Abstract
Giardia lamblia is recognized as one of the most prevalent parasites in dogs. The present study aimed to establish a loop-mediated isothermal amplification (LAMP) assay for rapid and specific detection of G. lamblia from dogs. The fecal samples were collected and prepared for microscopic analysis, and then the genomic DNA was extracted directly from purified cysts. The concentration of DNA samples of G. lamblia were diluted by 10-fold serially ranging from 10-1 to 10-5 ng/µl for LAMP and PCR assays. The LAMP assay allows the amplification to be finished within 60 min under isothermal conditions of 63℃ by employing 6 oligonucleotide primers designed based on G. lamblia elongation factor 1 alpha (EF1α) gene sequence. Our tests showed that the specific amplification products were obtained only with G. lamblia, while no amplification products were detected with DNA of other related protozoans. Sensitivity evaluation indicated that the LAMP assay was sensitive 10 times more than PCR. It is concluded that LAMP is a rapid, highly sensitive and specific DNA amplification technique for detection of G. lamblia, which has implications for effective control and prevention of giardiasis.
Keywords: Giardia lamblia, loop-mediated isothermal amplification (LAMP), PCR, EF1α gene, dog
Giardia lamblia (syn G. duodenalis) is a cosmopolitan enteric parasite with a very wide host range, including domestic and wild animals as well as human beings [1,2]. Most giardiasis is transmitted by fecal-to-oral route, directly from infected individuals or contaminated fomites, or by ingesting cysts in contaminated food or water [3]. Once infected, G. lamblia causes a generally self-limited clinical illness (i.e., giardiasis) characterized by diarrhea, bloating, weight loss, abdominal cramps, and malabsorption [4]. It is recently known that there are 7 major genotypic assemblages (A-G) of G. lamblia [5]. However, some new G. lamblia genotypes are still being described [6]. The assemblages A and B have a wide range of hosts, including humans and animals such as the livestock, dogs, cats, and other wild animals, but C-G genotypes of G. lamblia seem to be host-specific. The assemblages C and D are dog-specific genotypes, while the assemblage E has been identified in cattle, the assemblage F seems to be specific for cats, and G for rats [7].
Until now, microscopic examination has been the routine method for the detection of G. lamblia from dogs, which has limitation in that G. lamblia is difficult to be identified accurately particularly when there are concurrent infections with multiple parasite species in dogs. With the development of molecular techniques, PCR technique has been developed to detect G. lamblia infection in recent years. There are 5 major genes such as small subunit ribosomal RNA gene (ssu-rRNA), β-giardin (bg), triose phosphate isomerase (tpi), glutamate dehydrogenase (gdh), and EF1α genes, which are suitable for genetic markers for detecting and genotyping studies. However, these methods require expensive and high-precision instruments, expert techniques, and long reaction time (2-3 hr), which may not be readily available in rural endemic regions. Moreover, the Taq DNA polymerase used in PCR assay is easily inhibited by biological substances. Therefore, simple, rapid, and cost-effective detection method with high sensitivity is still needed to complement the limitations of PCR and other techniques.
A simple, sensitive, and rapid technique named loop-mediated isothermal amplification (LAMP) was first developed by Notomi et al. [8], and it relies on auto-cycling strand displacement DNA synthesis by Bst polymerase with displacement activity. This method allows amplification of target nucleic acids under isothermal conditions, and the amplification products are observed visually [8,9]. Therefore, LAMP assay has been applied successfully for the detection and identification of protozoan parasite infections, including Trypanosoma [10], Plasmodium falciparum [11], Cryptosporidium [12], and Toxoplasma gondii [13]. In addition, LAMP assay has been first developed to detect G. lamblia assemblages A and B cysts in environmental and human fecal samples in Japan [14]. After that, it was also used for detection of G. lamblia assemblages A and B specific DNA sequence in drinking water [15]. However, no information on LAMP assay for detection G. lamblia dog-specific genotypes has been available.
The objectives of the present study were to develop and evaluate a simple and cost-effective LAMP assay based on EF1α gene sequences for rapid detection of G. lamblia from dogs. The sensitivity and specificity of LAMP assay were evaluated by comparison with PCR method. LAMP method should supplement and enhance existing procedures to detect the G. lamblia infection.
