated and control phosphorylation of signal transducers and activators of transcription (STAT) proteins [78,79]. This results in formation and nuclear translocation of different DNA binding complexes important for transcription including the ISGF3 (IFN stimulated gene factor 3) complex consisting of phosphorylated STAT1, STAT2 and IRF-9, which regulates transcription via binding to ISREs (IFN stimulated response elements) [78,79]. IFNγ is the only known type II IFN, and it mediates its effects by binding to a cell surface receptor consisting of IFNGR1 and IFNGR2 chains which are associated with JAK1 and JAK2, respectively [78,79]. IFNγ-activated JAKs subsequently phosphorylate STATs that form either homodimers or heterodimers and activate transcription by binding to GAS (IFNγ activated sequences) sequences in target gene promoters [78,79].
Besides the JAK-STAT pathway, MAPK pathways play important roles in mediating the biological effects of both type I and type II IFNs. Engagemnet of the Type I IFNR results in activation of p38 MAPK in a Rac1- and MKK6-dependent manner [80–82], and such activation is ultimately required for optimal Type I IFN-mediated gene transcription and generation of the biological effects of Type I IFNs [82–85]. In the case of type II IFNs, engagement of the p38 MAPK is not required for transcription via GAS elements [86] but is essential for mediating its anti-proliferative responses on primitive hematopoietic cells [87].
Type I and type II IFNs have also been shown to engage the Erk MAPK to mediate their biological effects [88,89]. Interestingly, in the case of Type I IFNs, engagement of Erk is important for transcription-independent IFNα induced apoptosis [90], while activation of Erk can negatively regulate the anti-proliferative effects of IFNα on CD4+ T cells [91] as well as in human myeloma cell lines [92]. Additionally a downstream Erk target, RSK1 has been shown to regulate eIF4B phosphorylation in certain hematopoietic cell types and plays an important role in the control of IFN-induced mRNA translation and IFNα antileukemic responses [93]. In the case of IFNγ, Erk regulates IFNγ-dependent proteosomal degradatation of PPARδ [94] and enhances IFNγ regulated transcription by CCAAT-enhancer binding protein-β (C/CEBP-β) [88]. In addition, inhibition of Erk1 can partially reverse the antiproliferative effects of IFNγ on oligodendroglial progenitor cells [95].
As Mnk kinases are known effectors of both the p38 and Erk MAPK cascades, studies in our laboratory examined their potential involvement in IFN signaling. When U937 human myeloid leukemia cells or U266 human myeloma cells were treated with IFNα or IFNβ, we observed an increase in the phosphorylation of Mnk1 and its downstream target eIF4E [96]. Studies involving pharmacological inhibition of Mnk kinases or studies in mouse embryonic fibroblasts (MEFs) lacking both Mnk1 and Mnk2 demonstrated that the phosphorylation of eIF4E on Ser209 by type I IFNs is controlled by Mnk kinases [96]. In experiments employing pharmacological inhibition of p38 MAPK or p38α knockout MEFs we found that type I IFN- mediated engagement of Mnk1/eIF4E is p38 MAPK-independent [96]. On the other hand the engagement of Mnk1/eIF4E by type I IFNs was found to be MEK1/Erk dependent [96]. In further studies, it was established that pharmacological inhibition of Mnk kinases attenuates IFNα stimulated expression of ISG15, an ISG known to play roles in mediating the biological effects of type I IFNs [97]. IFNα-induced ISG15 expression was attenuated in MEFs with targeted disruption of either Mnk1 or Mnk2 or both Mnk1 and Mnk2 [96]. Transcriptional mRNA induction of ISG15 by IFNα was unaltered in MEFs with a targeted disruption of both Mnk1 and Mnk2; consistent with these results, Mnk kinases did not mediate IFNα induced phosphorylation of STAT1 [96]. Analysis of polysomal mRNA in the presence or absence of IFNα revealed that Mnk kinases are essential for the optimal translation of ISG15 as well as ISG54; and that such Mnk mediated translation of ISGs is essential for generation of the antiproliferative effects of IFNα on leukemic cell lines and primary hematopoietic progenitors derived from normal donors [96]. Thus ours results indicate an important role for Mnk1 in mediating biological responses to type I IFNs.
We also determined the role of the Mnk kinases in mediating cellular responses to IFNγ. Treatment of U937 cells with IFNγ was found to result in phosphorylation of Mnk1 and its downstream target eIF4E in an Erk-dependent manner [98]. IFNγ-induced IRF-1 protein expression was attenuated in the absence of Mnk expression or activity [98]. Analysis of polysomal mRNA revealed a requirement for both Mnk1 and Mnk2 in mRNA translation of IRF-1 [98]. Inhibition of Mnk activity or siRNA mediated knockdown of both Mnk1 and Mnk2 was found to partially abrogate the anti-proliferative effects of IFNγ on U937 cells and normal hematopoietic progenitors [98].
Both Type I and II IFNs have been shown to exert potent anti-tumorogenic effects [78,79] and therefore a better understanding of the signaling pathways involved in mediating such anti-proliferative responses could potentially allow optimization of their use in clinical medicine. The role of the Mnk kinases in mediating cellular responses to Type I and Type II IFNs is summarized in Figure 4. Our results indicate an important role for the Mnk kinases in the generation of the biological effects of IFNs, but the downstream mechanisms remain to be elucidated. The initiation factor eIF4E is an obvious candidate for future studies but other Mnk substrates such as Sprouty 2 [99], hnRNPA1 [34] and PSF [36] may also be important for IFN- responses. Notably, eIF4E has been shown to play an important role in mediating mRNA nuclear export [100], independently of its role in translation, and future studies should address a potential involvement of eIF4E in the nuclear export of ISGs.
