Abstract
Objective
To determine how physical activity at various ages over the life course is associated with cognitive impairment in late life.
Design
Cross-sectional study
Setting
Four US sites.
Participants
We administered a modified Mini-Mental State Examination (mMMSE) to 9344 women ≥65 years (mean 71.6 years) who self-reported teenage, age 30, age 50, and late life physical activity.
Measurements
We used logistic regressions to determine the association between physical activity status at each age and likelihood of cognitive impairment (mMMSE score >1.5SD below the mean, mMMSE≤22). Models were adjusted for age, education, marital status, diabetes, hypertension, depressive symptoms, smoking, and body mass index.
Results
Women who reported being physically active had lower prevalence of cognitive impairment in late life compared to women who were inactive at each time (teenage: 8.5% vs. 16.7%; adjusted Odds Ratio (95% Confidence Interval): 0.65 (0.53–0.80); age 30: 8.9% vs. 12.0%; 0.80 (0.67–0.96); age 50: 8.5% vs. 13.1%; 0.71 (0.59–0.85); old age: 8.2% vs. 15.9%; 0.74 (0.61–0.91)). When the four times were analyzed together, teenage physical activity was most strongly associated with lower odds of late-life cognitive impairment (OR=0.73 (0.58–0.92)). However, women who were physically inactive at teenage and became active in later life had lower risk than those who remained inactive.
Conclusions
Women who reported being physically active at any point over the life course, and especially at teenage, have lower likelihood of cognitive impairment in late life. Interventions should promote physical activity early in life and throughout the life course.
Keywords: Physical Activity, Exercise, Cognition, Cognitive Impairment, Life Course
Introduction
The prevalence of dementia is expected to rise dramatically in the upcoming decades, primarily due to changes in demographics and increasing longevity.1 Accordingly, interventions that decrease the risk of cognitive impairment are of the utmost importance. Physical activity is among the most promising strategies to decrease the risk of cognitive decline and cognitive impairment in old age.2 Numerous studies have reported that people who are physically active in mid- and late life have lower risk of developing cognitive impairment and dementia.3–5 In addition, physically active elderly people have slower rates of cognitive decline than those who are inactive.5, 6 Several randomized controlled trials confirm that exercise can improve cognition in elderly people, but the results are less consistent.7
Despite the large number of epidemiological studies that have examined physical activity in relation to cognition in old age, relatively few have included physical activity measures prior to mid-life8, 9 and none have included measures at multiple time points. However, there is reason to suggest that physical activity prior to mid-life may impact cognition in later life. Youth who are active have better cognitive and academic performance.10 It is possible that early-life physical activity—similar to early-life education11—could help to build ‘cognitive reserve’ that has long-lasting benefits. In addition, youth who are physically inactive have higher rates of obesity and type II diabetes,12 which are both risk factors for cognitive impairment in late life.13, 14
The objective of our study was to examine how physical activity at several points over the life course is related to cognitive performance and the prevalence of cognitive impairment in old age. Importantly, if early life physical activity is positively associated with late life cognitive function, then physical activity interventions to prevent cognitive impairment should target people as early in life as possible.
Materials and Methods
Population
Participants were enrolled in the Study of Osteoporotic Fractures (SOF), a multi-center, prospective, observational study of women aged 65 and older.15 There were 9,704 primarily white women recruited to the study between September 1986 and October 1988 from four metropolitan areas in the United States: Baltimore, Maryland; Minneapolis, Minnesota; Portland, Oregon; and Monongahela Valley, Pennsylvania. Women were excluded if they were unable to walk without help or had a bilateral hip replacement. All participants provided written informed consent and the study was approved by the committees on human research at each study site. Of the participants, 9395 provided self-reports of physical activity at each time point, of which 9344 (96%) women completed cognitive testing and constituted our study cohort.
Physical Activity
SOF participants were asked about teenage, age 30, age 50, and current (late life) yearly frequencies of low (e.g. walking or gardening), moderate (e.g. dancing or tennis), or high (jogging or skiing) intensity physical activities according to a modified Paffenbarger questionnaire.16 In preliminary analyses, there was no evidence of a dose response between physical activity at any age (either by frequency or intensity) and cognition performance or odds of cognitive impairment. As a result, the participants were dichotomized at each age into women who were physically active and inactive (those who reported no regular participation in any physical activity or sport at the relevant age) for the final analyses.
Cognition
Clinic staff administered a 26-point modified Mini-Mental State Examination (mMMSE) to assess cognitive function.17 The mMMSE is a brief test of global cognitive function that evaluates orientation, concentration, praxis, and memory that is based on the Mini-Mental State Examination (MMSE) but omits questions regarding language.18 Significant cognitive impairment was defined as an mMMSE score at least 1.5 standard deviations (SD) below the mean (mMMSE≤22).
