Abstract
Ferritin is a macromolecule and is responsible for the long term iron storage function in human serum and plasma. Recent studies have highlighted the role of cell phone exposure on central nervous system, immune function and reproduction. The aim of this study was to investigate whether the human serum ferritin level could be interfered by the exposure to the 900 MHz GSM cell phones. Fifty human serum wells from 25 normal healthy donors were labeled with ruthenium to form a sandwich complex based on an immunoassay technique. All of them were placed into two batches, and the well heads in the first batch were exposed to 900 MHz exposure emitted from a speech mode cell phone (Nokia, Model 1202, India) for 30 min. Unexposed batch was served as the control sample under identical conditions and was compared with the exposed one in quantitative determination of ferritin using the Wilcoxon test with criterion level of P = 0.050. Human serum wells in the exposed batch showed a significant decrease in serum ferritin relative to the control batch (P = 0.029). The average ± SD ferritin level in the exposed batch was 84.94 ± 1.04 μg/L while it was 87.25 ± 0.83 μg/L for the unexposed batch. Radiofrequency electromagnetic waves emitted from cell phones may lead to oxidative stress and rapid diffusion of the human ferritin level in an in vitro enzymun assay. Also, the enzyme activity can be affected. Effects of exposure from mobile phones must be considered further.
Keywords: Human ferritin, immunoassay test, radiofrequency radiation, ruthenium complex
INTRODUCTION
Iron (Fe) is an essential micronutrient in biological systems and plays an important role in several cellular processes, such as adenosine triphosphate (ATP), deoxyribonucleic acid (DNA), and neurotransmitter synthesis.[1,2] Tissue iron is stored inside a porous protein capsule called ferritin.[3] Ferritin is a macromolecule and is responsible for the long term iron storage function mainly in the liver, spleen, and bone marrow. Many diseases are associated with iron overload or iron deficiency. Determination of ferritin is a suitable method for ascertaining the iron metabolism situation which provides a representative measure of body's iron reserves.[4] For determination of ferritin, human serum is labeled with ruthenium to form a sandwich complex based on an immunoassay technique.[4]
In recent years, tremendous development and use of mobile phone telecommunication has drastically increased the amount of human exposition from the microwaves (MWs) radiation in everyday life. In this regard, a growing concern about the possible health hazards have increased considerably, since mobile phones MW may have health effects on almost everyone in the world, even those who do not have such phone.[5]
The World Health Organization (WHO) established the International Electro Magnetic Field (EMF) Project in 1996 to assess health and environmental effects of exposure to EMF in the frequency range from 0 to 300 Giga Hertz (GHz).[6,7] The mobile phone technology uses 880 and 1800 MHz.[8]
Many studies on different levels of organization (human populations, animal and plant, organs and sub-cellular) have been carried out or are in progress about the various effects of radiation emissions regarding the behavior, cancer, central nervous system, sleep, children, cardiovascular system, immune function, reproduction and development.[5,9–12]
The emitted microwaves have been shown to have many effects upon the mammalian brain,[12] sperm motility and morphology,[9] neurological pathologies syndrome[13] and immune functions.[5] However, many potential harmful effects of cell phone radiation remain controversial. Nittby et al. have investigated that this energy affects the mammalian blood-brain barrier permeability 7 days after exposure to the radiation from a 900 MHz global systems for mobile (GSM) communications.[14] Hinrikus et al. have shown that, the human brain EEG beta rhythms energies were increased by exposure to 450 MHz MW.[15] Lai et al. investigated the effects of microwave exposure on cholinergic systems in rat brain showing that, it is possible to establish a dose-response relationship for each brain region when different microwave power densities are used.[16–18] Recent experimental studies in mice have shown that exposure to EMF led to significant testicular germ cell apoptosis and morphological changes.[19–21] Lu et al., have found that household use of air conditioners, which generate significant amounts of EMF, has been associated with low semen quality.[22] Recently Shahbazi et al. have investigated that, mobile phones exposure results in reduction of chorionic gonadotropin level in human serum during the immunoenzymometric assay in laboratory.[23]
In addition, it is now generally accepted that weak EMF in the power frequency range can activate DNA to synthesize proteins.[24] Furthermore, recent studies have shown that long-term exposure decreases thyroid stimulating hormone (TSH) and tri-iodothyronine-thyroxin (T3–T4) levels.[25,26] Moreover, de Tommaso et al. found that, 900 MHz GSM exposure induces changes of contingent negative variation in healthy volunteers, especially reducing the arousal and expectation of warning stimulus.[12] Cespedes et al. found that, exposed proteins to the radio frequency (RF) magnetic fields of 1 MHz and 30 mT for several hours released more iron than control samples.[27] Using the same exposing protocol, in another research, Cespedes et al. showed that the rates of iron chelation with ferrozine, were reduced by up to a factor of 3 in the exposed proteins. They stated that, the observed effect was non-thermal and depended on the frequency-amplitude product of the magnetic field.[28] In addition, considering the natural superparamagnetic ferrihydrite nanoparticles of protein ferritin, Cespedes et al., demonstrated that superparamagnetic nanoparticles increased their internal energy when exposed to radio frequency magnetic fields due to the lag between magnetization and applied field.[29]
According to the best of our knowledge, no published articles have presented a study on this issue with the methodology and analysis described here on the effects of cell phone radiation on the immunoassays for the in vitro quantitative determination of human serum ferritin. The aim of this study was to investigate whether the ferritin levels in the complex could be interfered by the exposure to the 900 MHz GSM cell phones in the laboratory.