G. lamblia samples were collected directly from the feces of infected pet dogs in Guangdong Province in China. The fecal samples were prepared for microscopic analysis by floatation technique with saturated zinc sulfate, and then cysts were purified by sucrose gradient solution. The "heterologous control samples" to assess the specificity of LAMP assay were T. gondii, Neospora caninum, Cryptosporidium parvum, and Eimeria tenella. Genomic DNA (gDNA) was extracted directly from purified cysts using a QIAamp DNA Stool Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions.
LAMP primers (Table 1) for the specific amplification of the target DNA were designed from the EF1α gene sequences of G. lamblia using Primer Explorer V4 software (http://primerexplorer.jp/e). LAMP assay requires 4 sets of specific primers (B3, F3, BIP, and FIP) that recognize a total of 6 distinct sequences (B1, B2c, B3, F1c, F2, and F3). A 208 bp fragment of the EF1α gene was amplificated using PCR with the outer primers B3 and F3, and the specificity of the outer primers was confirmed by BLAST search (http://www.ncbi.nlm.nih.gov/Blast) in the NCBI database.
Table 1.
Sequences of LAMP primers for the amplification of G. duodenalis EF1α gene
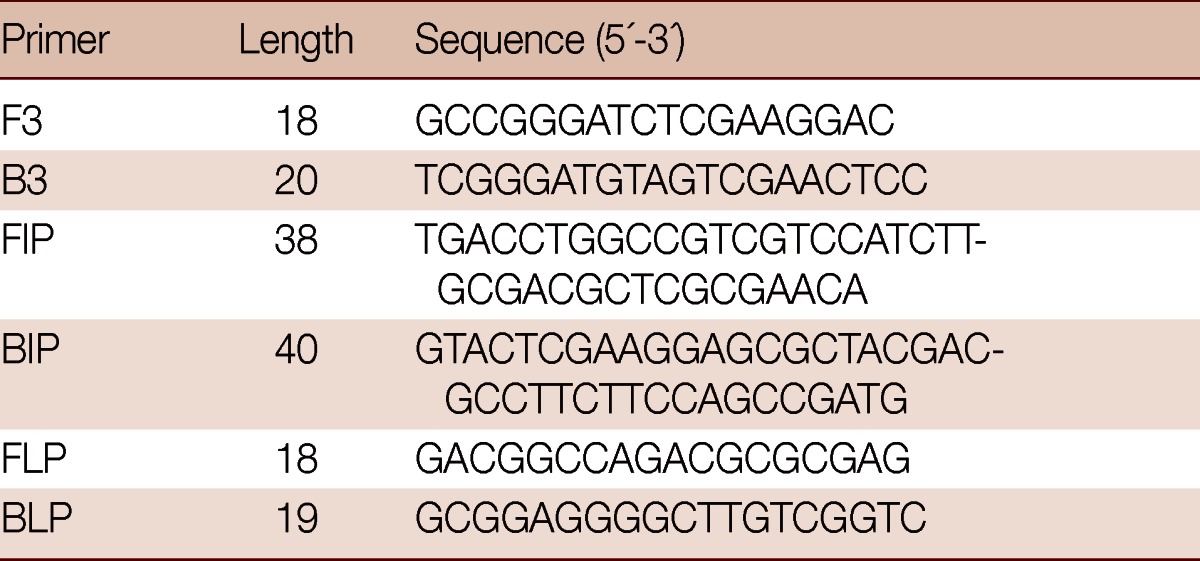
F3, forward outer primer; B3, backward outer primer; FIP, forward inner primer; BIP, backward inner primer; FLP, forward loop primer; BLP, backward loop primer.
LAMP assay was carried out in a total of 25 µl reaction mixture containing: 10× Bst-DNA polymerase buffer (2.5 mM each), betaine (1.6 M), deoxynucleotide triphosphates (2.5 mM each), MgSO4 (8 mM), F3 and B3 primers (0.2 µM each), FIP and BIP (1.6 µM each), loop-F and loop-B (0.8 µM each), Bst DNA polymerase (8 U) (New England Biolabs, Beverly, Massachusetts, USA), and template DNA (2 µl). No template DNA was added in the 'negative control' reaction. The mixture was incubated at 63℃ for 60 min, and then heated at 80℃ for 10 min. The LAMP products were visually detected further by adding 1 µl of 1:10 diluted 10,000× concentration of SYBR Green I (Invitrogen, Carlsbad, California, USA) to the reaction tube. Also, the products (5 µl) were examined on a 2% agarose gel with DL2000 (TaKaRa, Dalian, China) to estimate the sizes of amplification products and stained with ethidium bromide. The stained gel and the reaction tubes were photographed using UV Image system (UVItec, London, UK). The genotypes of tested G. lamblia samples were identified as assemblage D, which is dog-specific, based on the EF1α gene [16]. The specificity of LAMP test was assessed with DNA prepared from T. gondii, N. caninum, C. parvum, and E. tenella. The reaction tubes were photographed using UV Image system (UVItec).