Other Variables
The participants’ age and race was collected at the study visit. Participants also self-reported years of education, whether they had ever smoked regularly, their current marital status, and whether they lived arrangements. Medical history included whether a doctor had ever told them they had diabetes or Parkinson’s disease. In addition, the participant’s blood pressure, height, and weight were measured during the clinical visit. Participants were classified as hypertensive if their systolic blood pressure measurement was above 160mmHg, their diastolic blood pressure measurement was above 90mmHg, or they self-reported use of anti-hypertensive medication. Body weight and height were used to calculate the body mass index (BMI), which is the weight in kilograms divided by the square of height in meters (kg/m2). Depressive symptoms were evaluated using the 15-point Geriatric Depression Scale19 shortly after baseline. A higher score on the Geriatric Depression Scale indicates more symptoms of depression.
Statistical Analysis
The participants’ baseline characteristics were compared by physical activity status (active/inactive) at each age (teenage, age 30, age 50, late life) over the life course using analysis of variance (ANOVA), Kruskal-Wallis (skewed data), or chi-squared (χ2) as appropriate. In order to determine the relationship between physical activity at each age and cognitive function in late life, we conducted multiple linear regressions. Initially each time point of physical activity was entered in the model individually. Those variables that were significantly associated with physical activity status at the relevant time point in descriptive analyses (p<0.05) were entered into the model as potential confounders. In a final model, all physical activity time points were included in order to determine the relative strength of their association with late life cognition.
We conducted multiple logistic regressions to evaluate the odds of cognitive impairment associated with being physically active versus inactive at each age. Variables that were significantly associated with physical activity status at the relevant age were entered into the model as confounders. Finally, physical activity measures at all ages were entered into a final model to determine the relative strength of their association with late life cognitive impairment.
We examined whether the relationship between age 30, age 50, and late-life physical activity status and the odds of cognitive impairment was modified by strata of teenage physical activity using an interaction effect. All statistical analyses were conducted using SAS, version 9.1.3 (SAS Institute, Inc., Cary, NC).
Results
Of the participants, 15.5%, 29.7%, 28.1%, and 21.1% women reported being physically inactive at teenage, at 30 years, at 50 years, and in late life (mean age (±standard deviation, SD) =71.6 (5.2) years) respectively. Physical activity reports at each age were positively correlated with physical activity at all other ages but these correlations were low to moderate, ranging from 0.24 to 0.64 (p<0.05).
Women who were physically active as teenagers were, on average, younger, more educated, more likely to be married or to have ever smoked, and less likely to report a diagnosis of diabetes in late life than women who were physically inactive as teenagers (Table 1). In addition, women who were physically active as teenagers reported fewer depressive symptoms and had a lower average BMI in late life than women who were inactive as teenagers (Table 1). The pattern of participant differences between physically active and inactive groups at age 30, age 50, and late life were similar to the differences between groups at teenage except that age 30 physical activity status was not associated with age, marital status and late life physical activity status was associated with hypertension in addition to diabetes but not smoking status.
Table 1.
Late-Life Characteristics of 9344 Women
| Late-Life Characteristic | Mean (SD) or % |
|---|---|
| Age (y) | 71.6 (5.2) |
| Education (y) | 12.6 (2.8) |
| Married | 48.9% |
| Medical History | |
| Diabetes | 7.1% |
| Hypertension | 38.8% |
| Parkinson’s Disease | 0.6% |
| Depression score | 1.7 (2.2) |
| Ever smoker | 39.7% |
| Body mass index, kg/m2 | 26.4 (4.5) |
In separate models, women who were physically active at teenage, at 30 years, at 50 years, or in late life had higher average mMMSE score than those who were inactive at the same age in unadjusted analyses (p<0.001). In multivariable models, the difference remained significant (p<0.001) for all ages except for age 30 (p=0.055) (adjusted for variables significantly associated with physical activity at the relevant age—Teenage: age, education, marital status, diabetes, depressive symptoms, smoking, BMI; Age 30: education, diabetes, depressive symptoms, smoking, BMI; Age 50: age, education, marital status, diabetes, depressive symptoms, smoking, BMI; Late Life: age, education, marital status, diabetes, hypertension, depressive symptoms, BMI). The difference in mMMSE score by physical activity status was small but statistically significant at each time point (0.1 to 0.3 points higher for physically active vs. inactive at any point over the life course). When physical activity measures for all four ages were entered into a single model, adjusted for variables associated with physical activity status at any age (age, education, marital status, diabetes, hypertension, depressive symptoms, smoking, and BMI), only teenage physical activity status remained significantly associated with cognitive performance in old age (teenage p < 0.001 versus age 30 p=0.49, age 50 p=0.10, late life p=0.15).
