Abstract
The purpose of the present study was to evaluate the potential role of the 27-Kilodalton (KDa) antigen versus Fasciola gigantica adult worm regurge antigens in a DOT-Blot assay and to assess this assay as a practical tool for diagnosis fascioliasis in Egyptian patients. Fasciola gigantica antigen of an approximate molecular mass 27-(KDa) was obtained from adult worms by a simple elution SDS-PAGE. A Dot-Blot was developed comparatively to adult worm regurge antigens for the detection of specific antibodies from patients infected with F. gigantica in Egypt. Control sera were obtained from patients with other parasitic infections and healthy volunteers to assess the test and compare between the antigens. The sensitivity, specificity, positive and negative predictive values of Dot-Blot using the adult worm regurge were 80%, 90%, 94.1%, and 69.2% respectively, while those using 27-KDa were 100% which confirms the diagnostic potential of this antigen. All patients infected with Fasciola were positive, with cross reactivity reported with Schistosoma mansoni serum samples. This 27-KDa Dot-Blot assay showed to be a promising test which can be used for serodiagnosis of fascioliasis in Egyptian patients especially, those presenting with hepatic disease. It is specific, sensitive and easy to perform method for the rapid diagnosis particularly when more complex laboratory tests are unavailable.
Keywords: Fasciola gigantica, 27-KDa, elution SDS-PAGE, dot-blot
INTRODUCTION
Fascioliasis is a parasitic infection of great concern, not only because of its high prevalence and economic significance to the animal stock [1,2] but also because it appears as an emerging public health problem in many regions of the world [3]. It is estimated that 2.4 million people are infected worldwide and that the number of people at risk is more than 180 million [4]. Fasciola hepatica is predominating in temperate zones, while Fasciola gigantica is found mainly in tropical and subtropical regions of Asia and Africa [5]. Fascioliasis is an example of an emerging and re-emerging parasitic disease in many countries because of environmental changes as well as man-made modifications [6].
Diagnosis of fascioliasis by finding eggs in stool samples lacks sensitivity, as the eggs are absent in acute and ectopic stages and intermittently released in the chronic stage. Therefore, coprological and immunological methods have to be used to confirm suspected cases [7]. Specific antigens in the somatic extract and excretory-secretory (ES) product of F. gigantica adults were identified and characterized by SDS-PAGE and immunoblotting analysis [8]. An antigenic component from the adult ES product with an approximate molecular mass of 27 kDa was found to give a consistent reaction with human fascioliasis sera up to 100% sensitivity and 98% specificity [8].
The purpose of the present study was to isolate the 27 kDa antigen using a simple elution method to evaluate its potential role vs Fasciola gigantica adult worm regurge antigens in a dot-blot assay and to assess this assay as a practical tool for diagnosis of fascioliasis in Egyptian patients.
MATERIALS AND METHODS
Worm collection
Adult F. gigantica flukes were collected from the bile ducts of naturally infected cattle from Cairo abattoir, Egypt. About 37 flukes were used for the intestinal content preparation [9].
Collection of intestinal content as adult worm regurge
The antigens were obtained as described previously [10]. Briefly, worms were washed 3 times in 0.9 M NaCl at room temperature until the supernatant became clear. The supernatant was placed in a Petri dish with fresh cold 0.9 M NaCl and incubated at 4℃ for 45 min. The supernatant was discarded and the worms were then incubated at 37℃ for 30 min in 0.9 M NaCl. The supernatant with evident black intestinal content was collected. The collected material was centrifuged at 12,000 g for 30 min, and the supernatant was concentrated osmotically using dialysis membrane (Sigma-Aldrich, St. Louis, Missouri, USA) immersed in polyethylene glycol. The concentrated material was aliquoted and stored at -70℃ until use.