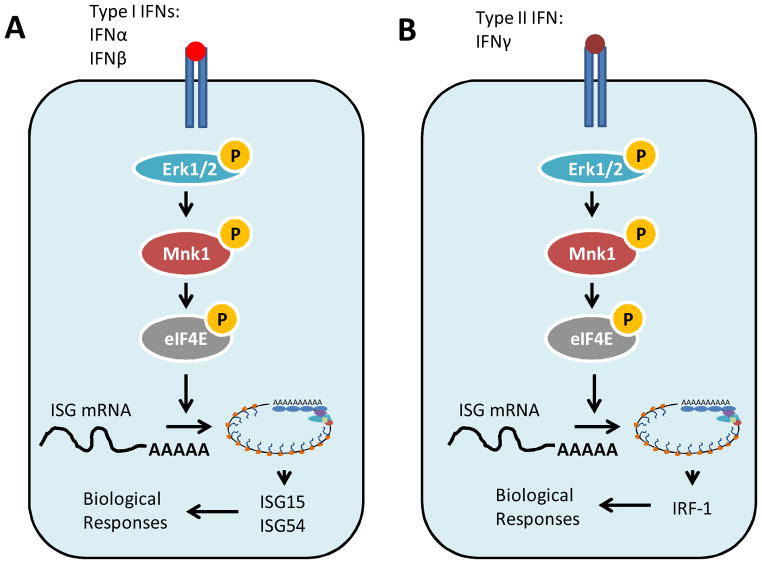
Figure 4. Role of Mnk kinases in Type I and type II IFN signaling.
(A) Type I IFN binding to the Type I IFNR leads to the engagement of Erk and its downstream targets Mnk1 leading to the phosphorylation of eIF4E. IFN-mediated engagement of the JAK-STAT pathway results in ISGF3-mediated transcriptional response and an increase in ISG15 and ISG54 mRNA. The engagement of Mnk1 by Type I IFNs is critical for optimal translation of ISG15 and ISG54 and plays an important role in mediating the anti-proliferative effects of type I IFNs. (B) Mnk1/eIF4E phosphorylation is induced via IFNγ mediated engagement of Erk. IFNγ mediated engagement of the JAK-STAT pathway results in IFNγ induced transcription of ISGs. The parallel engagement of Mnk1/eIF4E facilitates the optimal translation of IRF-1 which mediates the antiproliferative effects of IFNγ.
The Mnk kinases may also play roles in the production of IFNγ by NKT cells [18]. These observations combined with our results, raise the possibility of a positive feedback regulatory loop, in which Mnk kinases both regulate Type II IFN production and positively mediate Type II IFN responses. Therefore inhibition of the Mnk kinases in disorders, such as rheumatoid arthritis, multiple sclerosis, schizophrenia and other auto-immune skin disorders characterized by enhanced IFNγ production [101] may prove to have potent therapeutic implications in the future.
Mnk kinases in IL-2 and IL-15 Signaling
Interleukin-2 (IL-2) plays key roles in promoting T cell proliferation in response to mitogenic stimulation of T lymphocytes [102]. IL-15 was initially identified as a cytokine that could support the proliferation of an IL-2 dependent cell line in the presence of neutralizing antibodies against IL-2 [103,104]. Some similarities in the in vitro activities of IL-2 and IL-15 are mediated by a shared receptor subunit [105]; however studies in mice lacking IL-15 [106] or IL-2 [107] suggest that the two cytokines mediate distinct biological functions [108]. Similar to IL-2, IL-15 can mediate differentiation of NK cells as well as drive the proliferation of naïve T cells [108]. However while IL-2 plays a crucial role in the elimination of self-reactive T cells preventing auto-immunity [109], IL-15 is important for mediating the prolonged maintenance of CD8+ T cells [110] and can negatively regulate the anti-auto-immunity function of IL-2 [111]. Therefore a better understanding of the mechanisms regulating IL-2 and IL-15 signaling should have important implications in cancer immunotherapy as well as in the design of new approaches for the treatment of auto-immune diseases.
IL-2 and IL-15 signaling involves engagement of the JAK-STAT pathways [112,113]. Additionally IL-2 and IL-15 also engage the phosphatidylinositol-3-kinase–AKT pathway as well as the Ras/Raf/Erk and p38 MAPK pathways, which play important roles in mediating the biological effects of these cytokines [114–116]. Grund et al (2005), examined the role of Mnk1 in mediating IL-2 and IL-15 signaling in NK cells [117]. These investigators noted that mice with targeted disruption of IL-2Rβ, IL-2Rδ, IL-15 and Ets1 (Avian erythroblastosis virus E26 (v-ets) oncogene homolog-1) were all characterized by lack of NK cells, suggesting IL-2 and IL-15 regulate the expression of Ets-1 [117]. They also demonstrated that IL-2 and IL-15 treatment of NK cells results in an increase in Ets1 protein synthesis while Ets1 mRNA levels remain unchanged, suggesting post-transcriptional regulation of Ets1 mRNA by the two cytokines [117]. Studies involving inhibition of p38 MAPK, Akt and Erk MAPK revealed a role for Erk in mediating Ets1 protein synthesis in response to IL-2- or IL-15-treatment of cells [117]. IL-2 treatment of NK cells resulted in the phosphorylation of Mnk1/eIF4E in an Erk-dependent manner, while expression of a dominant negative Mnk1 construct attenuated IL-2 induced Ets-1 expression [117]. Altogether the results of this work suggest that IL-2 and IL-15 mediate NK cell proliferation by upregulating Ets-1 protein synthesis via the Erk/Mnk1/eIF4E pathway [117]. However this study did not directly address the role of Mnk1 in mediating NK cell proliferation and the data presented did not define whether Mnk1 mediated Ets-1 protein synthesis is regulated by increased translational efficiency or by controlling nuclear export or mRNA stability of Ets-1 mRNA. The Ets-1 transcription factor that been shown to induce a pro-inflammatory response [118] and promote the invasive behavior of endothelial cells, vascular smooth muscle cells and epithelial cancer cells [119]. Notably, Ets-1 hypomorphic mice exhibit enhanced B-cell activation and develop autoimmune disease [120]. Further research is required to understand the mechanism of Mnk1 mediated Ets-1 protein expression and such studies may ultimately prove to have therapeutic implications in cancer immunotherapy and in the prevention of autoimmune diseases.