Women who were physically active at each age were also less likely to have cognitive impairment in late life than those who were inactive in unadjusted analyses (teenage: 8.5% vs. 16.7%; Odds Ratio (95% Confidence Interval, CI): 0.46 (0.39–0.54); age 30: 8.9% vs. 12.0%; 0.71 (0.61–0.82); age 50: 8.5% vs. 13.1%; 0.62 (0.54–0.71); late life: 8.2% vs. 15.9%; 0.47 (0.41–0.55)) (Table 2). The associations remained significant at each age when potential confounders (as per linear regressions) were included in the model (teenage: 0.65 (0.53–0.80); age 30: 0.80 (0.67–0.96); age 50: 0.71 (0.59–0.85)); late-life 0.75 (0.61–0.91)) (Table 2). When physical activity status for all four ages (teenage, 30 years, 50 years, and late life) were entered into a single model, teenage physical activity status was most strongly associated with lower odds of cognitive impairment (Teenage: (95%CI): 0.73 (0.58–0.92)); the association between physical activity status at other ages and the odds of cognitive impairment was not statistically significant.
Table 2.
The Association Between Physical Activity Status Across the Life Course and the Odds of Late Life Cognitive Impairment, as Defined by >1.5 SD Below the Mean mMMSE (mMMSE≤22), In Older Women.
| Physical Activity | Odds Ratios (95% Confidence Interval) |
||
|---|---|---|---|
| Status | Prevalence (%) | Unadjusted | Adjusted |
|
Teenage Physical Activity | |||
| Inactive | 16.7 | 1.0 (Reference) | 1.0 (Reference) |
| Active | 8.5 | 0.46 (0.39–0.54) | 0.65 (0.53–0.80) |
|
Age 30 Physical Activity | |||
| Inactive | 12.0 | 1.0 (Reference) | 1.0 (Reference) |
| Active | 8.9 | 0.71 (0.61–0.82) | 0.80 (0.67–0.96) |
|
Age 50 Physical Activity | |||
| Inactive | 13.1 | 1.0 (Reference) | 1.0 (Reference) |
| Active | 8.5 | 0.62 (0.54–0.71) | 0.71 (0.59–0.85) |
|
Late Life Physical Activity | |||
| Inactive | 15.9 | 1.0 (Reference) | 1.0 (Reference) |
| Active | 8.2 | 0.47 (0.41–0.55) | 0.74 (0.61–0.91) |
Adjusted models include significant confounders from descriptive analyses:
Teenage: age, education, marital status, diabetes, depressive symptoms, smoking, BMI
Age 30: education, diabetes, depressive symptoms, smoking, BMI
Age 50: age, education, marital status, diabetes, depressive symptoms, smoking, BMI
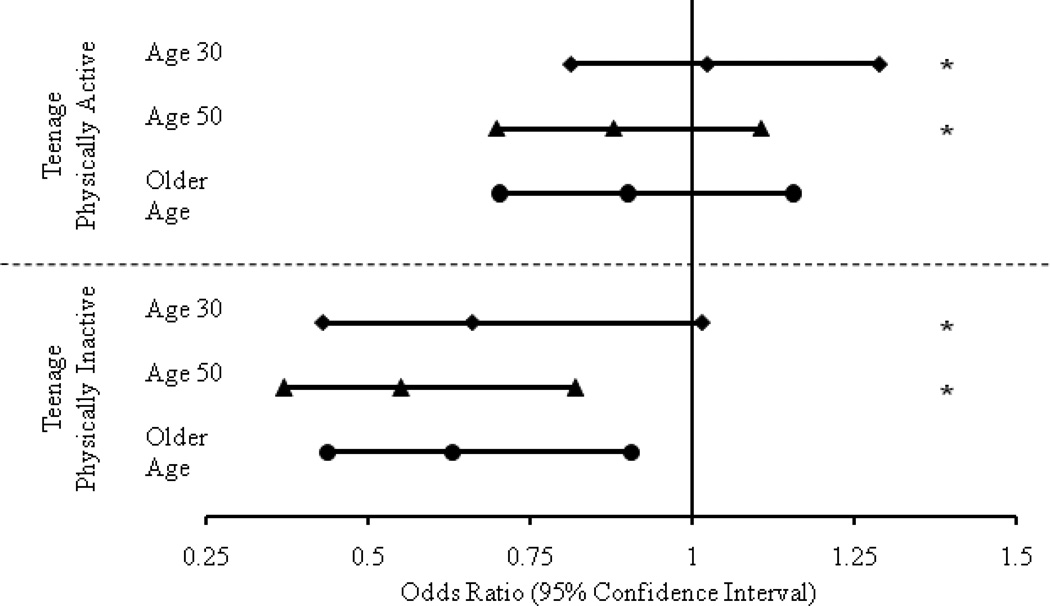
Teenage physical activity status significantly modified the relationship between age 30 (p=0.004) and age 50 physical activity (p=0.011), but not late-life physical activity (p=0.26), and the risk of cognitive impairment in late-life. Women who were physically inactive at teenage but became physically active at age 30 and age 50 had significantly reduced odds of cognitive impairment relative to those who remained physically inactive (Figure 1). In contrast, being physically active at age 30 and age 50 was not significantly associated with rates of cognitive impairment in those women who were already physically active at teenage (Figure 1).
