Abstract
The polymyxins (polymyxin B and E) are bactericidal polypeptide antibiotics first discovered in 1947 and used for the treatment of gram-negative bacterial infections. Renal and neurologic toxicities coupled with the increasing availability of effective alternatives led to declining use in the 1960s. The emergence of multidrug-resistant organisms in the past decade has resulted in a resurgence in the use of polymyxins in critically ill patients, yet the side effects are not well known. We report two cases of respiratory arrest likely due to polymyxin B infusions in the context of a 10-fold increase in the use of polymyxin B in our institution over the past 10 years.
Case 1
Patient 1 was a 48-year-old man who had a living related-donor liver transplant for hepatic cirrhosis. His course was complicated by hospital-acquired pneumonia with initial improvement on IV vancomycin, piperacillin/tazobactam, and tobramycin. However, his condition deteriorated 2 days later, with hypotension and severe leukocytosis. An exploratory laparotomy revealed a biliary leak, and on the same day, blood cultures grew carbapenem-resistant Klebsiella pneumoniae, sensitive to polymyxin B, and with intermediate sensitivity to amikacin. Both IV amikacin and polymyxin B were initiated in consultation with the infectious diseases service. Before infusion of polymyxin B, the patient’s serum creatinine level was 0.7 mg/dL and he was resting comfortably on oxygen by nasal cannula with a documented oxygen saturation (Sao2) of 100% and a respiratory rate of 18 to 21 breaths/min. Polymyxin 125 mg (1.6 mg/kg) IV every 12 h was started. An hour after initiation of the first polymyxin B infusion, he became apneic and unresponsive to sternal rub, with decreasing Sao2 and hypotension. He received emergency airway management including endotracheal intubation, and an infusion of epinephrine. Shortly after resuscitation, his Pao2 was 256 mm Hg (on an Fio2 of 100%), his sensorium was clear, and his lung mechanics were normal.
Case 2
Patient 2 was a 58-year-old man with chronic renal failure who received a cadaveric renal transplant. His postoperative course was complicated by a retroperitoneal hematoma and graft rejection, treated with IV immunoglobulin and steroids, ultimately resulting in acute renal failure that required transient renal replacement therapy. He had a history of multidrug-resistant (MDR) K pneumoniae urinary tract infections sensitive to carbapenems and he received pre- and postoperative meropenem, as well as intraoperative bladder irrigation with polymyxin B. After discharge to the surgical ward, he developed a large perinephric abscess that was drained, and the fluid culture was positive for MDR K pneumoniae, Enterococcus faecium, and Pseudomonas aeruginosa. He had been treated empirically with meropenem and tigecycline, and IV polymyxin B was added at a loading dose of 200 mg (2.9 mg/kg) IV followed by 80 mg (1.1 mg/kg) IV every 72 h because of his severe renal insufficiency. The day of the fourth infusion of polymyxin B, the patient’s serum creatinine level was 4.6 mg/dL. Three hours after the start of the infusion the patient had acute respiratory distress. He was speaking with two physicians in his hospital room and his voice suddenly became high-pitched and he then became apneic. He received emergency airway management including endotracheal intubation. An arterial blood gas drawn shortly after intubation revealed hypercapnia (pH 7.14, Pco2 65, and Pao2 244 on 100% Fio2). He did not require any vasopressor support during the respiratory arrest. The patient later recalled that at the time of the crisis he was fully aware but felt unable to breathe or move his arms. He was transferred to the ICU, where carotid Dopplers, transthoracic echocardiogram, and evaluation of his vocal cords and supraglottic region by an otolaryngologist were all normal. The endotracheal tube was removed the next day, and he was transferred to the floor 2 days later.
Patient 2 was readmitted to the ICU 2 weeks later with evidence of further severe sepsis, including hypotension, fever, and leukocytosis. His recent microbiologic results included MDR K pneumoniae (blood cultures, as well as tracheal aspirate and urine), resistant Bacteroides fragilis (blood cultures), and MDR Escherichia coli and P aeruginosa (abdominal fluid collection). Because his condition was worsening and he was currently being treated with tigecycline, levofloxacin, meropenem, metronidazole, and fluconazole, a consensus decision was made to administer a test dose of polymyxin under careful monitoring in the ICU, because the previous diagnosis of a polymyxin-induced neuromuscular weakness had been suspected but not proven and the therapeutic alternatives were limited. Before the infusion, his respiratory rate was 13 to 17 breaths/min, with an Sao2 of 100% on oxygen by nasal cannula. Two hours after commencing the infusion of polymyxin B, the patient developed a witnessed respiratory arrest requiring emergency airway management (including endotracheal intubation) followed by extubation without sequelae the next day. Polymyxin infusion rates were not documented in either of these cases; however, the pharmacy label indicated a 90-min infusion time.