MATERIALS AND METHODS
The study was approved by the Pathology Research Board at the Ahvaz Jundishapour University of Medical Sciences, Khozestan, Iran.
Data Collection and Exposure
This study was performed based on an immunoassay technique for the in vitro quantitative determination of human serum ferritin in a ruthenium sandwich complex. Human serum samples were collected from 25 donors and were labeled with ruthenium. Each sample was divided to two aliquots and was placed into two batches: The first batch (exposed group) was exposed to a commercially available cell phone (Nokia, Model 1202, India) with 1.09 Watt per kilogram (W/kg) of the tissue locally in the head specific absorption rate,[30] which produces 900 MHz RF radiation, to represent the exposure of GSM. Phone was in the talk mode during expose. The phone had a hidden antenna placed on the top back of its handset. The distance between the phone antenna and each specimen was kept at 2.5 cm [Figure 1]. The duration of exposure was 30 min. For the second batch (control group), no radiation was applied to the samples and they completed the assay cycle under identical conditions during the study period.
Figure 1.
Specimens were exposed to radiation emitted from the speech mode mobile phone at 2.5 cm distance
Immunoassay with Ruthenium Complex
For the assay two monoclonal antibodies M-4.184 and M-3.170 (Cobas, Roche Diagnostics Ltd, Mannheim, Germany) were used to form the sandwich complex. In the first incubation, 10 μL of each sample, a biotinylated monoclonal ferritin-specific antibody and a monoclonal ferritin-specific antibody were labeled with ruthenium to form a sandwich complex. In the second incubation, after addition of streptavidin-coated microparticles, the complex was became bound to the solid phase via interaction of biotin and streptavidin. The reaction mixture was aspirated into the measuring cell where the microparticles were magnetically captured onto the surface of the electrode. Following application of a voltage to the electrode the induced chemiluminescent emission was measured by a photomultiplier (Hitachi High-Technologies Corporation, Tokyo, Japan). All specimens (aliquots) were kept at room temperature to avoid the effect of temperature on the assay. Finally, results were determined via a calibration curve which was generated instrument-specifically with a master curve via the reagent barcode.
All experiments were performed comparing 1.09 W/kg exposed batch with the unexposed control batch. To avoid the variability inherent to the assay used, all tests were performed for two independent experiments.
Statistical Analysis
Mean values and standard deviations were calculated and statistical significance of the differences between exposed samples and controls was evaluated. A computer program (SPSS version 16.0, Chicago, IL, USA) was used for statistical analysis. Data were analyzed by Wilcoxon test (Nonparametric version of paired samples T-test). All hypotheses tested using a criterion level of P = 0.05.
Power Density
According to the International Commission for Non-Ionizing Radiation Protection (ICNIRP)[31] and the Federal Communications Commission (FCC),[32] the reference level for exposure of RF-EMW is peak power density. It is a commonly used term for characterizing an RF electromagnetic field. There were some irradiation sources in the laboratory (i.e., the wireless networks in the laboratory). Since, the study was performed in a distinct place of the laboratory and background radiation for all the batches (exposed and control groups) were identical, power density of these sources was not monitored during the study.
