Abstract
Chronic diarrhea with a 35 kg weight loss (75 kg to 40 kg) occurred during 2 years in an alcoholic patient was diagnosed with Isospora belli infection in the Republic of Korea. The patient, a 70-year old Korean male, had been a heavy drinker for more than 30 years. He was admitted to the Seoul National University Hospital because of long-standing diarrhea and severe weight loss. He had an increased white blood cell (WBC) count with high peripheral blood eosinophilia (36.8-39.9%) and lowered protein and albumin levels but without any evidence of immunosuppression. A parasitic infection was suspected and fecal examination was repeated 3 times with negative results. Peroral endoscopy with mural biopsy was performed in the upper jejunum. The biopsy specimens revealed villous atrophy with loss of villi together with various life cycle stages of I. belli, including trophozoites, schizonts, merozoites, macrogamonts, and microgamonts. The patient was treated successfully with oral doses of trimethoprim 160-320 mg and sulfamethoxazole 800-1,600 mg daily for 4 weeks. A follow-up evaluation at 2.5 years later revealed marked improvement of body weight (68 kg), increased protein and albumin levels, and normal WBC count with low eosinophils (3.1%). This is the first clinical case of isoporiasis with demonstration of various parasitic stages in the Republic of Korea.
Keywords: Isospora belli, isosporiasis, case report, diarrhea, alcoholism
INTRODUCTION
Isospora spp. are obligate intracellular protozoans infecting a variety of vertebrate hosts [1]. In humans, 2 species, Isospora belli and Isospora natalensis, have been found [1]. However, almost all human infections are due to I. belli, and humans are the only known host for I. belli [2]. Human infection with I. natalensis was reported from South Africa in the early 1950s and there were no further reports [1]. I. belli infection has a worldwide distribution, but is relatively rare and in most cases occurs in the tropics and subtropics [1,3]. In immunocompetent patients, I. belli infection is frequently asymptomatic, but can cause acute or chronic enteritis with variable clinical symptoms, including diarrhea, steatorrhea, headache, fever, malaise, abdominal pain, vomiting, dehydration, and weight loss [3]. In patients with immunocompromised conditions, including AIDS, malignancies, and organ transplantations, isosporiasis is an important opportunistic pathogen, and the patients experience a more severe clinical course, even with extraintestinal dissemination of parasites [3-7].
In the Republic of Korea (hereafter Korea), human I. belli infection was first reported in 2 diarrheal patients in 1960, erroneously as Isospora hominis (since synonymized with Sarcocystis hominis), and oocysts each containing 2 sporoblasts were detected in fecal examinations [8]. Thereafter, this type of oocysts (I. belli) has been reported several times in fecal examinations among Koreans from 1971 to 2006 [9-13]. However, life cycle stages of I. belli other than the fecal oocysts have never been demonstrated.
We recently encountered a clinical case of I. belli infection in an alcoholic patient who revealed no signs of immunosuppression. The patient experienced chronic enteritis with severe diarrhea that led to a loss of about 35 kg of body weight over the preceding 2 years. Peroral endoscopy with biopsy of the upper jejunum revealed various developmental stages of I. belli. We report here this case as the first human I. belli infection from whom the parasites were histopathologically confirmed in Korea.
CASE RECORD
A 70-year-old male presented with recurrent diarrhea and weight loss in March 2007. His diarrhea began 2 years previously and had exhibited repeated cycles of worsening and improvement. During these 2 years, he was recommended to have further evaluation. However, his symptoms improved after several days without specific treatment, and thus no evaluation was performed. He had been admitted to another hospital 3 months earlier; gastroduodenoscopy and colonoscopy had not led to a specific diagnosis. Fifteen days prior to the present admission, diarrhea and anorexia had been exacerbated. Stools were watery and foul-smelling, without blood or mucus. Stool frequency was 3-4 times per day. He did not complain of fever or abdominal pain. However, his diarrhea was aggravated after meals and was relieved with fasting. His body weight was 75 kg 2 years previously but had gradually decreased to 40 kg at admission.
The patient had a history of consuming raw fish and crabs 4 years earlier and was diagnosed with paragonimiasis and treated with praziquantel. He also had a 30-40 year history of alcohol abuse; he drank at least 3 times a week, each time more than 450 g of 20% alcoholic beverages. He was born and lived in Korea and did not travel abroad. He did not take any special medications.