SDS-PAGE and elution of the 27 kDa antigen
SDS-PAGE was carried out as described previously [11]. SDS-PAGE in 12% gel was carried using F. gigantica adult worm regurge antigens with a concentration of (0.6 mg/ml) and the electrophoretic run took place using the prestained wide range Bio-Rad marker to detect precisely the desired 27 kDa band (Fig. 1). A comb containing 3 lanes was used where the marker was applied to the 1st lane, and the antigen to the 2nd and 3rd lanes. The sample compromised 300 µl of the antigen was added to 300 µl sample buffer from which only 40 µl were added to the 3rd lane, and the rest applied to the 2nd lane. After cutting the stacking gel, the 3rd lane, which was used as a reference to locate the position of the desired protein band on the gel in the 2nd lane, was cut gently and stained in Coomassie blue then destained. It was then compared with the marker. As the destain shrinks the gel, because of its acetic acid content, the gel was resized by putting in distilled H2O (dH2O)or elution buffer (0.01 g SDS + 5 ml Tris 6.8+ 5 ml dH2O) before being compared with the marker. In a clean Petri dish containing elution buffer with the marker, the unstained and the destained gel were put side to side. The band in the unstained gel of the molecular weight of 27 kDa was cut gently and precisely. Then, the excised gel containing the specific band from the unstained gel was minced into very small pieces and transferred to a clean eppendorf tube containing elution buffer, and the content was homogenized entirely using a clean homogenizer and vortexed. The eluted homogenized protein trapped in the gel pieces was introduced to the Nanosep Micro Centrifugal Devices (0.45 µl) (Pall Life Sciences, Port Washington, New York, USA) and the kit was applied [12]. The sample reservoir was discarded, and the filtrate receiver was capped and stored at -70℃ till use after measuring the protein content.
Fig. 1.
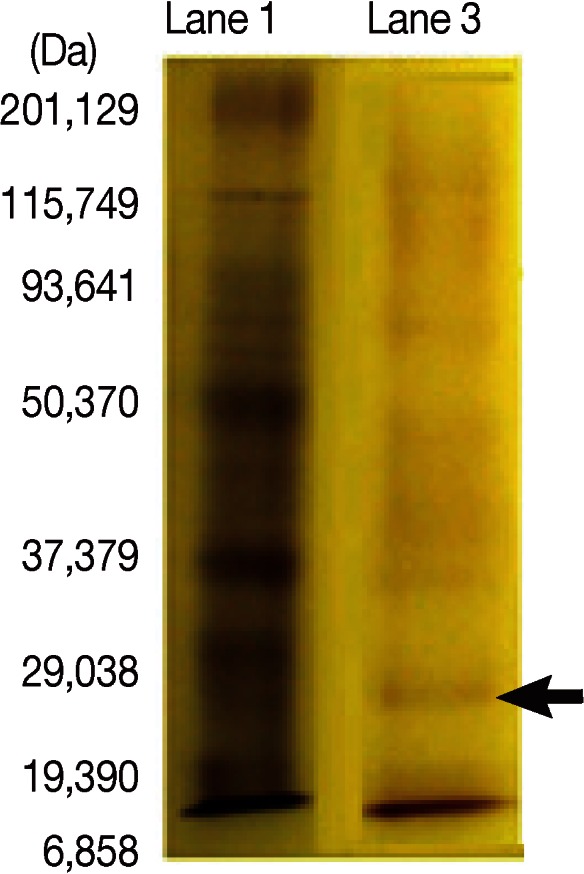
Characterization of 27-kDa band from Fasciola adult worm regurge separated by SDS-PAGE in 12% gel guided by wide range Bio-Rad marker. Lane 1. Molecular weight standard in kDa. Lane 3. Fractionated adult worm regurge antigens. Lanes are named according to the protocol of materials and methods. Arrow indicates 27 kDa band.
Determination of the protein content
Before antigen storage, the protein content was measured [13]. For the Fasciola adult worm regurge antigen, the protein concentration was 0.6 mg/ml, and for the 27 kDa band, the protein concentration was 0.06 mg/ml.
Collection of test and control sera
Serum samples required for study were separated from blood samples collected from cases referred to the Diagnostic and Research Unit, Parasitology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt. The study protocol was approved by the Scientific-Ethics Committee of Ain Shams University. Informed consent was obtained from the study subjects using standard guidelines. Whole blood was collected from each subject and centrifuged at 760 g at 4℃ for 10 min and the obtained serum samples were stored at -70℃ until use.
