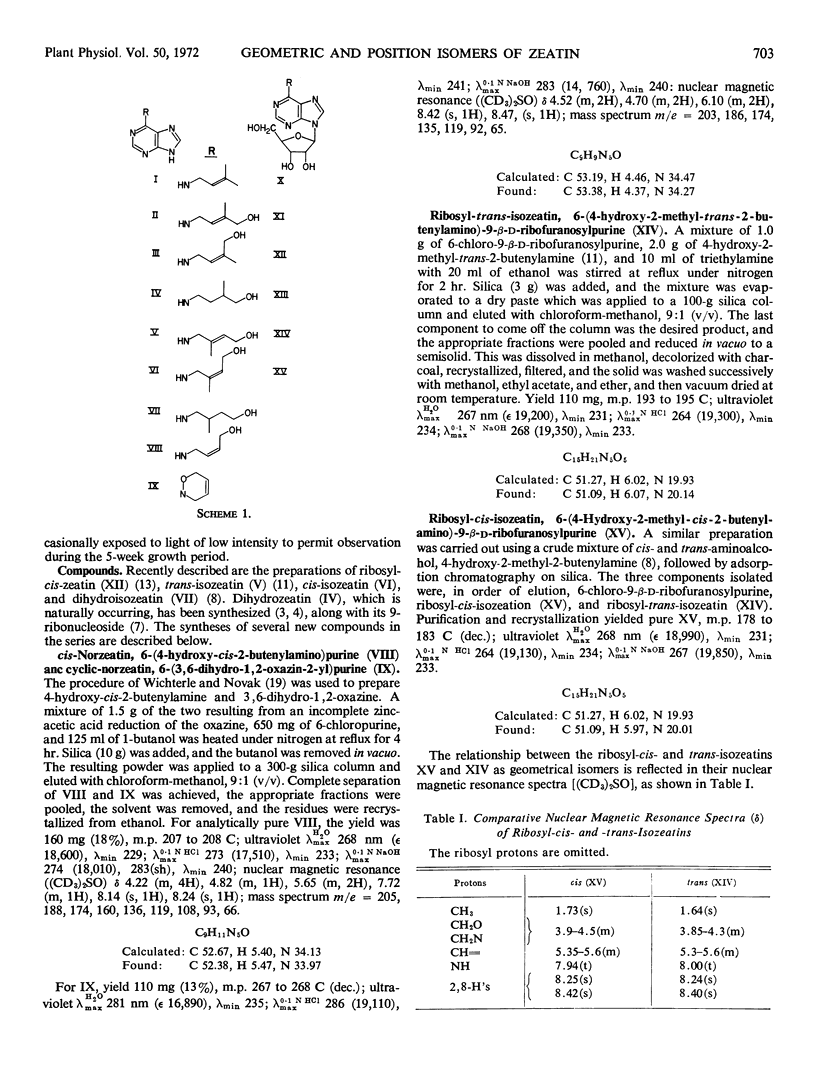
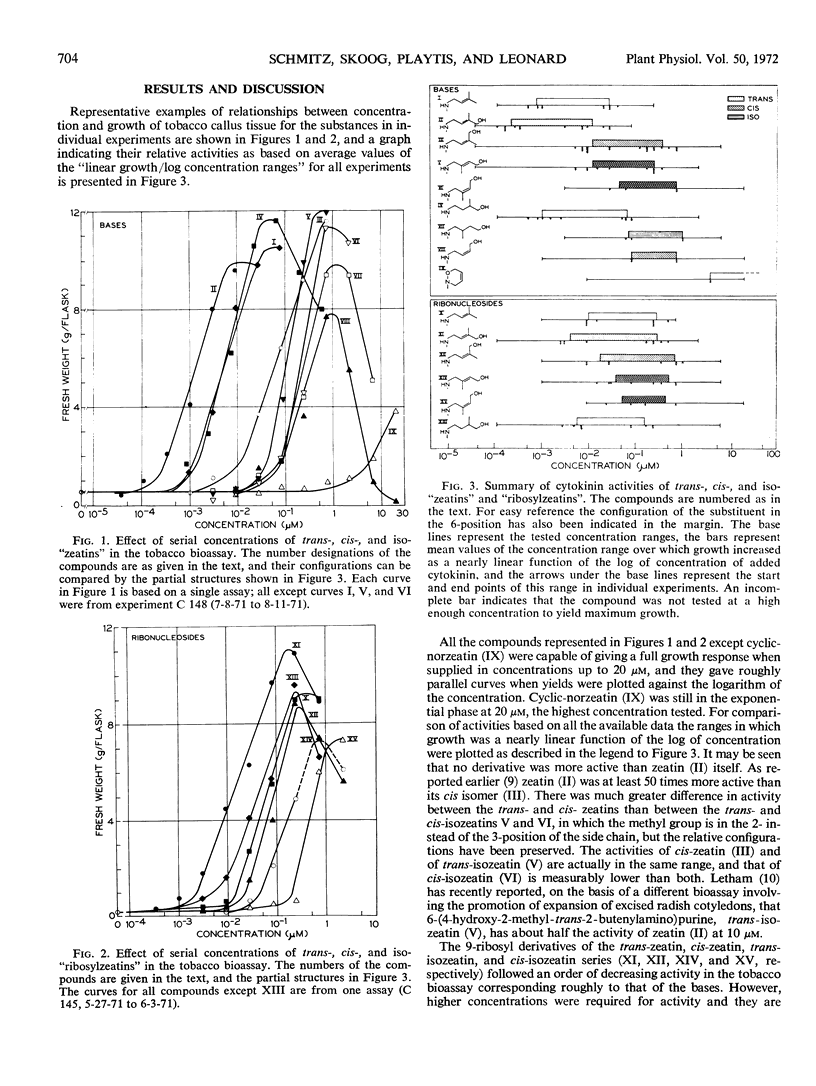
Abstract
Geometric and position isomers of zeatin and of ribosylzeatin and other compounds closely related to zeatin have been tested in the tobacco (Nicotiana tabacum var. Wisconsin No. 38) bioassay. None was more active than zeatin itself. There was a much greater difference in activity (> 50-fold) between trans- and cis-zeatin than between trans-isozeatin [6-(4-hydroxy-2-methyl-trans-2-butenylamino) purine] and cis-isozeatin [6-(4-hydroxy-2-methyl-cis-2-butenylamino) purine], the latter being less active than cis-zeatin and trans-isozeatin. Higher concentrations were required for equivalent callus growth stimulated by the 9-ribosyl derivatives, which followed an order of decreasing activity: ribosyl-trans-zeatin > ribosyl-cis-zeatin > ribosyl-trans-isozeatin > ribosyl-cis-isozeatin, corresponding roughly to that of the bases. The effect of side chain, double bond saturation was to diminish the activity, and in the dihydro series the shift of the methyl group from the 3- to the 2-position in going from dihydrozeatin to dihydroisozeatin [6-(4-hydroxy-2-methylbutylamino) purine] resulted in a 70-fold decrease in activity. cis-Norzeatin [6-(4-hydroxy-cis-2-butenylamino) purine], which was less than one-fifth as active as cis-zeatin, showed the effect of complete removal of the side chain methyl group, and cyclic-norzeatin [6-(3,6-dihydro-1,2-oxazin-2-yl) purine] was about 1/100 as active as cis-norzeatin. These findings delineate completely the effect on the cytokinin activity of zeatin of variation in side chain geometry, presence and position of the methyl substituent, presence