ABSTRACT
The mass spectrometry of a number of 6-substituted coumarins was studied in the context of correlating fragmentation pathways and electronic charges of atoms performed by AM1 semiempirical method. The atomic charges of atoms are found to be good predictors of the fragmentation pathways.
Keywords: AM1, coumarin, electronic charge, fragmentation, mass spectrometry
INTRODUCTION
Coumarins are important, naturally occurring heterocyclic compounds. Because of their several properties, they have been synthesized by several methods in laboratories. They exhibit important biologic properties and are extensively studied.[8–12] Their physical and chemical properties are also known,[2,12–15] but little attention has been paid to their properties in mass spectrometry. We describe below a study of mass spectral fragmentations in correlation with the electronic charges of atoms performed by AM1 semiempirical method.[16] AM1 semiempirical calculation has been used successfully for 2H and 13C NMR assignments,[17,18] for the study of tautomeric equilibrium,[19,20] reactions mechanism,[21] and so forth. In all these cases, AM1 calculations are consistent with the experimental results.
As a part of our continuing investigations about coumarins, we report herein some results obtained in the study of correlation between fragmentation processes in electronic impact mass spectrometry (eims) and electronic charges of atoms of some coumarins. The electronic charges are performed by AM1 semiempirical method. To our knowledge, no similar study has been reported about correlation between fragmentation processes in mass spectrometry of coumarin derivatives and electronic charges of their atoms.
MATERIALS AND METHODS
Synthesis of Coumarins
According to the Perkin reaction procedure,[4] coumarins are obtained with more than 70% yield as colorless crystal after purification. All the compounds have been identified by their IR and NMR (1H and 13C) data. Scheme 1 shows the materialization of the reaction.
SCHEME 1.
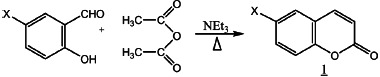
1a: X = H; 1b: X = CI; 1c: X = Br; 1d: X = NO2.
AM1 Calculation of Electronic Charges of Atoms
All the electronic charges of atoms are performed by Austin Model 1 (AM1) semiempirical method[16] from Chem3D Ultra8 software using a Pentium 4 computer.
Mass Spectra
All the mass spectra have been obtained by eims on a CPG-JSM AX505 apparatus for 1a, 1c, and 1d and on a Finnigan MAT ITD 880 MS apparatus at 70 eV for 1b.
RESULTS AND DISCUSSION
Tables 1, 2, and 3 summarize mass spectra of prepared coumarins and electronic charges of their atoms.
TABLE 1.
Relevant Mass Spectra of Coumarins 1
|
1a (X = H) |
1b (X = CI) |
1c (X = Br) |
1d (X = NO2) |
||||
|---|---|---|---|---|---|---|---|
| m/e | (%) | m/e | (%) | m/e | (%) | m/e | (%) |
| 146 (M∗%) | 96.8 | 182 (M∗%) | 56.52 | 226 (M∗%) | 96 | 191 (M∗%) | 62.22 |
| 131 | 1.01 | 180 | 93.47 | 224 | 100 | 185 | 2.22 |
| 118 | 100.00 | 179 | 1.08 | 198 | 58.73 | 175 | 2.44 |
| 92 | 2.38 | 154.6 | 44.56 | 196 | 57.14 | 173 | 2.22 |
| 90 | 19.04 | 152.5 | 100.00 | 181 | 1.10 | 163 | 24.44 |
| 89 | 52.38 | 128.5 | 0.54 | 179 | 0.15 | 161 | 26.66 |
| 63 | 18.25 | 126.5 | 6.52 | 172 | 2.38 | 154 | 1.25 |
| 39 | 10.08 | 124.5 | 16.30 | 170 | 2.38 | 145 | 4.44 |
| – | 117.5 | 3.26 | 143 | 1.58 | 143 | 3.21 | |
| – | 99.5 | 2.17 | 117 | 11.90 | 133 | 33.33 | |
| – | 89.3 | 71.73 | 89 | 73.01 | 117 | 46.66 | |
| – | 73.3 | 8.69 | 63 | 19.05 | 105 | 23.33 | |
| – | 63.3 | 34.78 | 39 | 3.17 | 89 | 95.55 | |
| – | 62.3 | 26.08 | – | 772 | 8.88 | ||
| – | 61.3 | 8.69 | – | 63 | 100.00 | ||
| – | – | – | 51 | 46.66 | |||
TABLE 2.