These serum samples were categorized into group I (20 patients with patent fascioliasis passing Fasciola eggs in their stool samples and negative for other parasitic infections), group II (10 patients negative for fascioliasis and positive for other parasitic infections by indirect hemagglutination test; IHAT) (3 cases of schistosomiasis mansoni, 3 hydatidosis, and 4 toxoplasmosis), and group III (normal controls that included 10 healthy individuals, free from fascioliasis by IHAT and other parasitic infections by stool examination).
Dot-blot assay
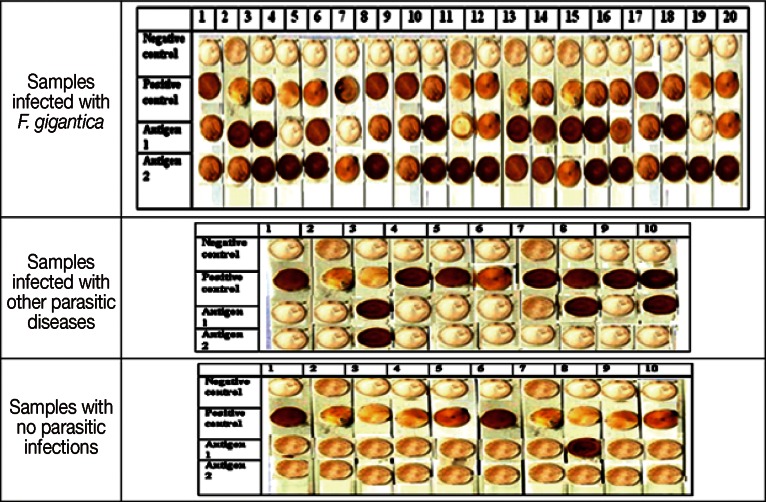
Dot-blot assay was applied with some modifications [14] comparatively using Fasciola adult worm regurge antigens and the eluted 27 kDa soluble antigen. A grid is drawn by a pencil on the nitrocellulose membrane (Bio-Rad, Hercules, California, USA) to indicate the region to be blotted. Two µl of antigen was spotted onto the nitrocellulose membrane at the center of the grid through minimizing the area that the solution penetrates (3-4 mm diameter) by applying it slowly. The membrane was dried. Non-specific sites were blocked by soaking in 5% skimmed milk in PBS-T (5 g milk + 100 ml PBS, pH 7.2 + 30 µl Tween 20) in a 10 cm Petri dish for reaction chamber, then incubated with primary antibody from serum samples diluted to 1:50 and dissolved in 5% skimmed milk in PBS-T for 1 hr at room temperature, washed 3 times with PBS-T 5min each. Then, it was incubated with the secondary antibody conjugated with peroxidase-labeled affinity purified antihuman IgG (KPL, Washington DC, Maryland, USA) and diluted to 1 µl: 1,000 µl in PBS-T followed by incubation 1 hr with shaking, washing 2 times with PBS-T 2 min each, and then once with PBS for 5 min. The colour reaction was developed with DAB (5 g from 3,3´-diaminobenzilidine dissolved in 100 ml dH2O and then aliquoted 500 µl in each tube)/H2O2 substrate solution (500 µl of DAB + 50 ml PBS+ 5 µl H2O2) for 2 min and stopped by several dH2O washing. Sera positive for F. gigantica infection showed purple dots on the membrane, while negative sera showed no dots.
Data management and analysis
The chi-square test was used to examine the relationship between 2 qualitative variables. Fisher's exact test was used to examine the relationship between 2 qualitative variables when the expected count was less than 5 in more than 20% of cells. McNemar test was used to assess the statistical significance of the difference between a qualitative variable measured twice for the same study group. The P-values set for the level of significance were P>0.05 (not significant; NS), P<0.05 (significant; S), and P<0.01 (highly significant; HS).
RESULTS
The results showed that, using the adult worm regurge antigen (antigen I) against serum of diseased patients in the dot-blot assay, 16 out of the 20 patients gave positive results with a sensitivity of 80%. Whereas, using the 27 KDa antigen (antigen II) in the dot-blot assay, all the diseased patients gave positive results with a sensitivity of 100% (Table 1).
Table 1.