Mnk kinases in TGF-β signaling
TGF-β is a cytokine that plays an important role in innate immunity and can regulate numerous cellular processes including hematopoiesis, cell proliferation and differentiation [121]. TGF-β can mediate an anti-inflammatory response by inhibiting T-cell proliferation and suppressing immune responses [122]. Binding of this cytokine to its cellular receptor leads to recruitment and phosphorylation of receptor-associated SMAD proteins which heterodimerize with co-SMADs, translocate to the nucleus and regulate the transcription of target genes by recruitment of co-activators or co-repressors (reviewed in [123]). Several studies have suggested an important role for the p38 MAPK and Erk pathways in the generation of TGF-β biological responses [124,125], raising the possibility that kinases downstream of these pathways may play important roles in TGF-β-signaling.
Grzmil and co-workers (2011) identified Mnk1 as a protein highly expressed in glioma cell lines as well as primary glioblastoma multiforme (GBM) [126]. Inhibition of Mnk kinases was found to suppress the growth of GBM cells and such suppression was enhanced by co-treatment of the cells with the mTOR inhibitor rapamycin [126]. As Mnk kinases are involved in translational regulation, the researchers conducted microarray analysis comparing mRNA expression in total and polysomal mRNA fractions in cells in the presence or absence of siRNA targeting Mnk1 [126]. Suppression of Mnk expression did not affect global mRNA translation but the mRNA translation of proteins involved in TGF-β signaling such as SMAD2, BMP8 as well as DP-1 were downregulated in Mnk1 knockdown cells [126]. Inhibition of Mnk activity or expression was found to suppress the TGF-β induced migratory ability of glioma cell lines in a wound healing assay [126]. Analysis of GBM patient samples revealed that Mnk1 overexpression correlates with elevated expression of the pro-migratory proteins vimentin and fibronectin and a decreased expression of the epithelial markers E-cadherin and tight junction protein 1 suggesting a role for Mnk1 in mediating TGF-β mediated transcriptional regulation [126]. Moreover, inhibition of SMAD2 phosphorylation by a specific inhibitor was found to exert anti-proliferative effects similar to the Mnk inhibitor indicating an important role for Mnk1-mediated SMAD2 upregulation in the generation of the pro-proliferative and pro-epithelial mesenchymal transition inducing effects of Mnk1 [126].
Expert Perspective
Mnk kinases represent a central node in the regulation of pro-inflammatory cytokines and are involved in signaling for IFNs, IL-2, IL-15 and TGFβ. As inflammation plays an important role in various human diseases, Mnk1 and Mnk2 are attractive candidates as therapeutic targets for autoimmune diseases, as well as anti-cancer agents. Notably, mice with a targeted disruption of either Mnk1 or Mnk2 or both Mnk1 and Mnk2 are characterized by a decrease or absence of eIF4E phosphorylation but otherwise exhibit no visible phenotype under unstressed conditions [8]. These observations suggest that clinical use of selective Mnk inhibitors may be feasible, possibly with limited toxicities in diseases or syndromes where the Mnk pathway is deregulated in affected cells (i.e. neoplastic cells). Many of the studies cited in this review used the Mnk inhibitor CGP57380 which targets both Mnk1 and Mnk2. CGP57380 is a low weight molecular compound that was identified from the Novartis Pharma compound collection by in vitro kinase assays [127] and the IC50 against Mnk1 is seen at a concentration of 2.2 μM [9]. Additionally CGP57380 inhibits casein kinase, MAP2K1 and BR serine/threonine-protein kinase 2 at concentrations comparable to those required for Mnk inhibition [128]. Therefore the results of studies solely based on that Mnk inhibitor should be interpreted with caution. Recently, Konicek et al. (2010) reported the identification of cercosporamide, an anti-fungal agent, as an inhibitor of Mnk kinases with a higher specificity [129]. Nevertheless, this compound exhibits higher specificity for Mnk2 as compared to Mnk1 [129]. Cercosporamide has been shown to exhibit anti-tumor effects in both in vitro and in vivo studies [129], suggesting that cercosporamide or similar compounds could ultimately progress to clinical trials and emerge as therapeutics for cancer and auto-immune diseases.
Most of Mnk mediated effects on translation reflect their ability to phosphorylate eIF4E; however Mnk substrates such as hnRNPA1, PSF may also play roles in mRNA translation or processing of their target mRNAs. Mnk kinases have been so also shown to phosphorylate Sprouty2 [99], a negative regulator of Erk phosphorylation [130]. Additionally it has also been suggested that the Mnk kinases mediate serine phosphorylation of cytosolic phospholipase A2 (cPLA2) in thrombin stimulated platelets, facilitating cPLA2-mediated arachidonate release [131]. cPLA2 can play an important role in regulating neutrophil responses by modulating the production of the platelet activating factor and other leukotrienes [132] and the potential involvement of the Mnk pathway in the process remains to be addressed in future studies.
Another area of potential translational relevance maybe the differences in function among members of the Mnk kinase family. Although Mnk1 and Mnk2 share a high degree of homology, there are differences in their regulation by MAPKs and in their basal kinase activity. Mnk1 can bind both p38 MAPK and Erk while Mnk2 can only bind Erk [7]. Engagement of Mnk kinases by external stimulu can be mediated by either p38 MAPK or Erk or both p38 MAPK and Erk [77]. As Mnk2 cannot bind p38 MAPK, it cannot be activated by stimuli that activate p38 MAPK. Therefore Mnk1 and Mnk2 may play distinct roles in mediating cytokine production and regulating cytokine responses. Such differences could be exploited by the development of specific inhibitors, selectively targeting Mnk1 or Mnk2. Experimental evidence also suggests the existence of alternative splicing of both Mnk1 and Mnk2. Mnk1 has two alternatively spliced isoforms Mnk1a and Mnk1b which differ at their carboxyl terminal end. The shorter Mnk1b isoform results from a lack of exon 19 resulting in a change in reading frame that introduces a premature stop codon [133]. Unlike Mnk1a, Mnk1b is also localized in the nuclear compartment, where it may regulate the phosphorylation of eIF4E and possibly other nuclear proteins [134]. Mnk1b exhibits higher basal activity as compared to Mnk1a possibly due to the lack of a C-terminal end containing negative regulatory elements [6]. Interestingly, Mnk1b lacks a MAPK domain and therefore cannot be activated by either p38 MAPK or Erk [6] suggesting regulation by other unknown mechanisms. As in the case of Mnk1, Mnk2 is also known to alternatively spliced into two isoforms Mnk2a and Mnk2b. The two isoforms differ in their carboxyl terminal ends due to an alternative exon 13 [5]. Mnk2b is shorter than Mnk2a, lacks a MAPK binding domain, exhibits low kinase activity towards eIF4E and is also localized in the nucleus [5]. Mnk2b is known to interact with the estrogen receptor (ER) β leading to the suggestion that Mnk2b mediated phosphorylation of ERβ may result in the transcriptional regulation of ER regulated genes [135]. Another study has shown that overexpression of the splicing factor SF2/ASF exerts oncogenic properties and preferentially mediates the expression of the Mnk2b isoforms [136].
