Abstract
Background:
The differences in digit ratio are proposed to arise due to differential effects of sex steroids on the growth of finger bones. In this study, we sought to examine the sex differences and the influence of family history of psychosis on digit ratio in patients with schizophrenia compared to matched healthy controls (HCs).
Materials and Methods:
Digit ratio (2D: 4D) was examined for a large sample of schizophrenia patients (n=200) and HC (n=177) to evaluate the potential effects of family history.
Results:
The right hand 2D: 4D digit ratio was lesser in schizophrenia patients compared to HC (0.97±0.05 vs 0.98±0.04, t=2.2, P=0.02). There was a significant difference in the right hand 2D: 4D digit ratio of female patients with schizophrenia when compared to female HCs (0.96±0.05 vs 0.98±0.03, t=2.1, P=0.03) while males showed no such difference on either hands. On the contrary, family history‑positive males showed a significantly greater digit ratio for the left hand (FH present (0.99±0.04) vs HC (0.97±0.04), t=2.15, P=0.03), while there was no difference between family history‑positive females and HC.
Conclusion:
Overall, in patients, reversal of expected “directionality” in digit ratio was observed in our study with greater left 2D: 4D in male patients having a family history of schizophrenia being a novel finding. Reversal of sexual dimorphism has been linked to the pathogenesis of schizophrenia. It is possible that such reversal might have a putative genetic basis, perhaps only in men with schizophrenia.
Keywords: Digit ratio, neurodevelopmental, schizophrenia, sex differences
INTRODUCTION
Schizophrenia is considered as a neurodevelopmental disorder and various somatic markers of aberrant neurodevelopment have been described in literature.[1] It is currently conceptualized that the pathophysiological mechanism of this condition is a delicate interplay between the genetic and environmental factors which shape the phenotype.[2] Prenatal endocrinal influence as a putative explanation for the pathogenesis of schizophrenia has been described.[3] It is important, in this context, to examine the reliable external biological markers which have link to the hormonal system to gain better understanding of the mechanisms behind neurodevelopmental pathology of schizophrenia. In this regard, one of the important markers is alteration in digit ratio.[4] Digit ratio is defined as the ratio of second digit length to fourth digit length (2D:4D). It is established by approximately week 14 of fetal development, remains relatively stable throughout adult life, and can be observed in both hands.[5] The typical pattern in men is shorter index finger than ring finger (“masculine pattern”); whereas in women, both fingers are more often close to equal length (“feminine pattern”). These sex differences in digit ratio are proposed to arise due to differential effects of sex steroids on the growth of finger bones. The intrauterine testosterone promotes the growth of the fourth digit, while estrogen promotes the growth of the second digit.[6] In this regard, lesser 2D: 4D and greater levels of fetal testosterone relative to fetal estrogen, and greater 2D: 4D with lesser fetal testosterone relative to fetal estrogen go hand in hand.[7]
Prenatal testosterone influences the neural mechanisms responsible for social and communicative behavior and in this context, digit ratio abnormalities have been examined in different psychiatric disorders,[8] including addiction, personality attributes,[9] schizophrenia,[4,10] and eating disorders.[11] Both men and women with schizophrenia showed a more “feminine” phenotype in both hands than same-sex controls.[12] In contrast, a gender difference in digit ratio abnormalities has been reported with female subjects displaying this neurodevelopmental marker.[4,13] In addition, 2D:4D asymmetry index was significantly lesser in male schizophrenia patients than male healthy controls (HCs) in a recent study from our group.[4] The neurodevelopmental abnormalities including digit ratio anomalies are considered to be the result of combined effect of physiological insults which occur during the intrauterine fetal development like viral infections, environmental toxins, nutritional compromise, etc., and genetically mediated resistance to stress or the presence of disease genes[14–16] suggesting a “two hit” hypothesis. Studies which looked into the model of inheritance in schizophrenia have found no single gene with major effect and supported the concept of a more obscure nature of inheritance than Mendelian model.[17] In such a case, some of the possible genetic mechanisms would include mitochondrial inheritance, genomic imprinting, and X-linked transmission.[18] It is postulated that this effect is mediated through these sex hormones' differential action on the Homeobox genes[19] and this aspect may suggest a possibility that family history/genetic loading may play an important role. We hypothesized that there could be sex dimorphism in the digit ratio in schizophrenia. In addition, we also speculated that family loading of psychosis could be an important determinant of expression of this marker. In this study, we sought to examine the sex differences of digit ratio in patients with schizophrenia compared to age- and sex-matched HCs in addition to examining the influence of family history of psychosis on digit ratio.