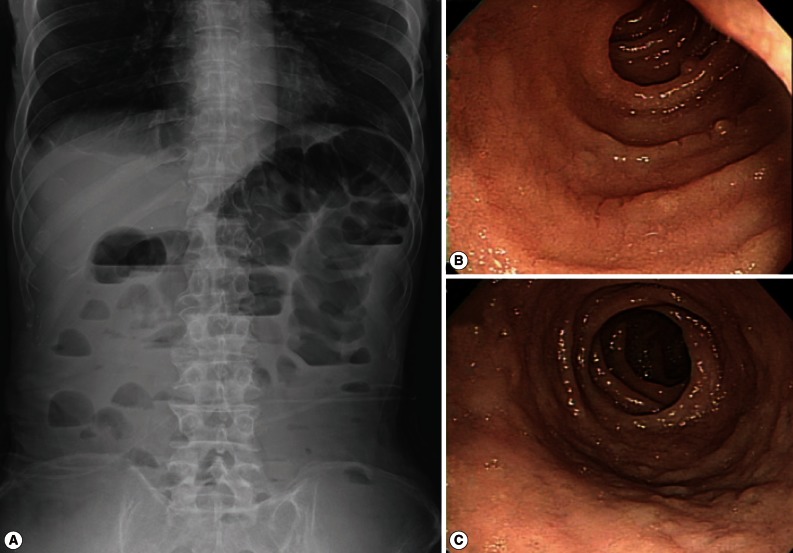
At admission, blood pressure was low (82/64 mm Hg). The pulse rate was 70 beats per min, temperature was 35.8℃, and respiratory rate was 18 breaths per min. His skin turgor was decreased and the abdomen was soft. The bowel sound was increased. Radiographs of the abdomen showed gaseous dilatation of the colon with signs of ileus (Fig. 1). A complete blood count revealed a WBC count of 10,280 per mm3 with 36.8% eosinophils. The patient had severe hypoalbuminemia (1.1 g/dl albumin) and elevation of aminotransferases (113 IU/L aspartate transaminase and 119 IU/L alanine transaminase). Serum sodium was decreased to 127 mM/L and potassium to 3.1 mM/L. The prothrombin time was prolonged to international normalized ratio (2.0), and fibrinogen was decreased to 93 mg/dl. Initial hemoglobin was 10.1 g/dl, and hematocrit was 35.7%.
Fig. 1.
(A) simple abdominal (erect) radiograph of the patient showing gaseous dilation of intestinal loops and signs of ileus. (B) Endoscopic view of the upper jejunum of the patient showing nodular appearance of the mucosal surface with loss of villi at the time of endoscopic biopsy. No erosion, ulcer, hemorrhage, or mass is seen. (C) Another endoscopic view of the jejunum of the patient showing nodular appearance of the mucosal surface taken at the time of endoscopic biopsy. No erosion, ulcer, hemorrhage, or mass is seen.
To manage dehydration and weight loss, intravenous crystalloid and supplementation of potassium and sodium were administered. After replacing deficient water, hemoglobin decreased to 9.6 g/dl. The serum levels of iron, total iron-binding capacity, folate, and vitamin B12 showed iron and folate deficiency. He continued fasting and total parenteral nutrition started. Abdominal discomfort was improved but stool character did not change appreciably. The stool output was 400 to 500 ml per day. Stool fatty acid contents were slightly increased. Stool culture was negative for any intestinal pathogen, and fecal exminations revealed no protozoan cysts or helminth eggs. Gastroduodenoscopy and colonoscopy were repeated. Multiple biopsies were done in the terminal ileum, transverse colon, and rectum but showed non-specific findings.