Outlook
Various studies have focused on the role of the Mnk kinases and their downstream substrate eIF4E in regulating cancer progression by controlling mRNA translation or nuclear export of pro-oncogenic proteins [137,138]. However immune responses controlling the secretion of pro- and anti-inflammatory cytokines can be important mediators of oncogenesis as well as tumor progression (reviewed in [139]). The experimental results discussed in this review point towards an important role for Mnk kinases in the production of pro-inflammatory cytokines. Tumor cells are known to secrete a variety of cytokines attracting lymphocytes and modulating their responses, leading to a pro-inflammatory and a pro-tumorogenic state [139]. Mnk kinases mediate the production of IL-6 [16] which is an important regulator of tumor initiation and progression [140]. Other Mnk regulated cytokines such as IL-8 [141], IL-1β [142], MCP-1 [143], IL-17 [144], RANTES [145], TNFα [146] and IL-4 [147] have also been shown to positively regulating cancer progression, metastasis and chemo-resistance. Therefore inhibiting Mnk kinases can potentially block the production of multiple pro-tumirogenic cytokines and exert potent anticancer effects on multiple cancer types. Besides auto-immune diseases and cancer, pro-inflammatory cytokines are also known to play important roles in metabolic regulation in adipose tissue and expression of TNFα, IL-6, IL-1β is elevated during obesity promoting insulin resistance (reviewed in [148]). As there is a well established link between obesity and cancer (reviewed in [149]), future studies to examine the potential roles of Mnk kinases in the process may provide interesting and therapeutically valuable information. Pro-inflammatory cytokine release by innate immune cells plays an important role in the induction of immune responses [150] and in light of the important role of the Mnk kinases in mediating pro-inflammatory cytokine release, it will be important to examine the role of Mnk mediated cytokine release in future studies in vivo.
Highlights.
Mnk kinases play an important role in pro-inflammatory cytokine production.
Mnk-mediated cytokine production is controlled by post-transcriptional regulation of cytokine expressions (i.e. TNFα, IL-17) or by transcription factors regulating cytokine production (i.e. RFLAT-1).
Cytokine signaling regulation by Mnk kinases promotes mRNA translation of cytokine stimulated genes (such as type I and type II ISGs) or transcription factors involved in cytokine mediated gene expression (i.e. Ets-1, SMAD-2).
There is emerging evidence that targeting Mnk kinases may have potential therapeutic applications in auto-immune diseases and cancer.
Acknowledgments
The research work of LCP is supported by NIH grants CA155566, CA77816, CA121192, CA161796 and a Merit Review grant from the Department of Veterans affairs; SJ was a Malkin’s Scholar award recipient.
References
- 1.Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–33. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–20. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol. 1999;19:1871–80. doi: 10.1128/mcb.19.3.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheper GC, Morrice NA, Kleijn M, Proud CG. The mitogen-activated protein kinase signal-integrating kinase Mnk2 is a eukaryotic initiation factor 4E kinase with high levels of basal activity in mammalian cells. Mol Cell Biol. 2001;21:743–54. doi: 10.1128/MCB.21.3.743-754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheper GC, Parra JL, Wilson M, Van Kollenburg B, Vertegaal AC, Han ZG, Proud CG. The N and C termini of the splice variants of the human mitogen-activated protein kinase-interacting kinase Mnk2 determine activity and localization. Mol Cell Biol. 2003;23:5692–705. doi: 10.1128/MCB.23.16.5692-5705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goto S, Yao Z, Proud CG. The C-terminal domain of Mnk1a plays a dual role in tightly regulating its activity. Biochem J. 2009;423:279–90. doi: 10.1042/BJ20090228. [DOI] [PubMed] [Google Scholar]
- 7.Parra JL, Buxade M, Proud CG. Features of the catalytic domains and C termini of the MAPK signal-integrating kinases Mnk1 and Mnk2 determine their differing activities and regulatory properties. J Biol Chem. 2005;280:37623–33. doi: 10.1074/jbc.M508356200. [DOI] [PubMed] [Google Scholar]
- 8.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24:6539–49. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knauf U, Tschopp C, Gram H. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol Cell Biol. 2001;21:5500–11. doi: 10.1128/MCB.21.16.5500-5511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonenberg N, Dever TE. Eukaryotic translation initiation factors and regulators. Curr Opin Struct Biol. 2003;13:56–63. doi: 10.1016/s0959-440x(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 11.Scheper GC, van Kollenburg B, Hu J, Luo Y, Goss DJ, Proud CG. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J Biol Chem. 2002;277:3303–9. doi: 10.1074/jbc.M103607200. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011;118:9–18. doi: 10.1182/blood-2010-08-265892. [DOI] [PubMed] [Google Scholar]
- 14.Banyer JL, Hamilton NH, Ramshaw IA, Ramsay AJ. Cytokines in innate and adaptive immunity. Rev Immunogenet. 2000;2:359–73. [PubMed] [Google Scholar]
- 15.Zhang YL, Dong C. MAP kinases in immune responses. Cell Mol Immunol. 2005;2:20–7. [PubMed] [Google Scholar]
- 16.Rowlett RM, et al. MNK kinases regulate multiple TLR pathways and innate proinflammatory cytokines in macrophages. Am J Physiol Gastrointest Liver Physiol. 2008;294:G452–9. doi: 10.1152/ajpgi.00077.2007. [DOI] [PubMed] [Google Scholar]