MATERIALS AND METHODS
Schizophrenia (DSM-IV) patients (N=200) who presented to the clinical services of the National institute of Mental Health and Neurosciences (NIMHANS), Bangalore, were recruited after informed consent for the study. The study was approved by the institute ethics committee of NIMHANS. The selection of subjects involved sampling during a period of 18 months ranging from March 2008 to September 2009. Diagnosis of schizophrenia was established using MINI Plus.[20] A detailed elicitation of family history with the help of Family Interview for Genetic Studies (FIGS)[21] was done, specifically exploring for schizophrenia or schizophrenia spectrum conditions in the first degree relatives. Using the available information, if non-affective psychosis was present in first degree relatives but, a confident diagnosis of schizophrenia could not be made, it was coded as schizophrenia spectrum disorder. Wherever possible, the information was collected from the mother. Whenever mother was not available, details were obtained from the father or the nearest relative. All available informants were interviewed for corroboration of the history.
Age-and sex-matched HC without any axis I psychiatric diagnosis/family history of psychiatric disorder in any of the first-degree relatives (N=177) were recruited from the community. Digit lengths were measured from the midpoint of the proximal crease to the tip of the finger, using a digital caliper (Aerospace™) which gives 0.01 mm precision in measurements. Inter-rater reliability was calculated for digit length assessments and intra-class correlation coefficient of >0.98 was obtained between two independent raters.
Statistical analysis
Data entry and statistical analyses were done using Statistical Package for Social Sciences (SPSS – 11.0). Data were assessed using Kolmogorov-Smirnov test and was found to be normatively distributed. Hence, parametric statistical tests were used – independent samples t-test for continuous variables and Chi square tests for categorical variables.
RESULTS
The results are presented in a hierarchical format with:
Comparison between the schizophrenia and HC groups
Comparison between family history positive and family history negative groups with HC
Analysis (2) was done with those with family history of schizophrenia alone and subsequently including the schizophrenia spectrum to the above groups.
The patients and controls were matched based on age (years) (31.61±8.53 vs 33.82±12.07, t=1.64, P=0.10). Among the patients, there were 106 men and 94 women, whereas HC consisted of 92 men and 85 women. There was no significant difference between the groups based on gender distribution (χ2=0.04, P=0.84). For the whole group, the right hand 2D: 4D digit ratio was significantly lesser in schizophrenia patients in comparison with HC (0.97±0.05 vs 0.98±0.04, t=2.2, P=0.02), while no difference in left hand digit ratio was observed (0.98±0.04 vs 0.98±0.03, t=0.4, P=0.65). Further sub-group analyses for sex-differential effects revealed that female patients with schizophrenia had significantly lesser right 2D: 4D than female HCs (0.96±0.05 vs 0.98±0.03, t=2.1, P=0.03). The right and left digit ratios of male patients and the left digit ratio of female patients did not differ from male and female controls, respectively [Table 1 and Figure 1].
Table 1.