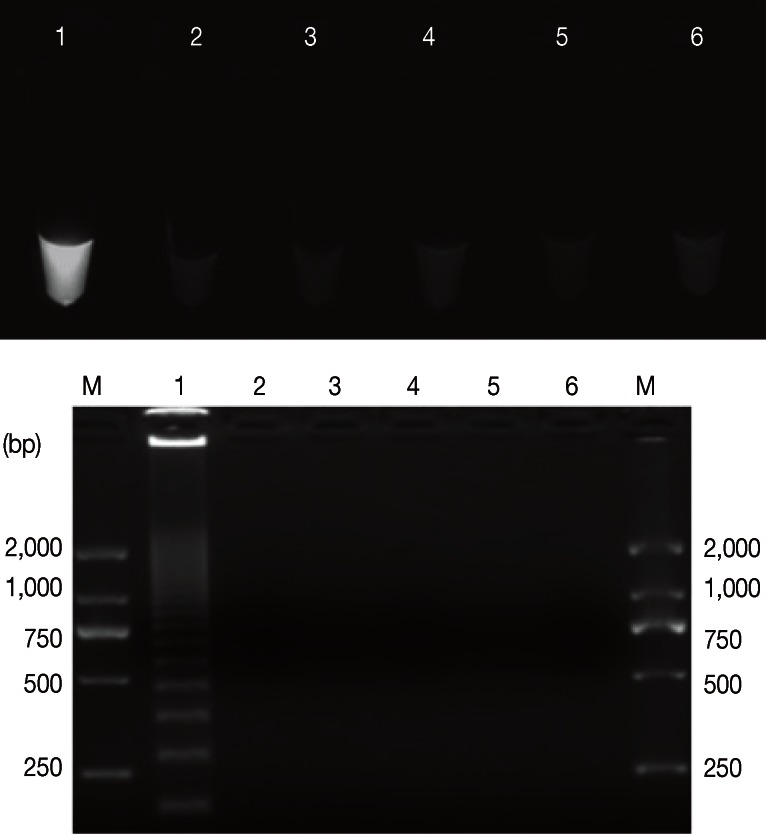
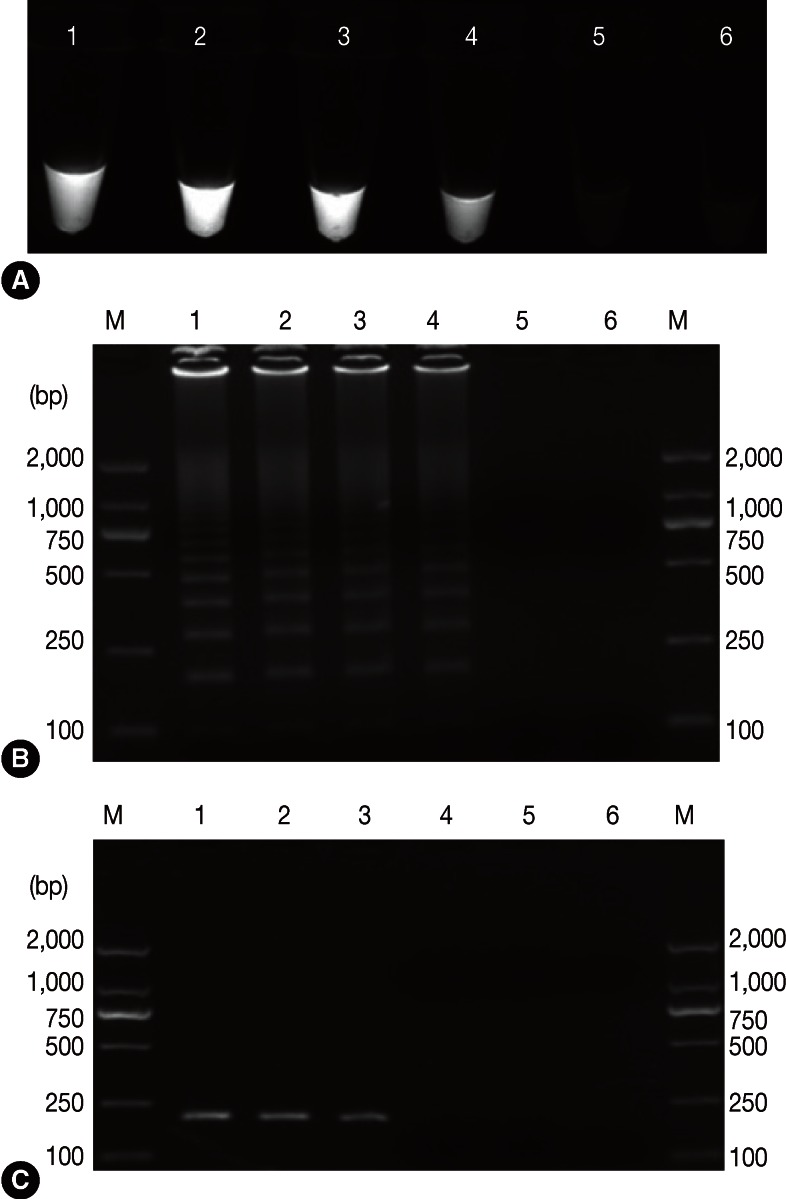
Positive results containing LAMP products were visually observed in tubes, and they presented with typical patterns of ladder-like bands on agarose gels. Whereas, no amplifications were found with DNA samples of 'heterologous control samples', namely, T. gondii, N. caninum, C. parvum, and E. tenella (Fig. 1). Our results demonstrated LAMP assay as a specific tool to detect G. lamblia. The sensitivity results showed that the detection limit of LAMP assay was 10-4 ng/µl (0.1 pg/µl) (Fig. 2A, B), which is more sensitive than 0.548 pg/µl and 0.8 pg/µl in a previous report by Plutzer et al. [15], whereas it was 10-3 ng/µl in PCR (Fig. 2C). Thus, LAMP was sensitive 10 times more than PCR assay.
Fig. 1.
Assessment of the pecificity of the LAMP assay for G. duodenalis. Upper: Visual examination of LAMP products. Bottom: Agarose gel electrophoresis of amplification products. Lanes 1-5 represent G. duodenalis, Toxoplasma gondii, Neospora caninum, Cryptosporidium parvum, Eimeria tenella, and no-DNA control, respectively. M represents a DNA size marker (ordinate values in bp).
Fig. 2.