RESULTS
Radiation exposure from mobile phones altered the serum levels in the exposed wells. Using the Wilcoxon test, a significant decrease in serum ferritin in the exposed group compared to the control group was seen (P = 0.029). The final average ± SD of ferritin level in the exposed group was 84.94 ± 1.04 μg/L while it was 87.25 ± 0.83 μg/L for the control group [Figure 2]. In other words, the present study showed that, the final ferritin measured level in the assay was affected by 30 min radiation exposure from 900 MHz mobile phone at 1.09 W/kg SAR.
Figure 2.
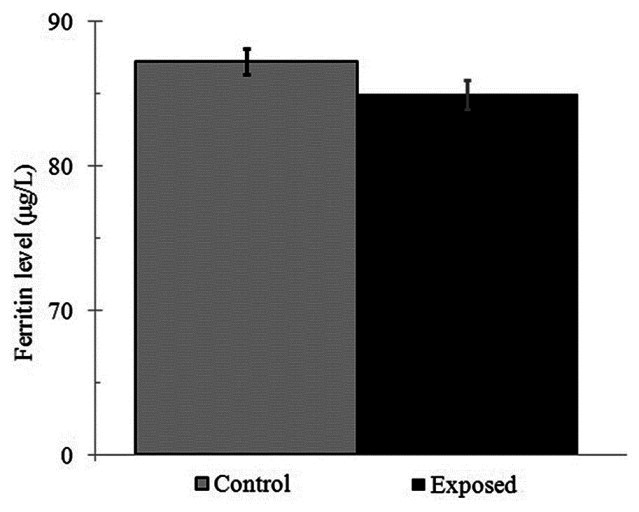
A comparison of average ±SD serum ferritin, among control and the exposed groups
DISCUSSION
Results of this study showed that, human serum ferritin levels labeled with ruthenium were affected following mobile phones exposure. The reason could be due to oxidative stress and rapid diffusion at high electromagnetic irradiation and field caused by mobile phone. Moreover, the enzyme activity can be affected.
Serum ferritin is widely used in diagnosing and monitoring neurodegenerative disorders-Parkinson's and Alzheimer's disease, progressive supranuclear palsy and neuroferritinopathy.[33] As most iron in the human is present as ferritin-bound iron, it is obvious that any change in the structure or function of ferritin may be related to oxidative stress and damage. However, the mechanisms and regulation of ferritins iron release and reutilization have not yet been fully elucidated.[34] For determination of ferritin, immunoassay technique based on sandwich complex of human serum and ruthenium is used.
It has been shown that RF fields at 300 MHz to several GHz, at which significant local and non-uniform absorption occurs, induce torques on molecules that can result in displacement of ions from unperturbed positions, vibrations in bound charges (both electrons and ions), and rotation and reorientation of dipolar molecules such as water.[5]
The findings here were in good agreement with previously published literature[31–33] Zagorskaia and Rodina, found that lowered concentrations of thyroid hormones labeled with Iodine-125 during 2 months after a single exposure of rats to 20 mT extremely low frequency EMF[35] Diem et al., reported DNA single- and double-strand induced breaks due to 1800 MHz RF-EMF exposure at 1.2 W/kg SAR.[36] Ammari et al., investigated the effects of a chronic GSM 900 MHz exposure on glia in the rat brain. They have concluded that chronic exposure to 900 MHz MW may induce persistent astroglia activation in the rat.[10] Barteri et al., studied the in vitro interaction between RF radiation and proteins of different species. They have demonstrated that mobile phone exposure affects the structural and biochemical characteristics of an important CNS enzyme.[37] Nittby et al. have investigated that albumin extravasation enhanced in the rats which were exposed to mobile phones at 12 mW/kg SAR.[14] While, Lantow et al. demonstrated that RF-EMF exposure of human monocytes and lymphocytes did not have any activating capacity to induce hsp70 expression.[38] Gurisik et al. found no significant differences between sham-exposed and RF-exposed cells in any of the assays or conditions examined.[39] Lee et al. reported that 1763 MHz RF radiation alone did not elicit any stress response.[40] Yuasa et al., investigated that the pulsed EMF field emitted by a mobile phone for 30 min has not short-term effects on the human somatosensory evoked potentials.[41] Possible reasons for these may be the lack of statistical power due to a small sample size, shorter exposure time and lower levels of SAR and serum concentrations. It has been shown that exposure at 1.09 W/kg SAR, especially in the higher levels of the human chorionic gonadotropin concentrations (>100 mIU/mL), caused a significant loss compared to 0.69 W/kg SAR exposure.[23] Moreover, considering the exposure time, an increased response in the alkaline comet assay in exposed human fibroblasts has been detected at 16 h compared to 4 h exposure time.[36] Therefore, perhaps, the trends seen for decrease in serum ferritin would have reached more statistical significance if exposure times had been longer.