Discussion
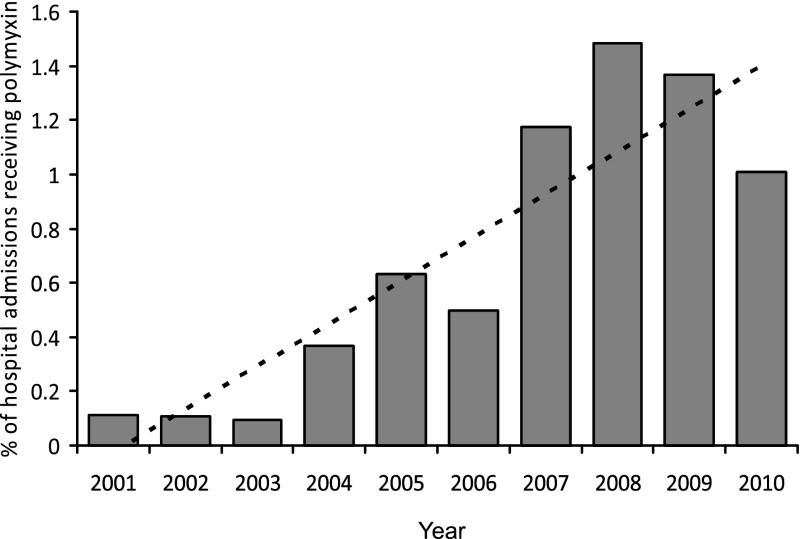
Polymyxin B and polymyxin E (colistin) are used clinically to treat serious infections caused by gram-negative bacilli. In the early 1960s, when gentamicin became available, the use of polymyxins decreased because of the significant renal and neurologic side effects.1 Several decades later, its use is now increasing as other antimicrobials become less helpful in the treatment of MDR bacteria.2 In our institution, prescriptions for polymyxin have increased dramatically over the past 10 years because of the increase in MDR gram-negative bacteria (Fig 1); the number of patients receiving either IV or inhalation polymyxin increased 10-fold, from one in 1,000 hospital admissions to one in 100. In the context of the increasing use of this antibiotic, we report two patients who experienced sudden respiratory arrests that were most likely secondary to polymyxin infusions.
Figure 1.
Prescriptions for IV and inhalational polymyxin B over 10 years (2001-2010) among all hospital admissions at Columbia University Medical Center. (Polymyxin E [colistin] was not on formulary during this time.)
Neuromuscular blockade is associated with many medications and several antimicrobial agents, including aminoglycosides and the polymyxins.3 Tests on human intercostal muscles comparing the paralytic effects of multiple antibiotics, including neomycin and polymyxin B, demonstrated that polymyxin B had the strongest neuromuscular blocking activity.4 Paralysis of the respiratory muscles in particular was described in patients receiving therapeutic doses of the polymyxins, primarily in the 1950s and early 1960s.5,6 However, one review noted that there were no case reports of respiratory arrest published in the past 15 years,7 likely because of the use of other antibiotics to treat gram-negative infections during this time period.7
The neurologic toxicity of polymyxin may manifest as dizziness, generalized muscle weakness, facial and peripheral parasthesias, partial deafness, visual disturbances, vertigo, confusion, hallucinations, seizures, ataxia, and neuromuscular blockade, usually described as a myasthenia-like clinical syndrome.7 The paralytic effects are thought to be due to a blockade at the myoneural end plate, producing a noncompetitive blockade that is resistant to cholinesterase inhibitors6,8; in animal models there is fade of the train-of-four twitch with polymyxin, yet potentiation (rather than reversal) of the block by cholinesterase inhibitors.9 Treatment of neurotoxicity associated with polymyxin is generally supportive. Injection of calcium has produced mixed results in attempts to reverse the respiratory paralysis associated with polymyxin.3,6,9
It is notable that there is no specific pattern as to when the complication may occur. In a case series reporting 11 patients with respiratory arrest associated with polymyxin, the authors noted that several of the episodes occurred after a single dose of antibiotic, but others occurred after receiving the drug for up to 45 days. Onset of paralysis occurred anywhere from 1 to 26 h after the start of the dose, with a “typical” timing of 1 to 3 h.6 In the two patients, the first had a respiratory arrest during the first infusion of the medication, and the second had received the drug previously, both IV and as bladder irrigation. Until November 2010, our institution diluted polymyxin B in 250 to 500 mL of 5% dextrose and water for intermittent IV infusions with suggested infusion rates ranging from 1 to 3 h. In November 2010, the diluent was changed to 100 mL.10 One of the first reports from the 1960s describes 100 mg diluted in 500 mL,5 whereas the case series from the same time period describes a variety of doses, most of which were likely higher than in current practice.6
The main risk factor known to be associated with development of a respiratory arrest secondary to polymyxin is renal disease (acute or chronic).6 However, polymyxin itself is a risk factor for acute kidney injury, with a recent estimate of injury occurring in 22% of patients who receive IV polymyxin B.11 It is notable that patient 2 had severe renal insufficiency, likely increasing the risk of toxicity from polymyxin, especially after multiple doses.11 Patient 1 had normal kidney function, suggesting that neurologic toxicity can also occur without known renal dysfunction.