Assessment of the sensitivity of the LAMP assay for G. duodenalis in comparison with conventional PCR which was performed with primers F3 and B3 (A, B: sensitivity analysis of the LAMP assay). Lanes 1-6 represent serial G. duodenalis DNA concentration of 10-1-10-5 ng/µl and no-DNA control, respectively (C: sensitivity analysis of a conventional PCR). M represents a DNA size marker (ordinate values in bp).
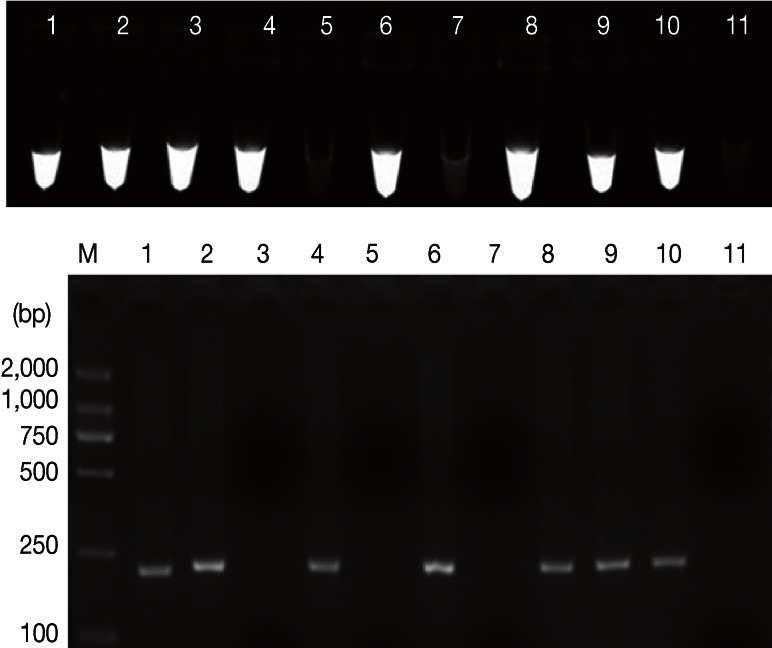
Positive microscopic identification was on 5 samples of 72 only (6.9% positive), while 7 out of 72 (9.7%) and 8 of 72 (11.1%) were positive by PCR and LAMP, respectively (Table 2). However, the 5 positive samples tested by microscopy were indicated as well by PCR (confirmed by sequencing). The nucleotide sequence has been deposited in the GenBank database under accession number JQ245136. Sequence analysis revealed that 7 samples were assemblage D that is dog-specific genotype, not a zoonotic genotype, and these PCR-positive samples were also positive by LAMP. Furthermore, 1 additional PCR-negative sample was also LAMP-positive (repeated 3 times). These results would be considered as a proofed index to believe that LAMP assay has superior sensitivity than PCR assay. The results of LAMP and conventional PCR assays are shown in Fig. 3.