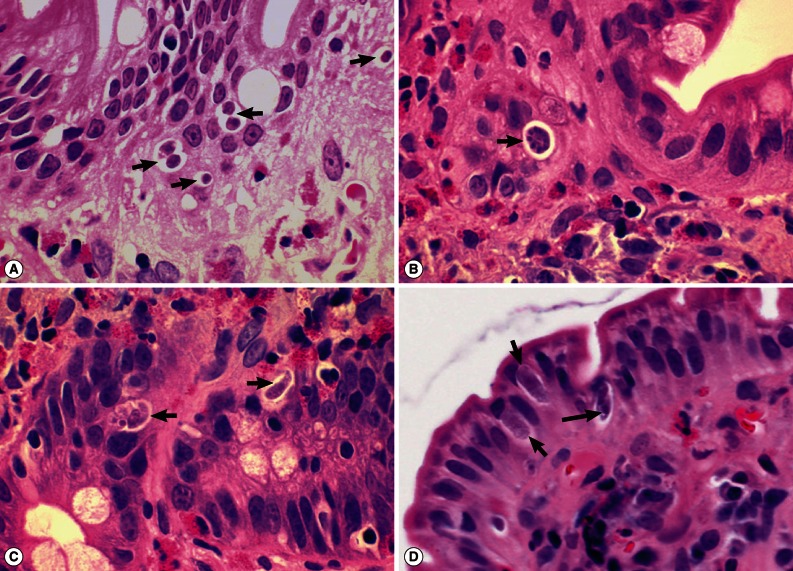
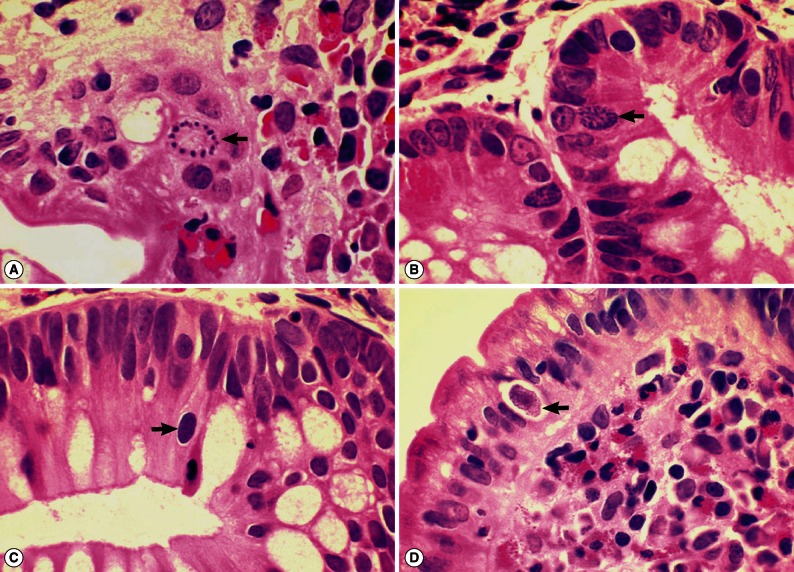
ELISA to detect the antigen and antibody of human immunodeficiency virus was negative. A capsule endoscopy to the small intestine was performed, which revealed loss of villi in the whole small bowel with hyperemic lesions, with the absene of other mucosal lesions, such as ulcer, erosion, or mass (Fig. 1C). On day 15 of hospitalization, an empirical treatment with ciprofloxacin was started. Two days later, complete blood count showed a high eosinophilia (3,910 eosinophils per mm3, 39.9% of the total WBC), without fever or exacerbation of abdominal pain. He had another endoscopic examination to the duodenum and jejunum and multiple biopsies were performed. The jejunal mucosa revealed nodular appearance and villous atrophy with loss of villi (Figs. 2, 3). The biopsy specimens histopathologically demonstrated I. belli protozoal infection, in which various life cycle stages, including trophozoites, schizonts, merozoites, and macro- and microgamonts, were detected (Fig. 2A-D; Fig. 3A-D). The mucosal tissues revealed villous atrophy, crypt hyperplasia, and diffuse infiltrations of eosinophils, lymphocytes, and plasma cells, which was consistent with isosporiasis.
Fig. 2.
Sections of the upper jejunum showing various developmental (asexual and sexual) stages of Isospora belli (×1,000, H-E stain). (A) Trophozoites (arrows), spherical in shape. (B) An immature schizont undergone nuclear division (arrow). Many eosinophils are infiltrated in the lamina propria. (C) A mature schizont with about 6 merozoites (left arrow) and a merozoite which entered an enterocyte (right arrow). Eosinophil infiltrations are also seen in this figure. (D) Two merozoites in an enterocyte (long arrow), 2 macrogamonts (short arrows), and a developing trophozoite (left lower) are seen in the jejunal epithelial layer.