Electronic Charges of Carbons Atoms Obtained by AM1
| No. | X | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | H | 0.541 | 0.124 | 0.053 | 0.029 | 0.068 | 0.021 | 0.095 | 0.263 | 0.012 |
| 1b | Cl | 0.533 | 0.124 | 0.047 | 0.135 | 0.146 | 0.093 | 0.087 | 0.213 | 0.009 |
| 1c | Br | 0.534 | 0.124 | 0.048 | -0.114 | 0.092 | 0.078 | 0.088 | 0.223 | 0.009 |
| 1d | NO2 | 0.533 | 0.293 | 0.086 | 0.085 | 0.465 | 0.201 | 0.266 | 0.301 | 0.087 |
TABLE 3.
Electronic Charges of Oxygen and Nitrogen Atoms
| Compound | X | O1 | O11 | N |
|---|---|---|---|---|
| 1a | H | 0.046 | 0.708 | – |
| 1b | Cl | 0.041 | –0.716 | – |
| 1c | Br | 0.042 | –0.714 | – |
| 1d | NO2 | 0.111 | 0.833 | 0.357 |
The 13C NMR data obtained are in good agreement with those of the literature.[22–25]
The m/z ratios of molecular ions of the whole compounds are in good agreement with the expected molecular weight and are an indication of the stability of this ion. According to the charges of different atoms, the most probable point of impact of the electron beam is the oxygen of the carbonyl (O11). That is the reason why most fragmentations are derived from the molecular ion (Scheme 2) obtained by this impact. This molecular ion has been described several times as the molecular ion of coumarins.[24–28]
SCHEME 2.
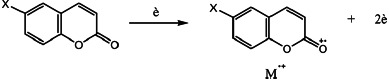
Formation of the molecular ions of Coumarins 1.
The carbons 3, 8, and 10 are the bearers of the most negative charge. The significant fragmentations, giving the most important fragments, take place on these carbons or are induced by heteroatoms (oxygen, nitrogen, or halogen). These carbons are the most electron-donating carbons. The carbon 6 gives also some fragmentations: loss of X or transposition of H when X = NO2. Some common fragmentations have been obtained giving the fragment A, which m/z ratio was M-28, and other fragments with m/z = 117, 89, 63, and 39.
The process with carbonyl loss has been described several times for coumarins[28,29] and for isocoumarins.[30,31] So has the contraction of the cycle of oxygenated fused heterocycles[22,26,32] or phenol derivatives.[30] Presence of the peak with m/z = M-28, obtained after expulsion of the CO group, described previously,[22,32–34] is another convincing argument. Thus, the fragmentation process of Scheme 3 is proposed.
SCHEME 3.
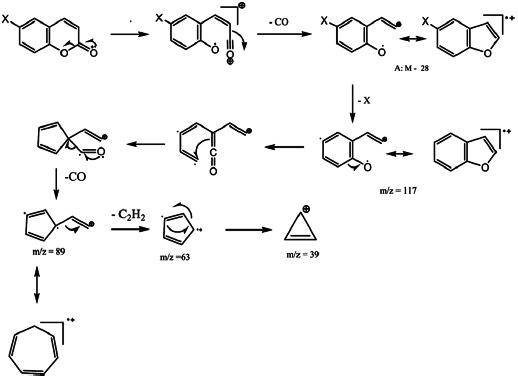
Formation of fragment A (M–28) and fragments with m/z = 117, 89, 63, and 39.
It is observed that the molecular ions of all the compounds and the base peaks come from the process of Scheme 3 and are very stable and present generally an important relative abundance (from 30% to 100%). In this scheme, the fragment m/z = M-17 appears at m/z = M-18 for compound 1a because the substituent X is a hydrogene. The same process (Scheme 4) takes place without losing the X group to give the B and C fragments.