Comparison of digit ratio between schizophrenia patients and healthy controls
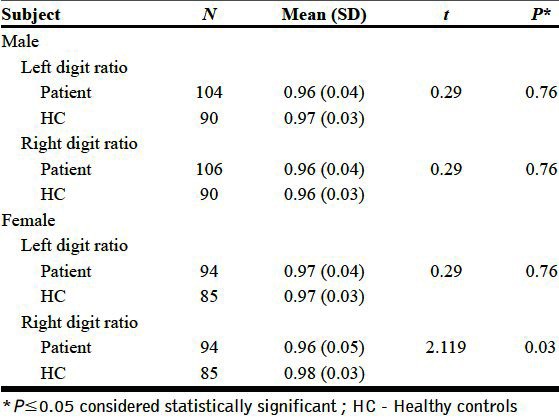
Figure 1.
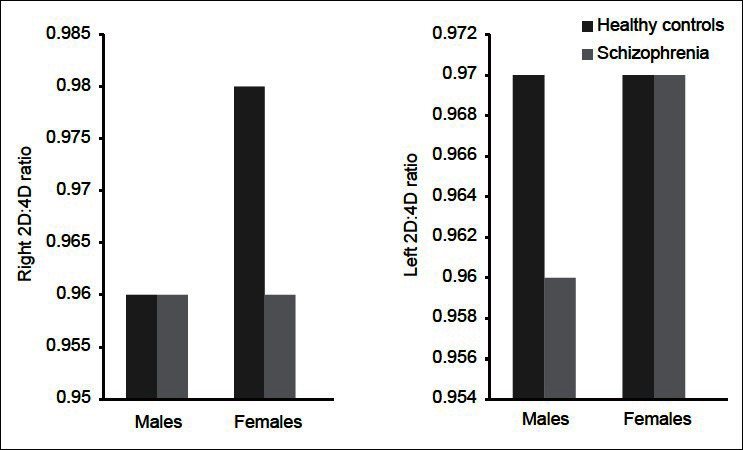
Difference in digit ratio between schizophrenia patients and healthy controls
Sub-group analysis for potential family history-related effects showed that family history-positive men with schizophrenia had a significantly greater left 2D: 4D (FH present (0.99±0.04) vs HC (0.97±0.04), t=2.15, P=0.03) in comparison with male controls. The left and right digit ratios of females did not show any difference compared to HC based on family history status [Tables 2 and 3].
Table 2.
Comparison between family history positive and healthy control groups
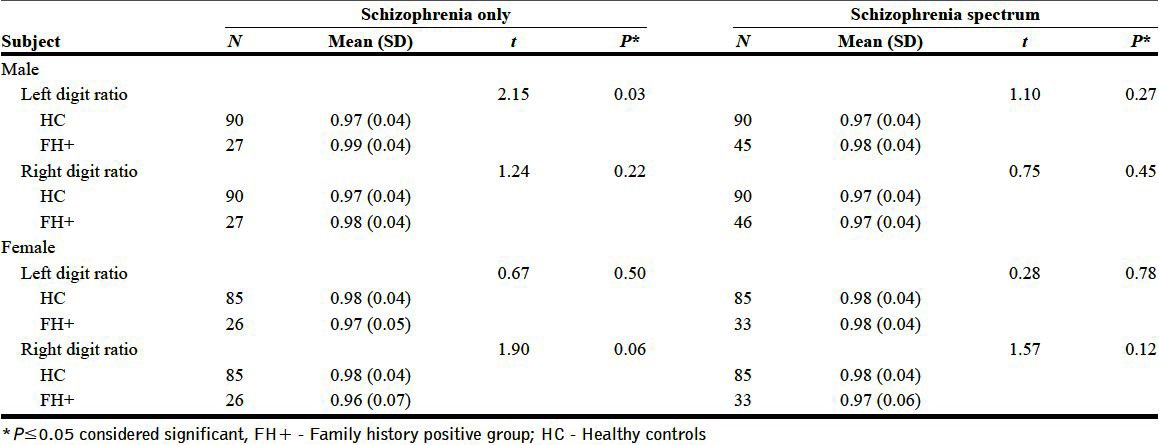
Table 3.