Fig. 3.
Sections of the upper jejunum showing various sexual stages of Isospora belli (×1,000, H-E stain). (A) A developing microgamont (arrow) with multiple nuclei that have migrated to the periphery. (B) A mature microgamont with multiple nuclei (arrow) in an enterocyte of the crypt layer. (C) A probable early macrogamont (arrow) in an enterocyte. (D) An old macrogamont or macrogamete (arrow) in a jejunal epithelial cell.
Treatment with trimethoprim-sulfamethoxazole was started on day 16 of hospitalization. Daily oral doses of trimethoprim 320 mg and sulfamethoxazole 1,600 mg (divided into 4 doses a day) were administered for 14 days and then a half-dose for 14 additional days. He was tested with oral feeding after 2 days of treatment and diarrhea did not recur. The patient's body weight had recovered to 48 kg when he was discharged from our hospital. Human immunodeficiency virus screening test was negative. Tests for human T-cell lymphotrophic virus type I and II were not performed because their seroprevalence is low in Korea. Esophagogastroduodenoscopy 3 months after the discharge still showed scant villi, nodular mucosal patterns, and hyperemic mucosal surfaces. In a follow-up 2.5 years later (October 2009), these endoscopic abnormal findings returned to normal. He did not develop any hematologic malignancy or other conditions related with immune suppression.
DISCUSSION
The present study suggests that risk factors for symptomatic I. belli infection may include a wider variety of conditions than have ever been documented [3,6,7]. In addition to immunocompromised conditions that include AIDS, adult T-cell leukemia, Hodgkin's lymphoma, non-Hodgkin's lymphoma, thymoma, transplantation, and corticosteroid-treated patients [6,7,14-21], various immunocompetent conditions can also be the risk factors for symptomatic isosporiasis. These factors include poor hygiene [3], malnutrition [22], and debilitating infectious diseases, for example dengue fever [23]. The present report adds chronic alcoholism as another risk factor for development of clinical isosporiasis.
Our patient presented with chronic enteritis with recurrent diarrhea and severe weight loss. However, stool frequencies and volumes were only marginally increased. Foul smelling odor and increased fat component of his stool suggested non-absorptive diarrhea. Endoscopy conducted twice showed severe loss of villi in the whole small bowel. Other evaluations showed all non-specific findings just compatible with chronic inflammatory diseases of the intestine and the resulting malabsorption and malnutrition. Repeated stool microscopy was negative for I. belli oocysts or other protozoan and helminth parasites.
The specific diagnosis of isosporiasis was possible in our case only after detection of various life cycle stages of parasites in endoscopic biopsy specimens of the upper jejunum. Virtually all developmental stages were identified in sectioned specimens, which included developing trophozoites, immature schizonts, mature schizonts, merozoites, micro- and macrogamonts, and micro- and macrogametes, with the exception of oocysts. The size, shape, and other morphologic characteristics were compatible with I. belli parasites [3]. The involved mucosa revealed villous atrophy, crypt hyperplasia, and mucosal inflammation with eosinophils, which were consistent with I. belli infection [1]. Peripheral blood eosinophilia, up to 39.9% before treatment, was a characteristic feature for our patient. Though lesser in degree, eosinophilia had also been commonly noted in isosporiasis cases [3,14,18,21]. Therefore, in patients with intestinal inflammatory diseases with diarrhea and peripheral blood eosinophilia, isosporiasis should be included among the list of differential diagnosis.
There has been no golden standard in diagnosing isosporiasis, and thus the sensitivity of diagnostic methods has not been determined until recently. However, in 2012, the stool oocyst positive rate was reported to be only 34% in 8 confirmed isosporiasis cases in which stool examinations were repeatedly performed, 2-13 times per case [19]. Meanwhile, the parasite positive rate in duodenal biopsies done up to 3 times per case was 63% [19]. This suggests that stool microscopy is not sufficient for excluding isosporiasis, and even intestinal biopsies can reveal false negative results. In our case, stool examinations were repeated more than 3 times; oocysts of I. belli were not detected.