Moreover, it should be noted that since each laboratory has its own conditions and instruments, results obtained in individual laboratories may differ from each other. Considering immunoenzymometric ferritin assay used in this study, it has been reported that repeatability and precision of the results by using different test methods and analyzers may varied from 1.9% and 2.7% to 3.0% and 4.4%, respectively.[39] At this work, serum ferritin level in the exposed group showed 2.7% decrease compared to the control group, which was within the used method's precision (P = 0.029).
The results of this study were obtained by using a commercial 1.09 W/kg at 900 MHz mobile phone to reproduce the reality of the human exposition. Today, an average SAR of 2 W/kg is selected, because it is the safety limit for the mobile-phone microwave-radiation emission as defined by ICNIRP.[31,42] However, results of this study suggested that the allowed radiation emission levels for mobile phones might not be sufficient to prevent biological effects. Of course, further investigations on SAR level dependence and dose dependence are required.
Mobile phones can affect the immunoassay a MW specific, non-thermal action, and a thermal molecular effect, or a combination of these mechanisms. Few studies that made the measurements before and after but not during exposure to EMF have ever demonstrated a significant effect of non-thermal levels of electromagnetic radiation on brain.[43] Panagopoulos et al., studied the mechanism of EMF action on cells plasma membrane using a biophysical model. Their results showed that, an external EMF causes forced-vibration of all the free ions on the membrane surface.[44]
The present results are consistent with our previous study which found significant effects of the mobile phone use on the immunoenzymometric assays.[23] Even though we studied only one aspect of the human serum, using available in vitro methods, we conclude that short term effects can be detected on the human ferritin after 30 min mobile phone exposure.
Due to limitations and difficulties associated with in vivo studies, at this work an in vitro experiment was performed to investigate the effects of exposure to the cell phone on the ferritin level. Nevertheless, in vivo studies, whether on animals or human beings, may provide more convincing evidence of the EMF effects.[12]
More accurate follow-up studies are needed for the evaluation of the effects of the mobile phone use. The results here should be confirmed in larger series, employing repeated exposure-dose related effect design and providing a detailed assessment of EMF magnetic fields produced by the phones.
On the basis of the present results, we can believe that mobile phone exposure may affect human long term iron storage function by reducing the ferritin level in the human plasma and serum. Considering that this is the first study dealing with the possible effects of EMF on the in vitro immunoassays for the quantitative determination of ferritin, further in vivo studies on mammalians are of utmost importance. However, the evaluation of the possible effects of cell phones radiation and the emitted MWs on the living organism is a complex process that needs the combined contributions of many scientific regulations.
BIOGRAPHIES

Jafar Fattahi-asl was born in Kohkiloie and Boierahmad, Iran, in 1970. He is now faculty member of Medical Physics group in Ahvaz Jundishapour University of Medical Sciences, Khozestan, Iran. His research interests include MRI and contrast agents, Monte Carlo simulation and non-ionizing radiation.
E-mail: jafarfatahi@yahoo.com

Molood Baradaran-Ghahfarokhi is now M.S student of Electrical Engineering in Isfahan University of Technology, Isfahan, Iran. Her research interest includes Biomedical Engineering.
E-mail: m.baradaran@ec.iut.ac.ir

Mojtaba Karbalae is now Ph.D student of Medical Physics in Isfahan University of Medical Sciences, Isfahan, Iran. His research interests include image processing, Monte Carlo simulation, radiotherapy physics, and non-ionizing radiation. He has published several research papers in these areas.