- 17.Johansen C, Kjellerup RB, Kragballe K, Iversen L. Pro-inflammatory cytokine release in keratinocytes is mediated through the MAPK signal-integrating kinases. Experimental Dermatology. 2008;17:498–504. doi: 10.1111/j.1600-0625.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 18.Nagaleekar VK, et al. Translational control of NKT cell cytokine production by p38 MAPK. J Immunol. 2011;186:4140–6. doi: 10.4049/jimmunol.1002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherla RP, Lee SY, Mees PL, Tesh VL. Shiga toxin 1-induced cytokine production is mediated by MAP kinase pathways and translation initiation factor eIF4E in the macrophage-like THP-1 cell line. J Leukoc Biol. 2006;79:397–407. doi: 10.1189/jlb.0605313. [DOI] [PubMed] [Google Scholar]
- 20.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–71. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 22.Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ, 3rd, Zentella A, Albert JD, Shires GT, Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–4. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 23.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–4. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 24.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 25.Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–41. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 27.Brynskov J, Foegh P, Pedersen G, Ellervik C, Kirkegaard T, Bingham A, Saermark T. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut. 2002;51:37–43. doi: 10.1136/gut.51.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruys V, Kemmer K, Shakhov A, Jongeneel V, Beutler B. Constitutive activity of the tumor necrosis factor promoter is canceled by the 3′ untranslated region in nonmacrophage cell lines; a trans-dominant factor overcomes this suppressive effect. Proc Natl Acad Sci U S A. 1992;89:673–7. doi: 10.1073/pnas.89.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000 Dec 22;103(7):1071–83. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 30.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 31.Han J, Brown T, Beutler B. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J Exp Med. 1990;171:465–75. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoareau L, et al. Signaling pathways involved in LPS induced TNFalpha production in human adipocytes. J Inflamm (Lond) 2010;7:1. doi: 10.1186/1476-9255-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meldrum KK, et al. p38 MAPK mediates renal tubular cell TNF-alpha production and TNF-alpha-dependent apoptosis during simulated ischemia. Am J Physiol Cell Physiol. 2001;281:C563–70. doi: 10.1152/ajpcell.2001.281.2.C563. [DOI] [PubMed] [Google Scholar]
- 34.Buxade M, et al. The Mnks are novel components in the control of TNF alpha biosynthesis and phosphorylate and regulate hnRNP A1. Immunity. 2005;23:177–89. doi: 10.1016/j.immuni.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Gillis P, Malter JS. The adenosine-uridine binding factor recognizes the AU-rich elements of cytokine, lymphokine, and oncogene mRNAs. J Biol Chem. 1991;266:3172–7. [PubMed] [Google Scholar]
- 36.Buxade M, Morrice N, Krebs DL, Proud CG. The PSF.p54nrb complex is a novel Mnk substrate that binds the mRNA for tumor necrosis factor alpha. J Biol Chem. 2008;283:57–65. doi: 10.1074/jbc.M705286200. [DOI] [PubMed] [Google Scholar]
- 37.Urban RJ, Bodenburg YH, Wood TG. NH2 terminus of PTB-associated splicing factor binds to the porcine P450scc IGF-I response element. Am J Physiol Endocrinol Metab. 2002;283:E423–7. doi: 10.1152/ajpendo.00057.2002. [DOI] [PubMed] [Google Scholar]
- 38.Santhanam AN, Bindewald E, Rajasekhar VK, Larsson O, Sonenberg N, Colburn NH, Shapiro BA. Role of 3′UTRs in the translation of mRNAs regulated by oncogenic eIF4E--a computational inference. PLoS One. 2009;4:e4868. doi: 10.1371/journal.pone.0004868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beretta L, Singer NG, Hinderer R, Gingras AC, Richardson B, Hanash SM, Sonenberg N. Differential regulation of translation and eIF4E phosphorylation during human thymocyte maturation. J Immunol. 1998;160:3269–73. [PubMed] [Google Scholar]
- 40.Emi Aikawa N, de Carvalho JF, Artur Almeida Silva C, Bonfa E. Immunogenicity of Anti-TNF-alpha agents in autoimmune diseases. Clin Rev Allergy Immunol. 2010;38:82–9. doi: 10.1007/s12016-009-8140-3. [DOI] [PubMed] [Google Scholar]
- 41.Schall TJ, Jongstra J, Dyer BJ, Jorgensen J, Clayberger C, Davis MM, Krensky AM. A Human T-Cell-Specific Molecule Is a Member of a New Gene Family. Journal of Immunology. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 42.Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–7. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 43.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–71. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 44.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+ mobilization, and enzyme release. J Immunol. 1996;156:322–7. [PubMed] [Google Scholar]
- 45.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–95. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, Brière F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998 Jul 20;188(2):373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 48.Song A, Chen YF, Thamatrakoln K, Storm TA, Krensky AM. RFLAT-1: a new zinc finger transcription factor that activates RANTES gene expression in T lymphocytes. Immunity. 1999;10:93–103. doi: 10.1016/s1074-7613(00)80010-2. [DOI] [PubMed] [Google Scholar]
- 49.Nikolcheva T, Pyronnet S, Chou SY, Sonenberg N, Song A, Clayberger C, Krensky AM. A translational rheostat for RFLAT-1 regulates RANTES expression in T lymphocytes. J Clin Invest. 2002;110:119–26. doi: 10.1172/JCI15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maruoka S, Hashimoto S, Gon Y, Takeshita I, Horie T. PAF-induced RANTES production by human airway smooth muscle cells requires both p38 MAP kinase and Erk. Am J Respir Crit Care Med. 2000;161:922–9. doi: 10.1164/ajrccm.161.3.9906059. [DOI] [PubMed] [Google Scholar]
- 51.Pazdrak K, Olszewska-Pazdrak B, Liu T, Takizawa R, Brasier AR, Garofalo RP, Casola A. MAPK activation is involved in posttranscriptional regulation of RSV-induced RANTES gene expression. Am J Physiol Lung Cell Mol Physiol. 2002;283:L364–72. doi: 10.1152/ajplung.00331.2001. [DOI] [PubMed] [Google Scholar]