Comparison between family history negative and healthy control groups
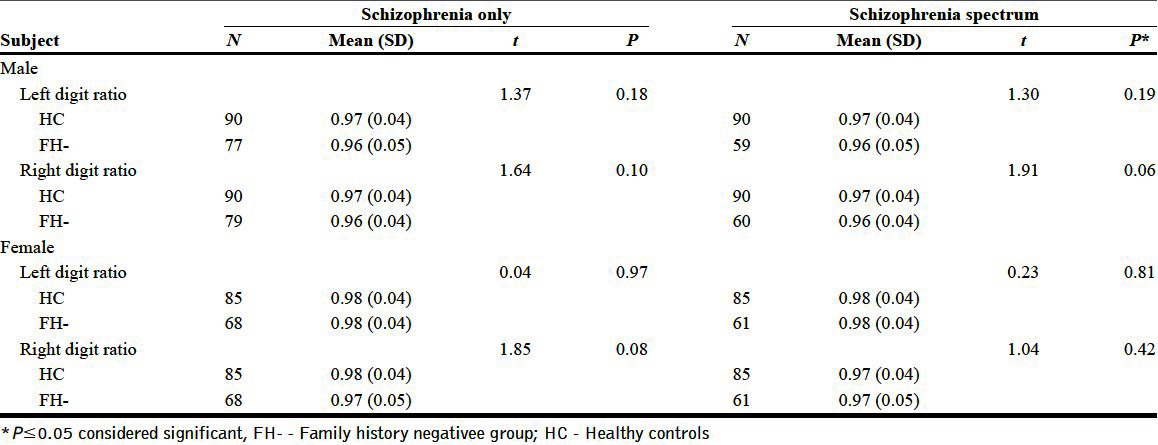
DISCUSSION
In this study, we observed women with schizophrenia to have significantly lesser right 2D: 4D replicating previous study findings.[4] Male patients with schizophrenia did not significantly differ from male controls in 2D: 4D; interestingly, schizophrenia patients with family history-positive status had significantly greater left 2D: 4D than male controls.
The differential effects of intrauterine testosterone and estrogen levels are considered to be responsible for digit ratio changes.[6] There is also emerging evidence for the influence of testosterone on the neural factors responsible for social and communicative behavior.[8] This could possibly link these hormones to behavioral deficits which are precursors to the development of schizophrenia. Schizophrenia patients have also been demonstrated to show disturbed cerebral sexual dimorphism—characterized by less hemispheric asymmetry and hence “dominance failure” for language,[22] which is thought to indicate the effects of sex hormones. Hence, altered digit ratios would provide indirect evidence of the prenatal hormonal changes producing the neurodevelopmental variations.[23]
Our study shows significant difference in digit ratio between schizophrenia and controls demonstrating the neurodevelopmental basis for the pathophysiology of schizophrenia.[10,12] Earlier studies have shown evidence for a “feminised” digit ratio in affected males.[24] On the contrary, affected women had a significantly “masculinised” right digit ratio than HCs in this study. In an earlier study,[12] such a difference has been shown in both the genders on the right hand. In a previous study from our group, a lesser 2D: 4D ratio was detected in female subjects with schizophrenia.[4] In the present study, those males who had a family history of either schizophrenia or a schizophrenia spectrum condition had significantly “feminized” digit ratio than those without. This effect was not apparent in female subjects. A similar finding was demonstrated by Collinson et al., even though not in familial schizophrenia, where males had a greater 2D: 4D ratio.[10] Collinson's paper discusses on the role of Hox genes which are critical for skeletal development. Testosterone and estrogen regulate the Hox expression in the early fetal life and any hormonal variations can dysregulate the expression of the gene. From a default female phenotype, a high level of prenatal testosterone is required for the formation of male phenotype[25] and this in turn may be mediated strongly by genetic factors. Overall, in patients, reversal of expected “directionality” in digit ratio was observed in our study with greater left 2D: 4D in male patients having a family history of schizophrenia being a novel finding. Interestingly, reversal of sexual dimorphism has been linked to the pathogenesis of schizophrenia.[23] It is possible that such reversal might have a putative genetic basis, perhaps only in men with schizophrenia. Thus, sex-specific responses to fetal experiences which occur during sensitive window periods of development may endorse long-term programming changes that cause diseases like schizophrenia. Altogether, it is possible to conceptualize that there could be a reversal in the intensity of the functions of the either sex hormones in a sex-specific manner in schizophrenia, partly mediated by genetic influence. This might thus explain the masculinization and feminization of digit ratios in women and men, respectively, in a pattern as observed in this study.