E-mail: mehdi_karbalaee@yahoo.com

Milad Baradaran-Ghahfarokhi was born in Isfahan, Iran, in 1983. He received the B.S degree in Mechanical Engineering and M.S degree in Medical Radiation Engineering from Shiraz University, Shiraz, Iran. He is now Ph.D student of Medical Physics in Isfahan University of Medical Sciences, Isfahan, Iran. His research interests include biomechanical Finite Element Modeling, Monte Carlo simulation, 4D radiotherapy, radiation shielding design and manufacturing and non-ionizing radiation.
E-mail: milad_bgh@yahoo.com

Hamid Reza Baradaran-Ghahfarokhi is from Molecular Medicine and Reproductive Endocrinology Research Center, Shahid Beheshty University of Medical Sciences, Tehran, Iran. His research interests include diagnosis, treatment and prevention of children metabolic disorders, prenatal screening and molecular techniques in diagnosis, treatment and prevention of cardiovascular disease. He has published several research papers in these areas.
E-mail: hmd_brdrn@yahoo.com
ACKNOWLEDGMENT
This research was supported by a Grant from the Ahvaz Jundishapour University of Medical Sciences, Khozestan, Iran (Grant No. U-91097).
Footnotes
Source of Support: Ahvaz Jundishapour University of Medical Sciences, Khozestan, Iran (Grant No. U-91097)
Conflict of Interest: None declared
REFERENCES
- 1.Beard JL, Connor JR, Jones BC. Iron in the brain. Nutr Rev. 1993;51:157–70. doi: 10.1111/j.1753-4887.1993.tb03096.x. [DOI] [PubMed] [Google Scholar]
- 2.Kauppi B, Nielsen BB, Ramaswamy S, Larsen IK, Thelander M, Thelander L, et al. The three-dimensional structure of mammalian ribonucleotide reductase protein R2 reveals a more-accessible iron-radical site than Escherichia coli R2. J Mol Biol. 1996;262:706–20. doi: 10.1006/jmbi.1996.0546. [DOI] [PubMed] [Google Scholar]
- 3.Clegg GA, Fitton JE, Harrison PM, Treffry A. Ferritin: Molecular structure and iron-storage mechanisms. Prog Biophys Mol Biol. 1980;36:56–86. [PubMed] [Google Scholar]
- 4.Jacobs A, Hodgetts J, Hoy T. Ferritins and isoferritins as biochemical markers. Amsterdam: Elsevier; 1984. pp. 113–27. [Google Scholar]
- 5.Repacholi MH. Health risks from the use of mobile phones. Toxicol Lett. 2001;120:323–31. doi: 10.1016/s0378-4274(01)00285-5. [DOI] [PubMed] [Google Scholar]
- 6.Hamblin DL, Croft RJ, Wood AW, Stough C, Spong J. The sensitivity of human event-related potentials and reaction time to mobile phone emitted electromagnetic fields. Bioelectromagnetics. 2006;27:265–73. doi: 10.1002/bem.20209. [DOI] [PubMed] [Google Scholar]
- 7.Dasdag S, Zulkuf Akdag M, Aksen F, Yilmaz F, Bashan M, Dasdag Mutlu M, et al. Whole body exposure of rats to microwaves emitted from a cell phone does not affect the testes. Bioelectromagnetics. 2003;24:182–8. doi: 10.1002/bem.10083. [DOI] [PubMed] [Google Scholar]
- 8.Valberg PA, van Deventer TE, Repacholi MH. Workgroup report: Base stations and wireless networks-radiofrequency (RF) exposures and health consequences. Environ Health Perspect. 2007;115:416–24. doi: 10.1289/ehp.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal A, Desai NR, Makker K, Varghese A, Mouradi R, Sabanegh E, et al. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: An in vitro pilot study. Fertil Steril. 2009;92:1318–25. doi: 10.1016/j.fertnstert.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Ammari M, Brillaud E, Gamez C, Lecomte A, Sakly M, Abdelmelek H, et al. Effect of a chronic GSM 900 MHz exposure on glia in the rat brain. Biomed Pharmacother. 2008;62:273–81. doi: 10.1016/j.biopha.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Chang KB, Huang AT, Joines WT, Kramer RS. The effect of microwave radiation (1.0 GHz) on the blood-brain barrier in dogs. Radio Sci. 1982;17:165–8. [Google Scholar]