- 52.Makino Y, Cook DN, Smithies O, Hwang OY, Neilson EG, Turka LA, Sato H, Wells AD, Danoff TM. Impaired T cell function in RANTES-deficient mice. Clin Immunol. 2002 Mar;102(3):302–9. doi: 10.1006/clim.2001.5178. [DOI] [PubMed] [Google Scholar]
- 53.Chihara J, et al. Elevation of the plasma level of RANTES during asthma attacks. J Allergy Clin Immunol. 1997;100:S52–5. doi: 10.1016/s0091-6749(97)70005-8. [DOI] [PubMed] [Google Scholar]
- 54.Makki RF, al Sharif F, Gonzalez-Gay MA, Garcia-Porrua C, Ollier WE, Hajeer AH. RANTES gene polymorphism in polymyalgia rheumatica, giant cell arteritis and rheumatoid arthritis. Clin Exp Rheumatol. 2000;18:391–3. [PubMed] [Google Scholar]
- 55.Gade-Andavolu R, Comings DE, MacMurray J, Vuthoori RK, Tourtellotte WW, Nagra RM, Cone LA. RANTES: a genetic risk marker for multiple sclerosis. Mult Scler. 2004;10:536–9. doi: 10.1191/1352458504ms1080oa. [DOI] [PubMed] [Google Scholar]
- 56.Lapteva N, Huang XF. CCL5 as an adjuvant for cancer immunotherapy. Expert Opin Biol Ther. 2010;10:725–33. doi: 10.1517/14712591003657128. [DOI] [PubMed] [Google Scholar]
- 57.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–56. [PubMed] [Google Scholar]
- 58.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- 59.Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol. 1999;162:494–502. [PubMed] [Google Scholar]
- 60.Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecque S. Interleukin-17. Int Rev Immunol. 1998;16:541–51. doi: 10.3109/08830189809043008. [DOI] [PubMed] [Google Scholar]
- 61.Van Kooten C, et al. Interleukin-17 activates human renal epithelial cells in vitro and is expressed during renal allograft rejection. J Am Soc Nephrol. 1998;9:1526–34. doi: 10.1681/ASN.V981526. [DOI] [PubMed] [Google Scholar]
- 62.Iyoda M, Shibata T, Kawaguchi M, Hizawa N, Yamaoka T, Kokubu F, Akizawa T. IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-alpha and IL-1beta. Am J Physiol Renal Physiol. 2010;298:F779–87. doi: 10.1152/ajprenal.00198.2009. [DOI] [PubMed] [Google Scholar]
- 63.Laan M, Lotvall J, Chung KF, Linden A. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br J Pharmacol. 2001;133:200–6. doi: 10.1038/sj.bjp.0704063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witowski J, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000;165:5814–21. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 65.Fridman WH, Tartour E. Macrophage- and lymphocyte-produced Th1 and Th2 cytokines in the tumour microenvironment. Res Immunol. 1998;149:651–3. doi: 10.1016/s0923-2494(99)80033-9. [DOI] [PubMed] [Google Scholar]
- 66.Hoshino H, Lotvall J, Skoogh BE, Linden A. Neutrophil recruitment by interleukin-17 into rat airways in vivo. Role of tachykinins. Am J Respir Crit Care Med. 1999;159:1423–8. doi: 10.1164/ajrccm.159.5.9806008. [DOI] [PubMed] [Google Scholar]
- 67.Brereton CF, Sutton CE, Lalor SJ, Lavelle EC, Mills KH. Inhibition of ERK MAPK suppresses IL-23- and IL-1-driven IL-17 production and attenuates autoimmune disease. J Immunol. 2009;183:1715–23. doi: 10.4049/jimmunol.0803851. [DOI] [PubMed] [Google Scholar]
- 68.Noubade R, et al. Activation of P38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood. 2011 doi: 10.1182/blood-2011-02-336552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henness S, van Thoor E, Ge Q, Armour CL, Hughes JM, Ammit AJ. IL-17A acts via p38 MAPK to increase stability of TNF-alpha-induced IL-8 mRNA in human ASM. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1283–90. doi: 10.1152/ajplung.00367.2005. [DOI] [PubMed] [Google Scholar]
- 70.Hashmi S, Zeng QT. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron Artery Dis. 2006;17:699–706. doi: 10.1097/01.mca.0000236288.94553.b4. [DOI] [PubMed] [Google Scholar]
- 71.Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, Barnes JL, Chandrasekar B. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta , NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol. 2007;293:H3356–65. doi: 10.1152/ajpheart.00928.2007. [DOI] [PubMed] [Google Scholar]
- 72.Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem. 2007;282:27229–38. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawaguchi M, Kokubu F, Fujita J, Huang SK, Hizawa N. Role of interleukin-17F in asthma. Inflamm Allergy Drug Targets. 2009;8:383–9. doi: 10.2174/1871528110908050383. [DOI] [PubMed] [Google Scholar]
- 74.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–9. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 75.Toda M, Leung DY, Molet S, Boguniewicz M, Taha R, Christodoulopoulos P, Fukuda T, Elias JA, Hamid QA. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003;111(4):875–81. doi: 10.1067/mai.2003.1414. [DOI] [PubMed] [Google Scholar]
- 76.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 77.Proud CG, Wang XM, Flynn A, Waskiewicz AJ, Webb BLJ, Vries RG, Baines IA, Cooper JA. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. Journal of Biological Chemistry. 1998;273:9373–9377. doi: 10.1074/jbc.273.16.9373. [DOI] [PubMed] [Google Scholar]
- 78.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 79.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–90. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uddin S, et al. Activation of the p38 mitogen-activated protein kinase by type I interferons. J Biol Chem. 1999;274:30127–31. doi: 10.1074/jbc.274.42.30127. [DOI] [PubMed] [Google Scholar]
- 81.Li Y, et al. Activation of mitogen-activated protein kinase kinase (MKK) 3 and MKK6 by type I interferons. J Biol Chem. 2005;280:10001–10. doi: 10.1074/jbc.M410972200. [DOI] [PubMed] [Google Scholar]
- 82.Uddin S, et al. The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon alpha-dependent transcriptional activation but not serine phosphorylation of Stat proteins. J Biol Chem. 2000;275:27634–40. doi: 10.1074/jbc.M003170200. [DOI] [PubMed] [Google Scholar]