Isosporiasis responds relatively well to treatment with trimethoprim-sulfamethoxazole [3]. We used a daily dose of 1,920 mg which consisted of 320 mg trimethoprim and 1,600 mg sulfamethoxazole for a total of 28 days. The results were satisfactory. However, chronic relapsing I. belli infections have been reported in AIDS patients despite treatment with trimethoprim-sulfamethoxazole and immune reconstitution [19]. In these patients, a double daily dose of 3,840 mg should be given up to 2 years while monitoring for myelotoxicity [19].
Immigrants from endemic areas are known to develop isosporiasis after several years due to newly developed immune suppressive conditions, such as lymphoma [16,17]. These findings suggest that in some individuals chronic infection state can persist with or without symptoms. Our patient had competent immune status but failed to eradicate the parasite and eventually sufferred from chronic relapsing enteritis symptoms. It has been suggested that unicellular cysts of I. belli dormant in mesenteric lymphoid tissues could be the source of a relapsing infection [20]. In our patient, follow-up studies were done longer than 2.5 years but his diarrheal episodes did not occur again.
Conclusively, we report here an isosporiasis case in an alcoholic patient without any immune deficiency conditions. Stool frequencies and volumes were moderately increased, and symptoms and signs of severe malabsorption and malnutrition with remarkable weight loss were characteristically shown. An endoscopic jejunal biopsy revealed various developmental stages of I. belli parasites. Treatment was successful with trimethoprim-sulfamethoxazole which led to complete resolution of symptoms. This is the first clinical case of I. belli infection in Korea in which different life cycle stages of parasites have been demonstrated.
References
- 1.Curry A, Smith HV. Emerging pathogens: Isospora, Cyclospora and microsporidia. Parasitology. 1998;117:S143–S159. doi: 10.1017/s0031182099004904. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira-Silva MB, Lages-Silva E, Resende DV, Prata A, Ramierez LE, Frenkel JK. Cystoisospora belli: in vitro multiplication in mammalian cells. Exp Parasitol. 2006;114:189–192. doi: 10.1016/j.exppara.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Klasse-Fischer MK, Neafie RC, Wear DJ, Meyers WM. Topics on the Pathology of Protozoan and Invasive Arthropod Diseases (e-book) Fairfax, Virginia, USA: Inova Central Laboratory; 2011. Chapter 13. Cryptosporidiosis, isosporiasis, cyclosporiasis, and sarcocystosis; pp. 1–19. [Google Scholar]
- 4.DeHovitz JA, Pape JW, Boncy M, Johnson WD., Jr Clinical manifestations and therapy of Isospora belli infection in patients with the acquired immunodeficiency syndrome. New Eng J Med. 1986;315:87–90. doi: 10.1056/NEJM198607103150203. [DOI] [PubMed] [Google Scholar]
- 5.Restrepo C, Macher AM, Radany EH. Disseminated extraintestinal isosporiasis in a patient with acquired immune deficiency syndrome. Am J Clin Pathol. 1987;87:536–542. doi: 10.1093/ajcp/87.4.536. [DOI] [PubMed] [Google Scholar]
- 6.Atambay M, Bayraktar MR, Kayabas U, Yilmaz S, Bayindir Y. A rare diarrheic parasite in a liver transplant patient: Isospora belli. Transplant Proc. 2007;39:1693–1695. doi: 10.1016/j.transproceed.2007.02.080. [DOI] [PubMed] [Google Scholar]
- 7.Gruz F, Fuxman C, Errea A, Tokumoto M, Fernandez A, Velasquez J, Nagel C, Ruf A, Mariňo E, Nachman F, Rumbo M, Gondolesi G. Isospora belli infection after isolated intestinal trnsplant. Transpl Infect Dis. 2009;12:69–72. doi: 10.1111/j.1399-3062.2009.00439.x. [DOI] [PubMed] [Google Scholar]
- 8.Park SH. The first observation of isosporiasis in Korea. Med Bull Natl Med Cent. 1960;1:73–77. [Google Scholar]
