Abstract
It is accepted practice to close large patent arterial ducts (PDA) with Amplatzer duct occluder devices, with extremely low rates of residual PDA. We report a child who required device closure of PDA with two Amplatzer PDA devices on two separate occasions, despite the first device deployment being a standard placement of an appropriately sized Amplatzer device in the usual position.
MeSH: Ductus Arteriosus, Patent Heart defects, congenital Embolization, Therapeutic, Instrumentation Heart Catheterization/Instrumentation
Introduction
Since the first case of transcatheter closure of patent ductus arteriosus (PDA) by Porstmann in 1967,1 device closure has become a mainstream form of intervention for this lesion with a wide variety of devices.2
The Amplatzer PDA occluder is a self-expanding nitinol double-disk device and consists of two disks of varying sizes with the larger disk positioned at the aortic end of the duct. Since its introduction, this device remains, to date, the device of choice for the transcatheter occlusion of large (>3mm) PDAs,3 and the exact technique for device deployment has been previously described.4
We report one child who required device closure of PDA with two Amplatzer PDA devices on two separate occasions.
Patient
Our patient was born in March 2001 and a murmur was noted shortly after birth. Echocardiography eventually showed a large PDA, and followup did not reveal signs of pulmonary hypertension. An Amplatzer PDA device (8 by 6 mm) was implanted at almost 3 years of age (figure 1).
Figure 1.
Large PDA on lateral view measured at 6 mm.
Clinical and echocardiographic signs of significant left heart volume overload persisted. Repeat cardiac catheterization showed a significant residual shunt (figures 2 and 3) and a second device (10 by 8 mm) was implanted at 4 years and 3 months of age, 1½ years after the first device was implanted (figures 4 and 5), with little residual shunting.
Figure 2.
Lateral view showing significant residual shunting through the PDA.
Figure 3.
Postero-anterior view showing significant residual shunting through the PDA.
Figure 4.
Lateral view showing two devices in place with little residual shunting.
Figure 5.
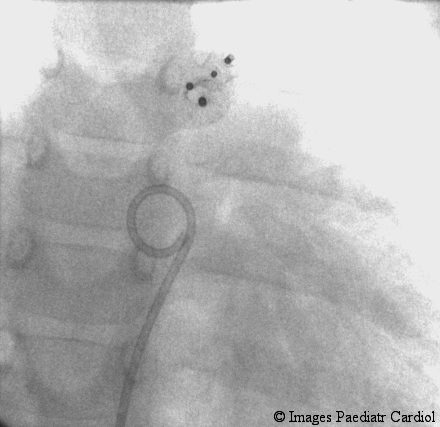
Postero-anterior view showing two devices in situ.
Discussion
The Amplatzer ductal occluder is a safe device and has been utilized extensively with few complications in competent hands.5 Closure rates of >99% have been documented,5 and follow-up has not revealed any episodes of delayed device migration, endocarditis, thromboembolism, or wire fracture/device disruption.6 Our patient is unusual in that despite standard placement of an appropriately sized Amplatzer device in the usual position, significant residual shunting necessitated the placement of a second device.
References
- 1.Porstmann W, Wierny L, Warnke H, Gerstberger G, Romaniuk PA. Transcathter closure of patent ductus arteriosus: 62 cases treated without thoractomy. Radiol Clin North Am. 1971;9:203–211. [PubMed] [Google Scholar]
- 2.Atiq M, Aslam N, Kazmi KA. Transcatheter closure of small-to-large patent ductus arteriosus with different devices: queries and challenges. J Invasive Cardiol. 2007;19:295–298. [PubMed] [Google Scholar]
- 3.Masura J, Walsh KP, Thanopoulous B, Chan C, Bass J, Goussous Y, Gavora P, Hijazi ZM. Catheter closure of moderate- to large-sized patent ductus arteriosus using the new Amplatzer duct occluder: immediate and short-term results. J Am Coll Cardiol. 1998;31:878–882. doi: 10.1016/s0735-1097(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 4.Boehm W, Emmel M, Sreeram N. The Amplatzer duct occluder for PDA closure: indications, technique of implantation and clinical outcome. Images Paediatr Cardiol. 2007;31:16–26. [PMC free article] [PubMed] [Google Scholar]
- 5.Bilkis AA, Alwi M, Hasri S, Haifa AL, Geetha K, Rehman MA, Hasanah I. The Amplatzer duct occluder: experience in 209 patients. J Am Coll Cardiol. 2001;37:258–261. doi: 10.1016/s0735-1097(00)01094-9. [DOI] [PubMed] [Google Scholar]
- 6.Faella HJ, Hijazi ZM. Closure of the patent ductus arteriosus with the amplatzer PDA device: immediate results of the international clinical trial. Catheter Cardiovasc Interv. 2000;51:50–54. doi: 10.1002/1522-726x(200009)51:1<50::aid-ccd11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]