and geometry of hydroxyl substitution, presence of the double bond, and of side chain cyclization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fleysher M. H. N 6 -Substituted adenosines: synthesis, biological activity, and some structure-activity relationships. J Med Chem. 1972 Feb;15(2):187–191. doi: 10.1021/jm00272a015. [DOI] [PubMed] [Google Scholar]
- Leonard N. J., Hecht S. M., Skoog F., Schmitz R. Y. CYTOKININS: SYNTHESIS OF 6-(3-METHYL-3-BUTENYLAMINO)-9-beta-D-RIBOFURANOSYLPURINE (3IPA), AND THE EFFECT OF SIDE-CHAIN UNSATURATION ON THE BIOLOGICAL ACTIVITY OF ISOPENTYLAMINOPURINES AND THEIR RIBOSIDES. Proc Natl Acad Sci U S A. 1968 Jan;59(1):15–21. doi: 10.1073/pnas.59.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard N. J., Hecht S. M., Skoog F., Schmitz R. Y. Cytokinins: synthesis, mass spectra, and biological activity of compounds related to zeatin. Proc Natl Acad Sci U S A. 1969 May;63(1):175–182. doi: 10.1073/pnas.63.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playtis A. J., Leonard N. J. The synthesis of ribosyl-cis-zeatin and thin layer chromatographic separation of the cis and trans isomers of ribosylzeatin. Biochem Biophys Res Commun. 1971 Oct 1;45(1):1–5. doi: 10.1016/0006-291x(71)90041-6. [DOI] [PubMed] [Google Scholar]
- Schmitz R. Y., Skoog F. The use of dimethylsulfoxide as a solvent in the tobacco bioassay for cytokinins. Plant Physiol. 1970 Apr;45(4):537–538. doi: 10.1104/pp.45.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreman H. J., Skoog F. Cytokinins in pisum transfer ribonucleic Acid. Plant Physiol. 1972 May;49(5):848–851. doi: 10.1104/pp.49.5.848. [DOI] [PMC free article] [PubMed] [Google Scholar]