Comparison between antigen I and antigen II as regard to sensitivity for disease diagnosis, specificity among positive controls, and specificity among negative controls with other parasitic diseases using dot-blot assay
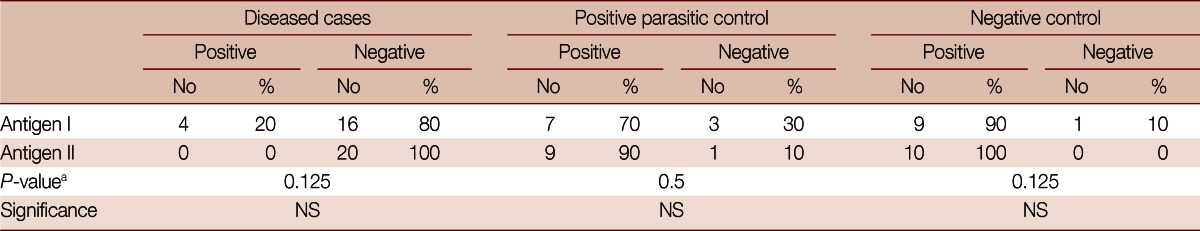
The sensitivity detected against Fasciola gigantica positive serum samples was 80% for antigen I compared to 100% for antigen II. The specificity detected against negative controls (no parasitic infection) was 90% for antigen I compared to 100% for antigen II. The specificity detected against other parasite positive samples was 70% for antigen I compared to 90% for antigen II.
aMcNemar test.
The dot-blot assay using antigen I among the negative control cases gave 9 negative results out of the 10 negative controls compared to 10 negative results using antigen II, showing a specificity of antigen I of 90% compared to 100% specificity using antigen II (Table 1). The results also showed that, using antigen I among positive controls (patients positive for other parasitic infections), 7 cases were negative with a specificity of 70% compared to 9 negative cases among 10 using antigen II with a specificity of 90% (Table 1).
The dot-blot assay done to compare between diseased patients and negative controls for antigen I showed that there was a highly significant difference between them as the test gave positive results in 80% of the diseased cases compared to only 10% in negative controls (Table 2). Comparison between diseased patients and negative controls for antigen II showed a highly significant difference between both groups as it gave positive results in 100% of diseased group compared to 0% in normal controls (Table 2).
Table 2.
Comparison of diseased cases to positive and to negative controls using antigen I and antigen II by dot-blot assay
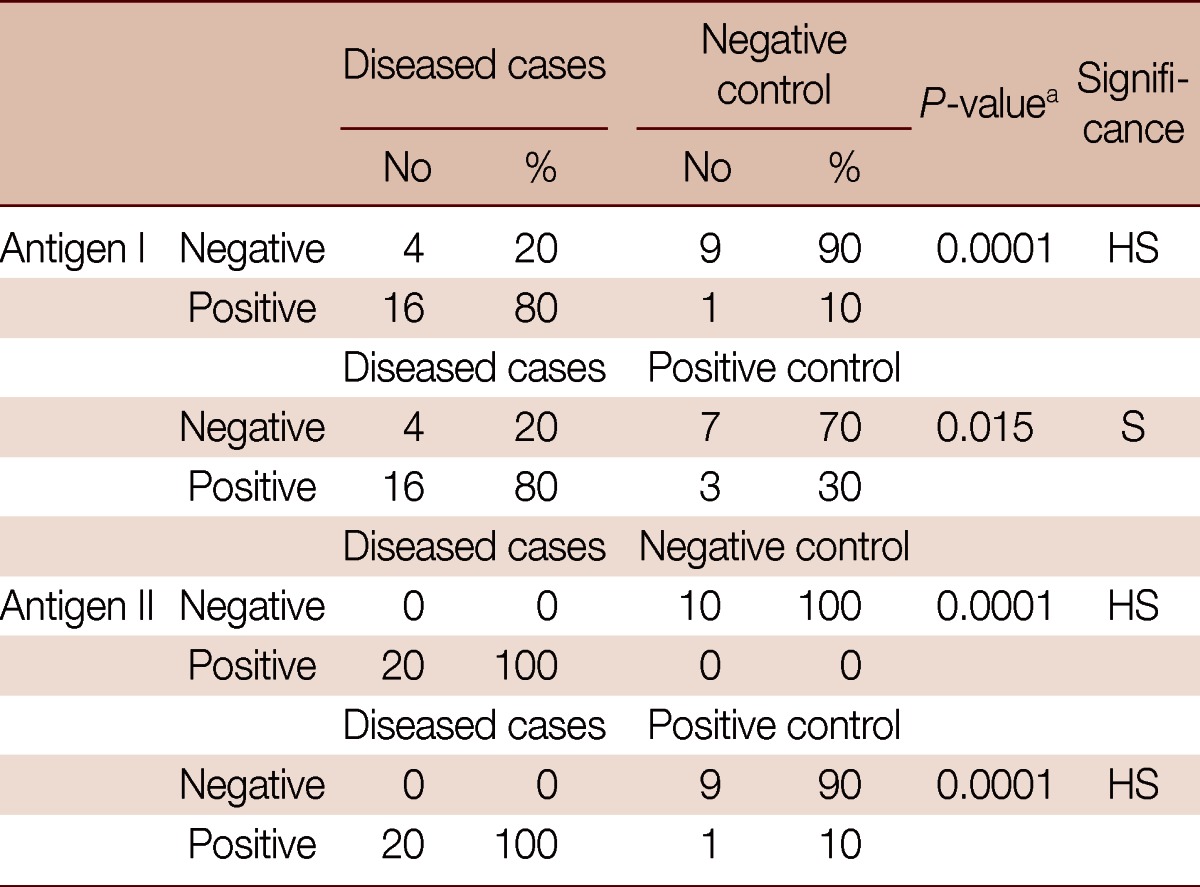
The comparison between diseased cases and negative controls for antigen I revealed 94.1% PPV (positive predictive value) and 69.2% NPV (negative predictive value). The comparison between diseased cases and negative controls for antigen II revealed 100% PPV and 100% NPV. The comparison between diseased cases and positive controls for antigen I revealed 84.2% (16/19) PPV and 63.6% (7/11) NPV. The comparison between diseased cases and positive controls for antigen II revealed 95.2% (20/21) PPV and 100% (9/9) NPV.
aFisher's exact test.