SCHEME 4.
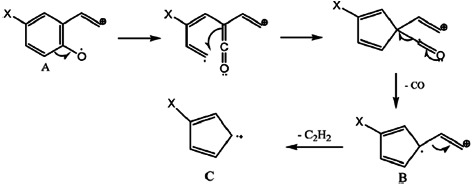
B: X = H; m/z = 90; X = CI, m/z = 126–124; X = Br: m/z = 170–168; X = NO2: m/z = 135; C: X = H m/z = 63; X = CI: m/z = 100–98 (as 99.5); X = Br, m/z = 144–142.
On the other hand, the M-CO fragment A can lose an acetylene molecule to give D. This process is given in Scheme 5.
SCHEME 5.
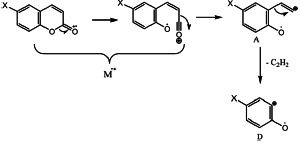
A: X = H; m/z = 118; X = CI: m/z = 154–152. X = Br: m/z = 198–196; X = NO2: m/z = 163. D: X = H m/z = 92; X = CI: m/z = 128–126; X = Br: m/z = 172–170; X = NO2: m/z = 137.
Three surprising fragments have been obtained in the spectrum of 1d (X = NO2). These are the fragments with m/z = 105, 77, and 51. This compound is the only one that gives these fragments. These pics are characteristics of the formation of a benzoyl fragment,[24,25,32] We assume that this phenomenon can be attributed to the presence of the nitro group, and the process give in Scheme 6 can explain it. The process starts from the fragment A by a hydrogen radical transfer.
SCHEME 6.
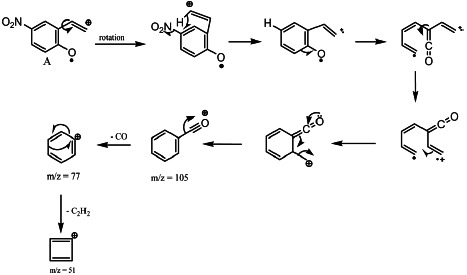
Formation of fragments m/z = 105, 77, and 51.
The high withdrawing character of the nitro group authorizes this hydrogen transfer. The high positive electronic charges of nitrogen and the very high negative one of C6 of the compound 1d are in good agreement with this process. A previous study of proton transfer has been made by mass spectrometry and by AM1 for amidines.[35] It has been found, according to heats of formation (?H) of each fragment in gas phase, that the transfer is favored by the electronic effect of substituents, and our results seem to conform to this observation.
Formation of the fragments m/z = 105, 77, and 51 is usually observed for monosubstituted benzoyl derivatives. This behavior has been observed recently for the 4-acyl isochroman-1,3-diones, another type of heterocyclic compound with fused rings.[36]
CONCLUSIONS
In this study, it has been found that the electronic charges of carbons and heteroatoms (oxygen, nitrogen, or halogen) obtained by AM1 semiempirical method are useful to suggest the site of electronic beam impact and to determine a fragmentation's starting points and its pathways. An unusual fragmentation of one of the coumarins synthesized has been obtained and explained. Those, the 6-nitro coumarin 1d reacts in mass spectrometry like mono-substituted benzoyl derivatives.
REFERENCES
- 1.Murray R. D. Coumarins. Nat. Prod. Rep. 1989;6:591–624. doi: 10.1039/np9890600591. [DOI] [PubMed] [Google Scholar]
- 2.Barry R. D. Isocoumarins: Development since 1950. Chem. Rev. 1964;64:229. [Google Scholar]
- 3.O'Kennedy R., Thornes R. D. Coumarins-Biology, Applications and Mode of Action. UK: John Wiley & Sons: Chichester; 1997. [Google Scholar]
- 4.Vogel A. I., Tatchell A. R., Furnis B. S., Hannaford A. J., Smith P. W. G. Vogel's Textbook of Practical Organic Chemistry. 4th ed. Longman: London and New York, 1981; 761 pp.