We cannot be sure that the respiratory arrests we report were specifically due to the polymyxin B infusions. Applying the Naranjo adverse drug reaction probability scale indicated a possible relationship for patient 1 and a probable relationship between the administration of polymyxin B and respiratory arrest for patient 2.12 The case of patient 2 appears to be more clear-cut in terms of the likely cause of his respiratory arrest, given the repetition of the event with a second dose of polymyxin B. In addition, because the rates of infusion were not well documented for either patient, it is possible that rapid infusions may have contributed to this observed toxicity. In neither case was muscle weakness assessed using a train-of-four twitch monitor, which would have helped clarify whether there was a specific neuromuscular blockade occurring at the time. Many patients sick enough to require polymyxin may already be receiving mechanical ventilation, making detection of muscle weakness or paralysis difficult and perhaps contributing to problems with ventilator weaning or early mobilization. We suggest that in such cases, assessment of a train-of-four may help rule out antibiotic-induced paralysis as a contributing factor in weakness.
As MDR bacteria continue to increase, the use of polymyxins to combat these infections is likely to become even more widespread.13 The two patients we describe were fortunate to have “witnessed” respiratory arrests, resulting in prompt recognition of the situation and immediate ventilatory support without long-term consequences. It is imperative that all health-care professionals who either prescribe or care for patients receiving polymyxin be aware of this potentially fatal complication and remain vigilant at all times in monitoring the patients.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: This study received approval at Columbia University from Institutional Review Board committee 2, IRB-AAAI1063.
Abbreviations
- MDR
multidrug resistant
- Sao2
oxygen saturation
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Chen LF, Kaye D. Current use for old antibacterial agents: polymyxins, rifamycins, and aminoglycosides. Infect Dis Clin North Am. 2009;23(4):1053–1075. doi: 10.1016/j.idc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362(19):1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokoll MD, Gergis SD. Antibiotics and neuromuscular function. Anesthesiology. 1981;55(2):148–159. doi: 10.1097/00000542-198108000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Sabawala PB, Dillon JB. The action of some antibiotics on the human intercostal nerve-muscle complex. Anesthesiology. 1959;20:659–668. doi: 10.1097/00000542-195909000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Pohlmann G. Respiratory arrest associated with intravenous administration of polymyxin B sulfate. JAMA. 1966;196(2):181–183. [PubMed] [Google Scholar]
- 6.Lindesmith LA, Baines RD, Jr, Bigelow DB, Petty TL. Reversible respiratory paralysis associated with polymyxin therapy. Ann Intern Med. 1968;68(2):318–327. doi: 10.7326/0003-4819-68-2-318. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care. 2006;10(1):R27. doi: 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmerman JC, Long JP, Pittinger CB. Neuromuscular blocking properties of various antibiotic agents. Toxicology. 1959;1(3):299–304. doi: 10.1016/0041-008x(59)90114-0. [DOI] [PubMed] [Google Scholar]
- 9.Lee C, Chen D, Nagel EL. Neuromuscular block by antibiotics: polymyxin B. Anesth Analg. 1977;56(3):373–377. doi: 10.1213/00000539-197705000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Zavascki AP, Goldani LZ, Cao G, et al. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis. 2008;47(10):1298–1304. doi: 10.1086/592577. [DOI] [PubMed] [Google Scholar]
- 11.Mendes CA, Cordeiro JA, Burdmann EA. Prevalence and risk factors for acute kidney injury associated with parenteral polymyxin B use. Ann Pharmacother. 2009;43(12):1948–1955. doi: 10.1345/aph.1M277. [DOI] [PubMed] [Google Scholar]
- 12.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 13.Giske CG, Monnet DL, Cars O, Carmeli Y. on behalf of the ReAct-Action on Antibiotic Resistance Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother. 2008;52(3):813–821. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]