In comparison of diseased cases and positive controls with antigen I, the results showed a significant difference between both groups as it was positive in 80% in diseased cases compared to 30% in positive controls (Table 2). The assay done using antigen II to compare diseased cases and positive controls showed a highly significant difference between the 2 groups as it was positive in 100% of diseased cases compared to 10% in positive controls (Table 2).
DISCUSSION
In humans, fascioliasis is becoming an increasingly important health problem with cases reported from South America, Europe, Africa, Australia, and the Far East [6,15,16]. In endemic areas, clinical examination and imaging techniques are used for the diagnosis of fascioliasis, but these methods cannot always differentiate between fascioliasis and other causes of hepatitis [16]. Therefore, coprological and immunological methods have to be used to confirm suspected cases of fascioliasis. However, diagnosis by coprological examination is complicated by the long prepatent period of Fasciola spp., during which no eggs are shed, and by the fact that in humans not all flukes develop to adult egg-laying worms or flukes migrate to non-hepatic areas [7]. Moreover, Fasciola eggs may be found in the stools of uninfected persons who have eaten raw infected livers leading to false positive diagnosis. Many factors impose the idea of evaluating and establishing serological methods for the diagnosis of fascioliasis, which allow diagnosis of the disease even in the acute stage, before the parasite eggs can be identified in feces [17].
The immunological techniques offer an interesting option for the diagnosis of fascioliasis [18,19,20]. Among these tests, ELISA has been developed using ES antigens from adult flukes, purified antigens, or recombinant antigens [8,21,22]. An ELISA using the 27 kDa native protein isolated from ES products of Fasciola spp. has shown high sensitivity and specificity in the diagnosis of human fascioliasis [23]. Several groups have reported the purification of the immunodominant antigens from the ES products of the liver fluke [24,25]. Of these, only one group described the use of a purified 27 kDa cysteine proteinase from Japanese Fasciola spp. and its evaluation as an antigen in the diagnosis of human disease [25]. This proteinase demonstrated a high sensitivity and specificity in ELISA for human fascioliasis, showing only 1 case of cross-reactivity with sera from schistosomiasis japonicum patients. The purified fractions Fas 1 and Fas 2, which contain a cysteine proteinase of F. hepatica with an approximate molecular mass of 25-26 kDa, are specific and sensitive antigens [26].