- 5.Chin-Neng H., Pai-Yu K., Chi-Hui L, Ding-Ya Y. Synthesis and characterisation of 2H-pyrano[3,2-c]coumarin derivatives and their photochromic and redox properties. Tetrahedron. 2007;63:10025–10033. [Google Scholar]
- 6.Kostova I. Synthetic and natural coumarins as cytotxic agents. Curr. Med. Chem. Anti-Cancer Agents. 2005. 5, 29–46. [DOI] [PubMed]
- 7.Stojadin V. D., Vidoslav S. D., Branimirka V., Biljana R. D., Milan S. D. Synthesis of New coumarin derivatives. Facta Universitatis Series Physics. Chemistry and Technology. 2007;5(1):85–88. [Google Scholar]
- 8.Behel F., Quelever G., Lelouard H., Petit A., da Costa C. A., Pourquie O., Checler F., Thellend A., Pierre P., Kraus J.-L. Synthesis of new 3-Alkoxy-7-amino-4-chloro-isocoumarin derivatives as new ?-amyloid peptides production inhibitors and their activities on various classes of proteases. Bioorg. Med. Chem. 2003;11:3141–3152. doi: 10.1016/s0968-0896(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 9.Al-Haiza M. A., Mostafa M. S., El-Kady M. Y. Preparation of some new coumarin derivatives with biological activity. Scientific Journal of King Faisal University (Basic and Applied Sciences) 2005;6(1):1426. [Google Scholar]
- 10.Reddy N. S., Gumireddy K., Muralidhar R., Mallureddygari S. C., Consenza P. R., Bell S. C., Reddy P., Reddy M. V. R. Novel coumarin-3-(N-aryl)carboxamides arrest breast cancer cell growth by inhibiting ErbB-2 and ERK1. Bioorg. Med. Chem. 2005;13(9):3141–3147. doi: 10.1016/j.bmc.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 11.Kollroser M., Shober C. Determination of coumarin-type anticoagulants in human plasma by HPLC-ESI-tandem mass spectrometry with an ion trap detector. Clin. Chem. 2002;48:84–91. [PubMed] [Google Scholar]
- 12.Nofal Z. M., El-Zahar M. I., Abd El-Karim S. S. Novel coumarin derivatives with expected biological activity. Molecules. 2000;5:99–113. [Google Scholar]
- 13.Tkach I. I., Luk'yanets E. A. Some new reactions of coumarins. Chem. Heteorcycl. Comp. 1992;28(8):881–883. [Google Scholar]
- 14.Takadate A., Masuda T., Murata C., Tanaka T., Irikura M., Goya S. Fluorescence characteristics of methoxycoumarins as novel fluorophores. Anal. Sci. 1995. pp. 97–101.
- 15.Yun J. J., Byung-Sik M., Min S. P., Bong-Gu K., Ji Y. K., Jay-Sung J. H., Yeo J. Y., Kap D. L., Juyoung Y. New Cavitand derivatives bearing four coumarin groups as fluorescent chemosensors for Cu2+ and recognition of dicarboxylates utilising Cu2+ complex. Tetrahedron. Lett. 2006;47:2707–2710. [Google Scholar]
- 16.Dewar M. J. S., Zoebish E. G., Healy E. F., Steward J. J. P. AM1: A new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985;107:3902–3909. [Google Scholar]
- 17.Saba A., Sib F. S., Aycard J. P. Isocoumarins: Structural study by NMR and by AM, semi-empirical method. Spectrosc. Lett. 1995;28(7):1053–1060. [Google Scholar]
- 18.Lin T., Peng B-X., Bai F. The structure and 13C NMR of indolenium squaraine dye. Dyes and Pigments. 1999;43(2):67–71. [Google Scholar]
- 19.Saba A., Sib F. S., Faure R., Aycard J. P. NMR and AM1 study of the tautomeric equilibrium of isochroman-i,3-diones. Spectrosc. Lett. 1996;29(8):1649–1657. [Google Scholar]
- 20.Allegretti P. E., Cortiso M. S., Guzman C., Castro E. A., Furlong J. J. P. Tautomerism of lactones and related compounds. Mass spectrometric data and theorical calculations. ARKIVOC. 2003;x:24–31. [Google Scholar]
- 21.Khz J., Dibal J., Sedlakova Z. NMR and AM1 quantum chemical study of the radioselectivity of reaction of 2-hydroxyethyl methacrylate with 3-nitrophthalic anhydride. Collect. Czech. Chem. Comm. 1997;62:69–82. [Google Scholar]
- 22.Sheng-Yu T., Mcgowan J. C., Singh M., Galatsi P., Ellis B. E., Boyd R. K., Brown S. A. Mass spectrometry of some furanocoumarins. Can. J. Chem. 1979;57(15):1995–2003. [Google Scholar]
- 23.Breimaier E., Voelter W. Carbon 13 NMR Spectroscopy. 3rd ed. VCH: Weinheim, 1991; 229 pp.