Table 2.
Comparison of microscopy, conventional PCR, and loop-mediated isothermal amplification (LAMP) for detection of G. lamblia isolates from dogs
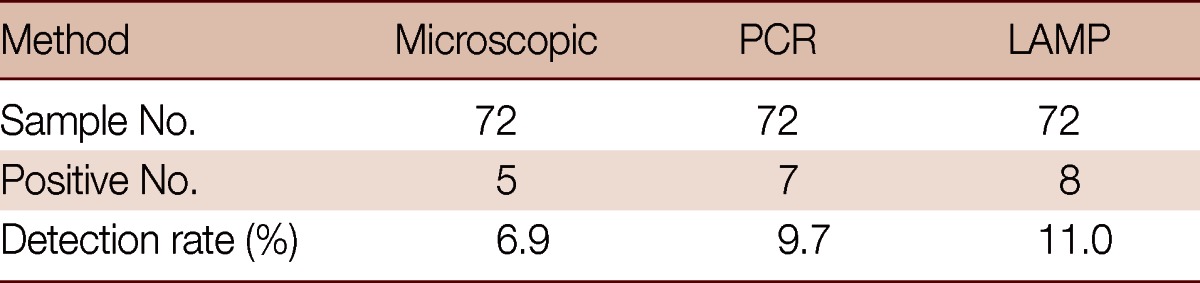
Fig. 3.
The clinical utility of the LAMP method for detection of EF1α gene of G. duodenalis from dogs. Upper: the examination of LAMP products, Lanes 1, 2, 3, 4, 6, 8, 9, and 10 represent G. duodenalis-infected. Lanes 5 and 7 represent negatives. Lanes 11 represents no-DNA control. Bottom: PCR performed with primers F3 and B3. M represents a DNA size marker (ordinate values in bp).
In this study, we report on the development of G. lamblia-specific LAMP assay based on EF1α genes. Through this study, we felt that LAMP is quite convenient to perform, and more sensitive than PCR assays in the case of G. lamblia detection in dogs. Where the amplification can be done within about 60 min with loop primers, which the amplification time is greatly decreased compared with 2 hr for detection of G. lamblia assemblages A and B cysts in environmental and human fecal samples [14], and it does not require a thermocycler, only a water bath or a heat block is required to carry out the amplification, which is readily available even in poorly equipped laboratories or in a large-scale epidemiological survey of giardiasis carried out in poor areas. Therefore, LAMP assays have an advantage with a lower cost and showed better cost-effectiveness compared with PCR assays. It is worth mentioning that 2 samples were negative by microscopic examinations, whereas they were positive by PCR assay. We believe that would be related to rapid destruction of trophozoites or interference of impurities in feces which is reducing the capability of microscopic detection.
The present LAMP assay depends on the Bst DNA polymerase, which is more resistant than the other DNA polymerases [11], such as Taq DNA polymerase, which is easily inhibited by biological substances. Additionally, not only the Bst enzyme is active at relatively high temperatures (63℃) which helps for reduction of non-specific pairing, but also LAMP primers recognizing different targets enhanced the sensitivity and specificity [17]. Furthermore, a large amwount of white magnesium pyrophosphate precipitate will generate for positive results, which allows the presence of G. lamblia DNA to be easily identified by visual inspection, and the positive amplification can be viewed by adding the fluorescent dyes, such as SYBR Green I.