- 12.de Tommaso M, Rossi P, Falsaperla R, Francesco Vde V, Santoro R, Federici A. Mobile phones exposure induces changes of contingent negative variation in humans. Neurosci Lett. 2009;464:79–83. doi: 10.1016/j.neulet.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 13.Leszczynski D, Joenvaara S, Reivinen J, Kuokka R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: Molecular mechanism for cancer- and blood-brain barrier-related effects. Differentiation. 2002;70:120–9. doi: 10.1046/j.1432-0436.2002.700207.x. [DOI] [PubMed] [Google Scholar]
- 14.Nittby H, Brun A, Eberhardt J, Malmgren L, Persson BR, Salford LG. Increased blood-brain barrier permeability in mammalian brain 7 days after exposure to the radiation from a GSM-900 mobile phone. Pathophysiology. 2009;16:103–12. doi: 10.1016/j.pathophys.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Hinrikus H, Bachmann M, Lass J, Karai D, Tuulik V. Effect of low frequency modulated microwave exposure on human EEG: Individual sensitivity. Bioelectromagnetics. 2008;29:527–38. doi: 10.1002/bem.20415. [DOI] [PubMed] [Google Scholar]
- 16.Lai H, Horita A, Chou CK, Guy AW. Low-level microwave irradiations affect central cholinergic activity in the rat. J Neurochem. 1987;48:40–5. doi: 10.1111/j.1471-4159.1987.tb13124.x. [DOI] [PubMed] [Google Scholar]
- 17.Lai H, Horita A, Guy AW. Acute low-level microwave exposure and central cholinergic activity: Studies on irradiation parameters. Bioelectromagnetics. 1988;9:355–62. doi: 10.1002/bem.2250090405. [DOI] [PubMed] [Google Scholar]
- 18.Lai H, Carino MA, Horita A, Guy AW. Low-level microwave irradiation and central cholinergic activity: A dose-response study. Bioelectromagnetics. 1989;10:203–8. doi: 10.1002/bem.2250100209. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Ahn SS, Jung KC, Kim YW, Lee SK. Effects of 60 Hz electromagnetic field exposure on testicular germ cell apoptosis in mice. Asian J Androl. 2004;6:29–34. [PubMed] [Google Scholar]
- 20.Hong R, Liu Y, Yu YM, Hu K, Weng EQ. Effects of extremely low frequency electromagnetic fields on male reproduction in mice. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2003;21:342–5. [PubMed] [Google Scholar]
- 21.Hong R, Zhang Y, Liu Y, Weng EQ. Effects of extremely low frequency electromagnetic fields on DNA of testicular cells and sperm chromatin structure in mice. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2005;23:414–7. [PubMed] [Google Scholar]
- 22.Lu M, Wu J, Yang Q, Zhou W, Gao E. The effect of air-conditioner exposure on semen quality. Reprod Contracept. 2004;24:279–84. [Google Scholar]
- 23.Shahbazi-Gahrouei D, Mortazavi SM, Nasri H, Baradaran A, Baradaran-Ghahfarokhi M, Baradaran-Ghahfarokhi HR. Mobile phone radiation interferes laboratory immunoenzymometric assays: Example chorionic gonadotropin assays. Pathophysiology. 2012;19:43–7. doi: 10.1016/j.pathophys.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Lin H, Blank M, Goodman R. A magnetic field-responsive domain in the human HSP70 promoter. J Cell Biochem. 1999;75:170–6. doi: 10.1002/(sici)1097-4644(19991001)75:1<170::aid-jcb17>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Koyu A, Cesur G, Ozguner F, Akdogan M, Mollaoglu H, Ozen S. Effects of 900 MHz electromagnetic field on TSH and thyroid hormones in rats. Toxicol Lett. 2005;157:257–62. doi: 10.1016/j.toxlet.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Rajkovic V, Matavulj M, Gledic D, Lazetic B. Evaluation of rat thyroid gland morphophysiological status after three months exposure to 50 Hz electromagnetic field. Tissue Cell. 2003;35:223–31. doi: 10.1016/s0040-8166(03)00029-6. [DOI] [PubMed] [Google Scholar]
- 27.Cespedes O, Inomoto O, Kai S, Ueno S. Effects of Cationization and 6-Hydroxydopamine on the reduced iron release rates from Ferritin by Radio-Frequency Magnetic Fields. IEEE Trans Magn. 2009;45:4865–8. [Google Scholar]