- 24.Silverstein R. M., Bassler G. C., Morrill T. C. Spectrometric Identification of Organic Compounds. 5th ed. New York: John Wiley & Sons; 1991. [Google Scholar]
- 25.Creswell C. J., Runquist 0., Campbell M. M. Spectral Analysis of Organic Compounds. 2nd ed. Minneapolis: Burgess Publishing Company; 1972. [Google Scholar]
- 26.Justensen U. Collision-induced fragmentation of deprotonated methoxylated flavonoids obtained by electrospray ionisation mass spectrometry. J. Mass Spectrom. 2001;36:169–178. doi: 10.1002/jms.118. [DOI] [PubMed] [Google Scholar]
- 27.Laure F. Thèse de doctorat. Université de Polynésie Française. 2005. pp. 236–250.
- 28.Elgamal H. A., Shalaby N. M. M., Shaban M. A., Duddeck H., Mikhova B., Simon A., Toth G. Synthesis and spectroscopic investigation of some dimeric coumarins and furanocoumarins models. Monatsh. Chem. 1997;128(6–7):701–712. [Google Scholar]
- 29.Vul'fson N. S., Golovkina L. S. Mass spectrometry of chromen-2-ones (coumarins) and of furo- and pyrano-chromenones. Russ. Chem. Rev. 1975;44:603–623. [Google Scholar]
- 30.El-Deen I. M., Ibrahim H. K. Synthesis and investigation of mass spectra of 3-(substituent)-benzopyran[3,2-c]-[1]-benzopyran-6,7-diones. J. Korean Chem. Soc. 2003;47(2):137–146. [Google Scholar]
- 31.Michel A. Thèse de Doctorat és Sciences. Université de Neuchâtel (Belgique); 2001. pp. 35–55. [Google Scholar]
- 32.Silverstein R. M., Webster F. X., Kiemle D. J. In: Identification Spectro-métrique de Composes Organiques. de Boeck, editor. Bruxelles; 2007. pp. 27–70. [Google Scholar]
- 33.Cuyckens F., May L, Pocsfalvi G., Claeys M. Tandem mass spectral strategies for the structural characterisation of flavonoids glycosides: Structure elucidation by LC-MS. Analysis. 2000;28(10):888–895. [Google Scholar]
- 34.Kutney P. J., Engendorf G., Inaba T., Dreyer D. L. Mass spectral fragmentation studies in monomeric and dimeric coumarins. Org. Mass Spectrom. 2005;5(3):249–263. [Google Scholar]
- 35.Taft R. W., Raczynska E. D., Maria P. C., Leito I., Lewadowski W., Kurg R., Gal J. F., Decouzon M., Anvia F. Application of experimental (FT-ICR) and theoretical (AM1) methods to the study of proton-transfert reactions for tautomerizing amidines in the gas phase. Fresenius J. Anal. Chem. 1996;355:412–414. doi: 10.1007/s0021663550412. [DOI] [PubMed] [Google Scholar]
- 36.Djande A., Kabore L., Saba A., Aycard J. P. Study of the fragmentation of 4-acyl isochroman-1,3-diones in mass spectrometry. Phys. Chem. News. 2006;31:125–131. [Google Scholar]