- 83.Ishida H, Ohkawa K, Hosui A, Hiramatsu N, Kanto T, Ueda K, Takehara T, Hayashi N. Involvement of p38 signaling pathway in interferon-alpha-mediated antiviral activity toward hepatitis C virus. Biochem Biophys Res Commun. 2004;321:722–7. doi: 10.1016/j.bbrc.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 84.Mayer IA, et al. The p38 MAPK pathway mediates the growth inhibitory effects of interferon-alpha in BCR-ABL-expressing cells. J Biol Chem. 2001;276:28570–7. doi: 10.1074/jbc.M011685200. [DOI] [PubMed] [Google Scholar]
- 85.Verma A, Deb DK, Sassano A, Uddin S, Varga J, Wickrema A, Platanias LC. Activation of the p38 mitogen-activated protein kinase mediates the suppressive effects of type I interferons and transforming growth factor-beta on normal hematopoiesis. J Biol Chem. 2002;277:7726–35. doi: 10.1074/jbc.M106640200. [DOI] [PubMed] [Google Scholar]
- 86.Li Y, et al. Role of p38alpha Map kinase in Type I interferon signaling. J Biol Chem. 2004;279:970–9. doi: 10.1074/jbc.M309927200. [DOI] [PubMed] [Google Scholar]
- 87.Verma A, et al. Cutting edge: activation of the p38 mitogen-activated protein kinase signaling pathway mediates cytokine-induced hemopoietic suppression in aplastic anemia. J Immunol. 2002;168:5984–8. doi: 10.4049/jimmunol.168.12.5984. [DOI] [PubMed] [Google Scholar]
- 88.Hu J, Roy SK, Shapiro PS, Rodig SR, Reddy SP, Platanias LC, Schreiber RD, Kalvakolanu DV. ERK1 and ERK2 activate CCAAAT/enhancer-binding protein-beta-dependent gene transcription in response to interferon-gamma. J Biol Chem. 2001;276:287–97. doi: 10.1074/jbc.M004885200. [DOI] [PubMed] [Google Scholar]
- 89.David M, Petricoin E, 3rd, Benjamin C, Pine R, Weber MJ, Larner AC. Requirement for MAP kinase (ERK2) activity in interferon alpha- and interferon beta-stimulated gene expression through STAT proteins. Science. 1995;269:1721–3. doi: 10.1126/science.7569900. [DOI] [PubMed] [Google Scholar]
- 90.Panaretakis T, Hjortsberg L, Tamm KP, Bjorklund AC, Joseph B, Grander D. Interferon alpha induces nucleus-independent apoptosis by activating extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase downstream of phosphatidylinositol 3-kinase and mammalian target of rapamycin. Mol Biol Cell. 2008;19:41–50. doi: 10.1091/mbc.E07-04-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romerio F, Zella D. MEK and ERK inhibitors enhance the anti-proliferative effect of interferon-alpha2b. FASEB J. 2002;16:1680–2. doi: 10.1096/fj.02-0120fje. [DOI] [PubMed] [Google Scholar]
- 92.Song L, Li Y, Shen B. Protein kinase ERK contributes to differential responsiveness of human myeloma cell lines to IFNalpha. Cancer Cell Int. 2002;2:9. doi: 10.1186/1475-2867-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kroczynska B, Kaur S, Katsoulidis E, Majchrzak-Kita B, Sassano A, Kozma SC, Fish EN, Platanias LC. Interferon-dependent engagement of eukaryotic initiation factor 4B via S6 kinase (S6K)- and ribosomal protein S6K-mediated signals. Mol Cell Biol. 2009;29:2865–75. doi: 10.1128/MCB.01537-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–8. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 95.Horiuchi M, Itoh A, Pleasure D, Itoh T. MEK-ERK signaling is involved in interferon-gamma-induced death of oligodendroglial progenitor cells. J Biol Chem. 2006;281:20095–106. doi: 10.1074/jbc.M603179200. [DOI] [PubMed] [Google Scholar]
- 96.Joshi S, Kaur S, Redig AJ, Goldsborough K, David K, Ueda T, Watanabe-Fukunaga R, Baker DP, Fish EN, Fukunaga R, Platanias LC. Type I interferon (IFN)-dependent activation of Mnk1 and its role in the generation of growth inhibitory responses. Proc Natl Acad Sci U S A. 2009;106:12097–102. doi: 10.1073/pnas.0900562106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ritchie KJ, Zhang DE. ISG15: the immunological kin of ubiquitin. Semin Cell Dev Biol. 2004;15:237–46. doi: 10.1016/j.semcdb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Joshi S, et al. Essential role for Mnk kinases in type II interferon (IFNgamma) signaling and its suppressive effects on normal hematopoiesis. J Biol Chem. 2011;286:6017–26. doi: 10.1074/jbc.M110.197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DaSilva J, Xu L, Kim HJ, Miller WT, Bar-Sagi D. Regulation of sprouty stability by Mnk1-dependent phosphorylation. Mol Cell Biol. 2006;26:1898–907. doi: 10.1128/MCB.26.5.1898-1907.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. J Cell Biol. 2005;169:245–56. doi: 10.1083/jcb.200501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skurkovich B, Skurkovich S. Anti-interferon-gamma antibodies in the treatment of autoimmune diseases. Curr Opin Mol Ther. 2003;5:52–7. [PubMed] [Google Scholar]
- 102.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–8. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 103.Bamford RN, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91:4940–4. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, Johnson L, Aldersom MR, Watson JD, Anderson DM, Giri JG. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 105.Carson WE, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–61. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 108.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–83. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 109.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6:1071–2. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 110.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 111.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:11445–50. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–10. [PubMed] [Google Scholar]
- 113.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 114.Yu TK, Caudell EG, Smid C, Grimm EA. IL-2 activation of NK cells: involvement of MKK1/2/ERK but not p38 kinase pathway. J Immunol. 2000;164:6244–51. doi: 10.4049/jimmunol.164.12.6244. [DOI] [PubMed] [Google Scholar]