- 28.Cespedes O, Ueno S. Effects of radio frequency magnetic fields on iron release from cage proteins. Bioelectromagnetics. 2009;30:336–42. doi: 10.1002/bem.20488. [DOI] [PubMed] [Google Scholar]
- 29.Cespedes O, Inomoto O, Kai S, Nibu Y, Yamaguchi T, Sakamoto N, et al. Radio frequency magnetic field effects on molecular dynamics and iron uptake in cage proteins. Bioelectromagnetics. 2010;31:311–7. doi: 10.1002/bem.20564. [DOI] [PubMed] [Google Scholar]
- 30.Dasdag S, Ketani MA, Akdag Z, Ersay AR, Sari I, Demirtas OC, et al. Whole-body microwave exposure emitted by cellular phones and testicular function of rats. Urol Res. 1999;27:219–23. doi: 10.1007/s002400050113. [DOI] [PubMed] [Google Scholar]
- 31.Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). International Commission on Non-Ionizing Radiation Protection. Health Phys. 1998;74:494–522. [PubMed] [Google Scholar]
- 32.Cleveland RF, Ulcek JL., Jr . Questions and answers about biological effects and potential hazards of radiofrequency electromagnetic fields. Washington, D.C: Federal Communications Commission; 1999. Aug, [Google Scholar]
- 33.Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochim Biophys Acta. 2010;1800:760–9. doi: 10.1016/j.bbagen.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman A, Arosio P, Finazzi D, Koziorowski D, Galazka-Friedman J. Ferritin as an important player in neurodegeneration. Parkinsonism Relat Disord. 2011;17:423–30. doi: 10.1016/j.parkreldis.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Zagorskaia EA, Rodina GP. Reaction of the endocrine system and peripheral blood of rats to a single and chronic exposure to pulsed low-frequency electromagnetic field. Kosm Biol Aviakosm Med. 1990;24:56–60. [PubMed] [Google Scholar]
- 36.Diem E, Schwarz C, Adlkofer F, Jahn O, Rudiger H. Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res. 2005;583:178–83. doi: 10.1016/j.mrgentox.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Barteri M, Pala A, Rotella S. Structural and kinetic effects of mobile phone microwaves on acetylcholinesterase activity. Biophys Chem. 2005;113:245–53. doi: 10.1016/j.bpc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Lantow M, Lupke M, Frahm J, Mattsson M, Kuster N, Simko M. ROS release and Hsp70 expression after exposure to 1,800 MHz radiofrequency electromagnetic fields in primary human monocytes and lymphocytes. Radiat Environ Biophys. 2006;45:55–62. doi: 10.1007/s00411-006-0038-3. [DOI] [PubMed] [Google Scholar]
- 39.Gurisik E, Warton K, Martin DK, Valenzuela SM. An in vitro study of the effects of exposure to a GSM signal in two human cell lines: Monocytic U937 and neuroblastoma SK-N-SH. Cell Biol Int. 2006;30:793–9. doi: 10.1016/j.cellbi.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Lee JS, Huang TQ, Kim TH, Kim JY, Kim HJ, Pack JK, et al. Radiofrequency radiation does not induce stress response in human T-lymphocytes and rat primary astrocytes. Bioelectromagnetics. 2006;27:578–88. doi: 10.1002/bem.20235. [DOI] [PubMed] [Google Scholar]
- 41.Yuasa K, Arai N, Okabe S, Tarusawa Y, Nojima T, Hanajima R, et al. Effects of thirty minutes mobile phone use on the human sensory cortex. Clin Neurophysiol. 2006;117:900–5. doi: 10.1016/j.clinph.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Non-ionizing radiation, Part 1. Static and extremely low-frequency (ELF) electric and magnetic fields. IARC Monogr Eval Carcinog Risks Hum. 2002;80:1–395. [PMC free article] [PubMed] [Google Scholar]
- 43.Terao Y, Okano T, Furubayashi T, Yugeta A, Inomata-Terada S, Ugawa Y. Effects of thirty-minute mobile phone exposure on saccades. Clin Neurophysiol. 2007;118:1545–56. doi: 10.1016/j.clinph.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Panagopoulos DJ, Karabarbounis A, Margaritis LH. Mechanism for action of electromagnetic fields on cells. Biochem Biophys Res Commun. 2002;298:95–102. doi: 10.1016/s0006-291x(02)02393-8. [DOI] [PubMed] [Google Scholar]