In the present study, the dot-blot assay using F. gigantica adult worm regurge antigens (antigen I) vs the partially purified fractions of the antigens with an approximate molecular mass of 27 kDa (antigen II) obtained by continuous elution SDS-PAGE, were used in a trial to evaluate their potential role in the diagnosis of fascioliasis. The sensitivity, specificity, positive and negative predictive values using the adult worm regurge (antigen I) were 80%, 90%, 94.1%, and 69.2%, respectively (Table 2; Fig. 2),while those using 27 kDa (antigen II) were 100% (Table 2; Fig. 2),which confirmed the diagnostic potential of this antigen.
Fig. 2.
Dot-blots showing reactivity of 20 human samples infected with F. gigantica (1st figure), 10 human samples infected with other parasites (positive control samples); samples no.1, 2, 4 (hydatidosis by IHAT), samples no 3,8,10 (S. mansoni infection by IHAT), samples no 5, 6, 7, 9 (toxoplasmosis by IHAT) (2nd figure), and 10 human samples with no parasitic infections (negative controls) (3rd figure) against antigen I (adult worm regurge) and antigen II (27 kDa). Negative control lane shows negative kit guide for colour results, so if a same colour appears it is a negative result. Positive control lane shows positive kit guide for colour results, so if a same colour appears it is a positive result.
All patients infected with Fasciola were positive, with cross-reactivity reported only in 1 schistosomiasis mansoni serum sample. There was significant difference between diseased cases and positive controls in regard to the results of antigen I test, as it was positive in 80% of the diseased group compared to 30% in the positive controls; PPV=84.2% and NPV=63.6% (Table 2; Fig. 2). There was a highly significant difference between diseased cases and positive controls in regard to the results of antigen II test, as it was positive in 100% of the diseased group compared to 10% in positive controls; PPV=95.2% and NPV=100% (Table 2; Fig. 2).
The cross-reactivity with schistosomiasis was previously reported [27]. This cross-reactivity was explained by the presence of cross-reactive epitopes between Fasciola ES products and S. mansoni. There was a relatively high cross-reactivity (25.9%) with the 27 kDa protein ELISA in serum samples from cholangiocarcinoma patients in Thailand [8]. Those patients might have been infected with Opisthorchis viverrini in association with cholangiocarcinoma development [28]. In another study, 2 out of 3 serum samples from Clonorchis sinensis infections gave a false positive result confirming that cross reactions with this parasite should be taken into accounts [29]. However, in contrast to fascioliasis, clinical manifestations of clonorchiasis and opisthorchiasis occur mainly in the chronic phase of the infection and are usually associated with fecal egg excretion.
There were cross reactions between Fasciola crude worm antigen and patients with schistosomiasis mansoni, toxoplasmosis, and ascariasis by western blot technique [30]. Two normal human subjects showed also false reactivity. The presence of cross reactions in sera of normal individuals may be explained by previous subclinical infections with Fasciola or other parasites that share Fasciola in these cross reactive epitopes or may be due to the presence of a common antigenic band in Fasciola crude worm antigen. Other cross reactions were also detected with Trichinella spiralis and Echinococcus granulosus patients sera [31].
In our study, the dot-blot immunoassay was found to be sensitive and specific and valuable for the serodiagnosis of human fascioliasis. It is simple, rapid, and practical for use. It may be used as a positive or a negative test for the presence of proteins but not for determining concentrations. It also gives a long-term result, since the color on the nitrocellulose membrane does not fade. It is fast and cost-effective and can be more importantly used in the field survey. The good performance of the test should be confirmed by applying the test on a larger number of documented samples.
In conclusion, the 27 kDa dot-blot immunoassay showed to be a promising test which can be used for serodiagnosis of fascioliasis in Egyptian patients, especially, those presenting with hepatic diseases.