- 115.Yano S, Komine M, Fujimoto M, Okochi H, Tamaki K. Interleukin 15 induces the signals of epidermal proliferation through ERK and PI 3-kinase in a human epidermal keratinocyte cell line, HaCaT. Biochem Biophys Res Commun. 2003;301:841–7. doi: 10.1016/s0006-291x(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 116.Gomez-Nicola D, Valle-Argos B, Nieto-Sampedro M. Blockade of IL-15 activity inhibits microglial activation through the NFkappaB, p38, and ERK1/2 pathways, reducing cytokine and chemokine release. Glia. 2010;58:264–76. doi: 10.1002/glia.20920. [DOI] [PubMed] [Google Scholar]
- 117.Grund EM, Spyropoulos DD, Watson DK, Muise-Helmericks RC. Interleukins 2 and 15 regulate Ets1 expression via ERK1/2 and MNK1 in human natural killer cells. J Biol Chem. 2005;280:4772–8. doi: 10.1074/jbc.M408356200. [DOI] [PubMed] [Google Scholar]
- 118.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115:2508–16. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29. doi: 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang D, John SA, Clements JL, Percy DH, Barton KP, Garrett-Sinha LA. Ets-1 deficiency leads to altered B cell differentiation, hyperresponsiveness to TLR9 and autoimmune disease. Int Immunol. 2005;17:1179–91. doi: 10.1093/intimm/dxh295. [DOI] [PubMed] [Google Scholar]
- 121.de Visser KE, Kast WM. Effects of TGF-beta on the immune system: implications for cancer immunotherapy. Leukemia. 1999;13:1188–99. doi: 10.1038/sj.leu.2401477. [DOI] [PubMed] [Google Scholar]
- 122.Wahl SM. Transforming growth factor beta (TGF-beta) in inflammation: a cause and a cure. J Clin Immunol. 1992;12:61–74. doi: 10.1007/BF00918135. [DOI] [PubMed] [Google Scholar]
- 123.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 124.Burch ML, Yang SN, Ballinger ML, Getachew R, Osman N, Little PJ. TGF-beta stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad2. Cell Mol Life Sci. 2010;67:2077–90. doi: 10.1007/s00018-010-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fujita H, Omori S, Ishikura K, Hida M, Awazu M. ERK and p38 mediate high-glucose-induced hypertrophy and TGF-beta expression in renal tubular cells. Am J Physiol Renal Physiol. 2004;286:F120–6. doi: 10.1152/ajprenal.00351.2002. [DOI] [PubMed] [Google Scholar]
- 126.Grzmil M, Morin P, Jr, Lino MM, Merlo A, Frank S, Wang Y, Moncayo G, Hemmings BA. MAP kinase-interacting kinase 1 regulates SMAD2-dependent TGF-beta signaling pathway in human glioblastoma. Cancer Res. 2011;71:2392–402. doi: 10.1158/0008-5472.CAN-10-3112. [DOI] [PubMed] [Google Scholar]
- 127.Tschopp C, Knauf U, Brauchle M, Zurini M, Ramage P, Glueck D, New L, Han J, Gram H. Phosphorylation of eIF-4E on Ser 209 in response to mitogenic and inflammatory stimuli is faithfully detected by specific antibodies. Mol Cell Biol Res Commun. 2000;3:205–211. doi: 10.1006/mcbr.2000.0217. [DOI] [PubMed] [Google Scholar]
- 128.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Konicek BW, et al. Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res. 2011;71:1849–57. doi: 10.1158/0008-5472.CAN-10-3298. [DOI] [PubMed] [Google Scholar]
- 130.Yusoff P, Lao DH, Ong SH, Wong ES, Lim J, Lo TL, Leong HF, Fong CW, Guy GR. Sprouty2 inhibits the Ras/MAP kinase pathway by inhibiting the activation of Raf. J Biol Chem. 2002;277:3195–201. doi: 10.1074/jbc.M108368200. [DOI] [PubMed] [Google Scholar]
- 131.Hefner Y, et al. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. J Biol Chem. 2000;275:37542–51. doi: 10.1074/jbc.M003395200. [DOI] [PubMed] [Google Scholar]
- 132.Geijsen N, Dijkers PF, Lammers JJ, Koenderman L, Coffer PJ. Cytokine-mediated cPLA(2) phosphorylation is regulated by multiple MAPK family members. FEBS Lett. 2000;471:83–8. doi: 10.1016/s0014-5793(00)01373-9. [DOI] [PubMed] [Google Scholar]
- 133.O’Loghlen A, Gonzalez VM, Pineiro D, Perez-Morgado MI, Salinas M, Martin ME. Identification and molecular characterization of Mnk1b, a splice variant of human MAP kinase-interacting kinase Mnk1. Exp Cell Res. 2004;299:343–55. doi: 10.1016/j.yexcr.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 134.O’Loghlen A, Gonzalez VM, Jurado T, Salinas M, Martin ME. Characterization of the activity of human MAP kinase-interacting kinase Mnk1b. Biochim Biophys Acta. 2007;1773:1416–27. doi: 10.1016/j.bbamcr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 135.Slentz-Kesler K, Moore JT, Lombard M, Zhang J, Hollingsworth R, Weiner MP. Identification of the human Mnk2 gene (MKNK2) through protein interaction with estrogen receptor beta. Genomics. 2000;69:63–71. doi: 10.1006/geno.2000.6299. [DOI] [PubMed] [Google Scholar]
- 136.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–93. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bianchini A, Loiarro M, Bielli P, Busa R, Paronetto MP, Loreni F, Geremia R, Sette C. Phosphorylation of eIF4E by MNKs supports protein synthesis, cell cycle progression and proliferation in prostate cancer cells. Carcinogenesis. 2008;29:2279–88. doi: 10.1093/carcin/bgn221. [DOI] [PubMed] [Google Scholar]
- 138.Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, Lowe SW. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–7. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 142.Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892–902. doi: 10.1038/onc.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, Ochiai A. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276–84. doi: 10.1002/ijc.24378. [DOI] [PubMed] [Google Scholar]
- 144.Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, Jackson AM. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62:1093–102. [PubMed] [Google Scholar]
- 146.Suganuma M, Okabe S, Marino MW, Sakai A, Sueoka E, Fujiki H. Essential role of tumor necrosis factor alpha (TNF-alpha) in tumor promotion as revealed by TNF-alpha-deficient mice. Cancer Res. 1999;59:4516–8. [PubMed] [Google Scholar]
- 147.Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92:921–8. doi: 10.1038/sj.bjc.6602416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–56. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 149.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–78. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]