The existing literature favors epigenetic mechanisms involving hormone expression in the intra-uterine life in mediating sexually dimorphic development and aberrations of this pattern may underlie pathogenetic mechanisms seen in schizophrenia.[26–30] Digit ratios reflect the complex interactions of the environment with the genome. The mechanism of differential growth induced by the relative levels of steroid hormones is yet to be elucidated, but even with these short comings and inconsistencies across studies, the digit ratio continues to be an important tool to study the organizational effects of hormones on the developing brain. The present study has methodological advantages that the digit lengths were measured by digital calipers with a high accuracy and high inter-rater reliability. Relatively small numbers of family history positive probands and the use of family history method for the ascertainment of psychiatric diagnosis of family members remain the short-comings of the study.
In conclusion, it seems possible that the genetic loading for psychosis might have an influence on digit ratio profile in men with schizophrenia with the family history-positive status being associated with relatively “feminine” pattern. Importantly, this observation needs replication. Also, we propose that future studies on schizophrenia should include this dimension and sample larger numbers and study sex-specific subgroup differences. Such approaches involving larger number of familial vs non-familial schizophrenia patients with concurrent assessment of digit ratio and similar measures putatively indicative of fetal environment could yield better understanding on the role of genetic diathesis and related hormonal interplay within the paradigm of fetal programming.
ACKNOWLEDGMENT
This study was funded by Indian Council of Medical Research (ICMR) under Financial Assistance toward MD Thesis Program for Dr. Anjith Divakaran.
Footnotes
Source of Support: Indian Council of Medical Research (ICMR)
Conflict of Interest: None.
REFERENCES
- 1.Weinberger DR. From neuropathology to neurodevelopment. Lancet. 1995;346:552–7. doi: 10.1016/s0140-6736(95)91386-6. [DOI] [PubMed] [Google Scholar]
- 2.Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: Contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29:653–69. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- 3.Brown JS., Jr Association of increased prenatal estrogen with risk factors for schizophrenia. Schizophr Bull. 2011;37:946–9. doi: 10.1093/schbul/sbp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatasubramanian G, Arasappa R, Rao NP, Gangadhar BN. Digit ratio (2D: 4D) asymmetry and schneiderian first rank symptoms: Implications for cerebral lateralisation theories of schizophrenia. Laterality. 2011;16:499–512. doi: 10.1080/1357650X.2010.499910. [DOI] [PubMed] [Google Scholar]
- 5.Manning JT, Bundred PE. The ratio of 2nd to 4th digit length: A new predictor of disease predisposition? Med Hypotheses. 2000;54:855–7. doi: 10.1054/mehy.1999.1150. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre MH. The use of digit ratios as markers for perinatal androgen action. Reprod Biol Endocrinol. 2006;4:10. doi: 10.1186/1477-7827-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum Dev. 2004;77:23–8. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Manson JE. Prenatal exposure to sex steroid hormones and behavioral/cognitive outcomes. Metabolism. 2008;57(Suppl 2):S16–21. doi: 10.1016/j.metabol.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Walder DJ, Andersson TL, McMillan AL, Breedlove SM, Walker EF. Sex differences in digit ratio (2D: 4D) are disrupted in adolescents with schizotypal personality disorder: Altered prenatal gonadal hormone levels as a risk factor. Schizophr Res. 2006;86:118–22. doi: 10.1016/j.schres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Collinson SL, Lim M, Chaw JH, Verma S, Sim K, Rapisarda A, et al. Increased ratio of 2nd to 4th digit (2D: 4D) in schizophrenia. Psychiatry Res. 2010;176:8–12. doi: 10.1016/j.psychres.