In conclusion, LAMP assay is a highly sensitive, rapid, and reliable method, and an ideal tool to detect G. lamblia from dogs. The assay has the potential as an alternative tool for clinical diagnosis and epidemiological investigations. Hence, it can contribute to the effective control and prevention of dog giardiasis.
ACKNOWLEDGMENTS
The present research was supported by a grant from National Natural Science Foundation of China (grant no. 30972179, 31272551). We thank Lin Ai for his excellent technical assistance.
References
- 1.Appelbee AJ, Thompson RC, Olson ME. Giardia and Cryptosporidium in mammalian wildlife-current status and future needs. Trends Parasitol. 2005;21:370–376. doi: 10.1016/j.pt.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becher KA, Robertson ID, Fraser DM, Palmer DG, Thompson RC. Molecular epidemiology of Giardia and Cryptosporidium infections in dairy calves originating from three sources in Western Australia. Vet Parasitol. 2004;123:1–9. doi: 10.1016/j.vetpar.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Ballweber LR, Xiao L, Bowman DD, Kahn G, Cama VA. Giardiasis in dogs and cats: update on epidemiology and public health significance. Trends Parasitol. 2010;26:180–189. doi: 10.1016/j.pt.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011;24:110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson RC. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet Parasitol. 2004;126:15–35. doi: 10.1016/j.vetpar.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Thompson J, Yang R, Power M, Hufschmid J, Beveridge I, Reid S, Ng J, Armson A, Ryan U. Identification of zoonotic Giardia genotypes in marsupials in Australia. Exp Parasitol. 2008;120:88–93. doi: 10.1016/j.exppara.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Cacciò SM, Thompson RC, McLauchlin J, Smith HV. Unravelling Cryptosporidium and Giardia epidemiology. Trends Parasitol. 2005;21:430–437. doi: 10.1016/j.pt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289:150–154. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- 10.Kuboki N, Inoue N, Sakurai T, Di Cello F, Grab DJ, Suzuki H, Sugimoto C, Igarashi I. Loop-mediated isothermal amplification for detection of African trypanosomes. J Clin Microbiol. 2003;41:5517–5524. doi: 10.1128/JCM.41.12.5517-5524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poon LL, Wong BW, Ma EH, Chan KH, Chow LM, Abeyewickreme W, Tangpukdee N, Yuen KY, Guan Y, Looareesuwan S, Peiris JS. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem. 2006;52:303–306. doi: 10.1373/clinchem.2005.057901. [DOI] [PubMed] [Google Scholar]
- 12.Karanis P, Thekisoe O, Kiouptsi K, Ongerth J, Igarashi I, Inoue N. Development and preliminary evaluation of a loop-mediated isothermal amplification procedure for sensitive detection of cryptosporidium oocysts in fecal and water samples. Appl Environ Microbiol. 2007;73:5660–5662. doi: 10.1128/AEM.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sotiriadou I, Karanis P. Evaluation of loop-mediated isothermal amplification for detection of Toxoplasma gondii in water samples and comparative findings by polymerase chain reaction and immunofluorescence test (IFT) Diagn Microbiol Infect Dis. 2008;62:357–365. doi: 10.1016/j.diagmicrobio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Plutzer J, Karanis P. Rapid identification of Giardia duodenalis by loop-mediated isothermal amplification (LAMP) from faecal and environmental samples and comparative findings by PCR and real-time PCR methods. Parasitol Res. 2009;104:1527–1533. doi: 10.1007/s00436-009-1391-3. [DOI] [PubMed] [Google Scholar]
- 15.Plutzer J, Törökné A, Karanis P. Combination of ARAD microfibre filtration and LAMP methodology for simple, rapid and cost-effective detection of human pathogenic Giardia duodenalis and Cryptosporidium spp. in drinking water. Lett Appl Microbiol. 2010;50:82–88. doi: 10.1111/j.1472-765X.2009.02758.x. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Wang PY, Zhang P, Liu YJ, Guo JC, Meng XL, Li GQ. Cloning and sequence analysis of EF1α gene of the Giardia lamblia from dogs. Prog Vet Med. 2012;33:53–56. in Chinese. [Google Scholar]
- 17.Nagamine K, Hase T, Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16:223–229. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]