- 9.Kim CH, Park CH, Kim HJ, Chun HB, Min HK, Koh TY, Soh CT. Prevalence of intestinal parasites in Korea. Korean J Parasitol. 1971;9:25–38. doi: 10.3347/kjp.1971.9.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Yoon DH, Yang SJ, Kim JS, Hong ST, Chai JY, Lee SH, Chi JG. A case of fatal malabsorption syndrome caused by strongyloidiasis complicated with isosporiasis and human cytomegalovirus infection. Korean J Parasitol. 1992;30:53–58. doi: 10.3347/kjp.1992.30.1.53. [DOI] [PubMed] [Google Scholar]
- 11.Choi HJ, Park SW, Kim HB, Kim NJ, Shin DH, Oh MD, Jung HC, Song IS, Choe KW. The gastrointestinal infections in Korean patients with human immunodeficiency virus infection. Korean J Gastroenterol. 1999;33:482–488. [Google Scholar]
- 12.Guk SM, Seo M, Park YK, Oh MD, Choe KW, Kim JL, Choi MH, Hong ST, Chai JY. Parasitic infections in HIV-infected patients who visited Seoul National University Hospital during the period 1995-2003. Korean J Parasitol. 2005;43:1–5. doi: 10.3347/kjp.2005.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai JY, Park JH, Guk SM, Kim HJ, Kim WH, Kim JL, Gu YS, Shin EH, Park HM, Hong KS, Kim SD, Lee SH. Status of intestinal parasite infections among 4,137 residents from provinces nationwide and metropolitan areas in the Republic of Korea (2004) Infect Chemother. 2006;38:198–203. [Google Scholar]
- 14.Greenberg SJ, Davey MP, Zierdt WS, Waldmann TA. Isospora belli enteric infection in patients with human T-cell leukemia virus type I-associated adult T-cell leukemia. Am J Med. 1988;85:435–438. doi: 10.1016/0002-9343(88)90603-1. [DOI] [PubMed] [Google Scholar]
- 15.Peng CY, Tsai W. Isospora belli infection in a patient with Hodgkin's disease: report of a case. J Formos Med Assoc. 1991;90:260–263. [PubMed] [Google Scholar]
- 16.Resiere D, Vantelon JM, Bouree P, Chachaty E, Nitenberg G, Blot F. Isospora belli infection in a patient with non-Hodgkin's lymphoma. Clin Microbiol Infect. 2003;9:1065–1067. doi: 10.1046/j.1469-0691.2003.00742.x. [DOI] [PubMed] [Google Scholar]
- 17.Ud Din N, Torka P, Hutchison RE, Riddell SW, Wright J, Gajra A. Severe Isospora (Cystoisospora) belli diarrhea preceding the diagnosis of human T-cell-leukemia-virus-1-associated T-cell lymphoma. Case Repts Infect Dis. 2012;2012:640104. doi: 10.1155/2012/640104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meamar AR, Rezaian M, Zare-Mirzaei A, Zahabiun F, Faghihi AH, Oormazdi H, Kia EB. Severe diarrhea due to Isospora belli in a patient with thymoma. J Microbiol Immunol Infect. 2009;42:526–529. [PubMed] [Google Scholar]
- 19.Boyles TH, Black J, Meintjes G, Mendelson M. Failure to eradicate Isospora belli diarrhoea despite immune reconstitution in adults with HIV--a case series. PLoS One. 2012;7:e42844. doi: 10.1371/journal.pone.0042844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frenkel JK, Silva MB, Saldanha J, de Silva ML, Filjo VDC, Barata CH, Lages E, Ramirez LE, Prata A. Isospora belli infection: observation of unicellular cysts in mesenteric lymphoid tissues of a Brazilian patient with AIDS and animal inoculation. J Eukaryot Microbiol. 2003;50(suppl):682–684. doi: 10.1111/j.1550-7408.2003.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 21.Navaneethan U, Kenkatesh PGK, Downs-Kelly E, Shen B. Isospora belli superinfection in a patient with eosinophilic gastroenteritis-a diagnostic challenge. J Crohns Colitis. 2012;6:236–239. doi: 10.1016/j.crohns.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Kochhar A, Saxena S, Malhotra VL, Deb M. Isospora belli infection in a malnourished child. J Commun Dis. 2007;39:141–143. [PubMed] [Google Scholar]
- 23.Pérez-Ayala A, Monge-Maíllo, Diáz-Menéndez M, Norman F, Pérez-Molina JA, López-Vélez R. Self-limited travelers' diarrhea by Isospora belli in a patient with dengue infection. J Travel Med. 2011;18:212–213. doi: 10.1111/j.1708-8305.2010.00494.x. [DOI] [PubMed] [Google Scholar]