References
- 1.Schweizer G, Braun U, Deplazes P, Torgerson PR. Estimating the financial losses due to bovine fasciolosis in Switzerland. Vet Rec. 2005;157:188–193. doi: 10.1136/vr.157.7.188. [DOI] [PubMed] [Google Scholar]
- 2.Mungube EO, Bauni SM, Tenhagen BA, Wamae LW, Nginyi JM, Mugambi JM. The prevalence and economic significance of Fasciola gigantica and Stilesia hepatica in slaughtered animals in the semi-arid coastal Kenya. Trop Anim Health Prod. 2006;38:475–483. doi: 10.1007/s11250-006-4394-4. [DOI] [PubMed] [Google Scholar]
- 3.Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. Bull World Health Organ. 1999;77:340–346. [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organisation. Control of foodborne trematode infections. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1995;849:1–157. [PubMed] [Google Scholar]
- 5.Andrews SJ. The life cycle of Fasciola hepatica. In: Dalton JP, editor. Fasciolosis. Walling Ford, UK: CABI Publishing; 1999. pp. 1–20. [Google Scholar]
- 6.Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–1278. doi: 10.1016/j.ijpara.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Maleewong W, Wongkham C, Intapan PM, Pipitgool V. Fasciola gigantica-specific antigens: purification by a continuous-elution method and its evaluation for the diagnosis of human fascioliasis. Am J Trop Med Hyg. 1999;61:648–651. doi: 10.4269/ajtmh.1999.61.648. [DOI] [PubMed] [Google Scholar]
- 8.De Almeida MA, Ferreira MB, Planchart S, Terashima A, Maco V, Marcos L, Gotuzzo E, Sánchez E, Náquira C, Scorza JV, Incani RN. Preliminary antigenic characterisation of an adult worm vomit preparation of Fasciola hepatica by infected human sera. Rev Inst Med Trop Sao Paulo. 2007;49:31–35. doi: 10.1590/s0036-46652007000100006. [DOI] [PubMed] [Google Scholar]
- 9.Planchart S, Incani RN, Ballen D, Matos C, Cesari I. Preliminary biochemical characterization of a Schistosoma mansoni adult worm vomit (AWV) preparation and immunological specificity of a mouse anti-AWV monoclonal antibody; International symposium on schistosomiasis, 9th; Salvador: 2003. [Google Scholar]
- 10.Jeno P, Horst M. Electroelution of Proteins from Polyacrylamide Gels. In: Walker JM, editor. The Protein Protocols Handbook. 2nd ed. Totowa, New Jersey, USA: Humana Press; 2002. pp. 207–214. [Google Scholar]
- 11.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 13.Wright SF, Morton JB. Detection of vesicular-arbuscular mycorrhizal fungus colonization of roots by using a dot-immunoblot assay. Appl Environ Microbiol. 1989;55:761–763. doi: 10.1128/aem.55.3.761-763.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayu B, Asnake S, Woretaw A, Ali J, Gedefaw M, Fente T, Getachew A, Tsegaye S, Dagne T, Yitayew G. Cases of human fascioliasis in North-West Ethiopia. Ethiop J Health Dev. 2005;19:237–240. [Google Scholar]
- 15.Marcos L, Romani L, Florencio L, Terashima A, Canales M, Nestares J, Huayanay L, Gotuzzo E. Hyperendemic and mesoendemic zones of Fasciola infection surrounding urban Lima: an emerging disease? Rev Gastroenterol Peru. 2007;27:31–36. [PubMed] [Google Scholar]
- 16.Marcos LA, Tagle M, Terashima A, Bussalleu A, Ramirez C, Carrasco C, Valdez L, Huerta-Mercado J, Freedman DO, Vinetz JM, Gotuzzo E. Natural history, clinicoradiologic correlates, and response to triclabendazole in acute massive fascioliasis. Am J Trop Med Hyg. 2008;78:222–227. [PubMed] [Google Scholar]
- 17.Mas-Coma S, Bargues MD, Esteban JG. Human fasciolosis. In: Dalton JP, editor. Fasciolosis. Walling Ford, UK: CABI Publishing; 1999. pp. 411–433. [Google Scholar]