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Oinonen KA, Bird JL. Age at menarche and digit ratio (2D: 4D): Relationships with body dissatisfaction, drive for thinness, and bulimia symptoms in women. Body Image. 2012;9:302–6. doi: 10.1016/j.bodyim.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Arato M, Frecska E, Beck C, An M, Kiss H. Digit length pattern in schizophrenia suggests disturbed prenatal hemispheric lateralization. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:191–4. doi: 10.1016/j.pnpbp.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Procopio M, Davies RJ, Marriott P. The hormonal environment in utero as a potential aetiological agent for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256:77–81. doi: 10.1007/s00406-005-0604-8. [DOI] [PubMed] [Google Scholar]
- 14.Schaumann BA, Opitz JM. Clinical aspects of dermatoglyphics. Birth Defects Orig Artic Ser. 1991;27:193–228. [PubMed] [Google Scholar]
- 15.Chakraborty R. The role of heredity and environment on dermatoglyphic traits. Birth Defects Orig Artic Ser. 1991;27:151–91. [PubMed] [Google Scholar]
- 16.Tsuang M. Schizophrenia: Genes and environment. Biol Psychiatry. 2000;47:210–20. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- 17.Karmakar B, Malkin I, Kobyliansky E. Inheritance of dermatoglyphic diversity in 500 Indian pedigrees: complex segregation analysis. Anthropol Anz. 2009;67:237–51. doi: 10.1127/0003-5548/2009/0023. [DOI] [PubMed] [Google Scholar]
- 18.Curley JP, Mashoodh R. Parent-of-origin and trans-generational germline influences on behavioral development: The interacting roles of mothers, fathers, and grandparents. Dev Psychobiol. 2010;52:312–30. doi: 10.1002/dev.20430. [DOI] [PubMed] [Google Scholar]
- 19.Buck JJ, Williams RM, Hughes IA, Acerini CL. In-utero androgen exposure and 2nd to 4th digit length ratio-comparisons between healthy controls and females with classical congenital adrenal hyperplasia. Hum Reprod. 2003;18:976–9. doi: 10.1093/humrep/deg198. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 21.Maxwell ME. Clinical Neurogenetics branch, Intramural Research Program, NIMH. 1992. Family interview for genetic studies. [Google Scholar]
- 22.Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20:339–43. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- 23.Mendrek A. Reversal of normal cerebral sexual dimorphism in schizophrenia: Evidence and speculations. Med Hypotheses. 2007;69:896–902. doi: 10.1016/j.mehy.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 24.Daly MP, Gooding DC, Jessen HM, Auger AP. Indicators of developmental deviance in individuals at risk for schizophrenia. Schizophr Res. 2008;101:152–60. doi: 10.1016/j.schres.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Grumbach MM, Hughes IA, Conte FA. Williams Textbook of Endocrinology. 10th ed. Philadelphia: Saunders; 2003. Disorders of sex differentiation. [Google Scholar]
- 26.Keverne EB, Curley JP. Epigenetics, brain evolution and behaviour. Front Neuroendocrinol. 2008;29:398–412. doi: 10.1016/j.yfrne.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Moore T. Genetic conflict, genomic imprinting and establishment of the epigenotype in relation to growth. Reproduction. 2001;122:185–93. doi: 10.1530/rep.0.1220185. [DOI] [PubMed] [Google Scholar]
- 28.Petronis A. The origin of schizophrenia: Genetic thesis, epigenetic antithesis, and resolving synthesis. Biol Psychiatry. 2004;55:965–70. doi: 10.1016/j.biopsych.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Qureshi IA, Mehler MF. Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility. Prog Brain Res. 2010;186:77–95. doi: 10.1016/B978-0-444-53630-3.00006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