- 18.Rahimi MT, Ashrafi K, Koosha S, Abdi J, Rokni MB. Evaluation of Fast-ELISA versus standard-ELISA to diagnose human fasciolosis. Arch Iran Med. 2011;14:18–21. [PubMed] [Google Scholar]
- 19.Espino AM, Díaz A, Pérez A, Finlay CM. Dynamics of antigenemia and coproantigens during a human Fasciola hepatica outbreak. J Clin Microbiol. 1998;36:2723–2726. doi: 10.1128/jcm.36.9.2723-2726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almazán C, Avila G, Quiroz H, Ibarra F, Ochoa P. Effect of parasite burden on the detection of Fasciola hepatica antigens in sera and feces of experimentally infected sheep. Vet Parasitol. 2001;97:101–112. doi: 10.1016/s0304-4017(01)00376-4. [DOI] [PubMed] [Google Scholar]
- 21.Dixit AK, Yadav SC, Sharma RL. 28 kDa Fasciola gigantica cysteine proteinase in the diagnosis of prepatent ovine fasciolosis. Vet Parasitol. 2002;109:233–247. doi: 10.1016/s0304-4017(02)00202-9. [DOI] [PubMed] [Google Scholar]
- 22.Maleewong W, Intapan PM, Wongkham C, Tomanakan K, Daenseekaew W, Sukeepaisarnjaroen W. Comparison of adult somatic and excretory-secretory antigens in enzyme-linked immunosorbent assay for serodiagnosis of human infection with Fasciola gigantica. Southeast Asian J Trop Med Public Health. 1996;27:566–569. [PubMed] [Google Scholar]
- 23.Dalimi A, Hadighi R, Madani R. Partially purified fraction (PPF) antigen from adult Fasciola gigantica for the serodiagnosis of human fascioliasis using Dot-ELISA technique. Ann Saudi Med. 2004;24:18–20. doi: 10.5144/0256-4947.2004.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva E, Castro A, Lopes A, Rodrigues A, Dias C, Conceição A, Alonso J, Correia da Costa JM, Bastos M, Parra F, Moradas-Ferreira P, Silva M. A recombinant antigen recognized by Fasciola hepatica-infected hosts. J Parasitol. 2004;90:746–751. doi: 10.1645/GE-136R. [DOI] [PubMed] [Google Scholar]
- 25.Yamasaki H, Aoki T, Oya H. A cysteine proteinase from the liver fluke Fasciola spp.: purification, characterization, localization and application to immunodiagnosis. Jpn J Parasitol. 1989;38:373–384. [Google Scholar]
- 26.Cordova M, Herrera P, Nopo L, Bellatin J, Naquira C, Guerra H, Espinoza JR. Fasciola hepatica cysteine proteinases: immunodominant antigens in human fascioliasis. Am J Trop Med Hyg. 1997;57:660–666. doi: 10.4269/ajtmh.1997.57.660. [DOI] [PubMed] [Google Scholar]
- 27.Sampaio-Silva ML, Da Costa JM, Da Costa AM, Pires MA, Lopes SA, Castro AM, Monjour L. Antigenic components of excretory-secretory products of adult Fasciola hepatica recognized in human infections. Am J Trop Med Hyg. 1996;54:146–148. doi: 10.4269/ajtmh.1996.54.146. [DOI] [PubMed] [Google Scholar]
- 28.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen TG, Le TH, De NV, Doan TT, Dao TH, Vercruysse J, Dorny P. Assessment of a 27-kDa antigen in enzyme-linked immunosorbent assay for the diagnosis of fasciolosis in Vietnamese patients. Trop Med Int Health. 2010;15:462–467. doi: 10.1111/j.1365-3156.2010.02468.x. [DOI] [PubMed] [Google Scholar]
- 30.Farghaly AM, Nada SMM, Emam WA, Mattar MA, Mohamed SMA, Sharaf EM, El Gamal RLE. Role of fast-ELISA and western blot in diagnosis of human fascioliasis using crude adult worm and excretory/secretory Fasciola antigens. Parasitol United J. 2009;2:55–65. [Google Scholar]
- 31.Abdel-Rahman EH, Abdel-Megeed KN, Abuel-Ezz NM. Cross-reaction: a common trait among helminthes. J Egypt Soc Parasitol. 2003;33:457–471. [PubMed] [Google Scholar]