Figure 1. Adjusted odds of cognitive impairment in older women who were physically active versus inactive over the life course, stratified by teenage physical activity status.
* Interaction terms are significant for Age 30 and 50: Teenage*Age 30: p=0.004; Teenage*Age 50: p=0.011
** The models were adjusted for:
Age 30: education, diabetes, depressive symptoms, smoking, BMI
Age 50: age, education, marital status, diabetes, depressive symptoms, smoking, BMI
Late Life: age, education, marital status, diabetes, hypertension, depressive symptoms, BMI
Discussion
In this study of older women, those who were physically active at any age (teenage, age 30, age 50, late life) across the life course, and particularly as teenagers, had better cognitive performance and lower likelihood of cognitive impairment in late life than women who were physically inactive. However, women who were physically inactive at teenage had lower rates of cognitive impairment in late life if they were physically active at age 30, at age 50, or in late life. Our results suggest that physical activity should be encouraged from early life and across the life course in order to minimize the risk of cognitive impairment in old age.
This is the first study to examine the association of physical activity at several ages across the life course with late life cognitive function. As in previous studies, our results indicated that people who were physically inactive in mid- and late life had higher likelihood of cognitive impairment in late life.3, 4 In addition, our findings are in line with previous results indicating that early life physical activity (age 15 to 25 years and age 36 years respectively) is associated with better information processing speed and slower memory decline in older age.8, 9 However, our study is the first to show that early life physical activity may also be associated with lower risk of cognitive impairment in old age.
The mechanisms by which physical activity across the life course is related to late life cognition are likely to be multi-factorial. There is evidence to suggest that physical activity has a positive effect on synaptic plasticity and cognition and this may be mediated by brain-derived neurotrophic factor (BDNF).20–22 In addition, physical activity reduces the rates and severity of vascular risk factors, such as hypertension, obesity, and type II diabetes,23 which are each associated with increased risk of cognitive impairment.13, 14, 24 Finally, in animal models, physical activity has been reported to reduce β-amyloid accumulation, a hallmark of Alzheimer’s disease.25
Of the four ages we examined, teenage physical activity appeared to be most strongly related to better cognitive function and the lower prevalence of cognitive impairment in old age. It may be that teenage physical activity positively affects brain development and as a result, enhances cognition in early life and then across the life course. Indeed, teenage physical activity has been shown to be positively associated with cognitive performance in youth,26 and this, in turn, has been linked with reduced rates of cognitive impairment in old age.27, 28 Our results are consistent with the hypothesis that teenage physical activity, similar to early-life education,11 may build a ‘cognitive reserve’ that protects against cognitive impairment in late life.
Our results suggest that women who are physically inactive in teenage can reduce their likelihood of cognitive impairment by becoming active in later life. Among women who were physically inactive at teenage, those who were physically active in later life had approximately half the risk of late-life cognitive impairment compared to those who remained inactive. Hence, physically activity should be promoted particularly to those who were sedentary in early life in order to prevent cognitive impairment. Counter intuitively, women who were physically active at teenage appeared to gain no additional benefit by remaining physically active in later life. Why this is so is unclear. However, given that later life physical activity is also associated with reduced rates of cardiovascular disease, some cancers, and depression,29 physically activity should continue to be encouraged over the life course regardless of physical activity status at teenage.
Our study has several strengths. Most importantly, we had reports of physical activity at four different time points. As a result, we were able to determine the association between physical activity over the life course and cognitive impairment in late life. In addition, our cohort of older women is large and well-characterized. Consequently, we were able to adjust for many possible confounders that other studies often neglect.
Our study also has some important limitations. We relied on self-reports of physical activity, which may not be highly accurate. In particular, non-exercise physical activity, such as daily chores and child rearing, may not have been adequately captured, underestimating physical activity levels. Non-exercise physical activity may be particularly relevant at age 30 where child rearing would be common. Even so, self reports are the most common measure of physical activity in epidemiological studies,30 particularly in studies evaluating physical activity in relation to cognition in old age.3–6 Furthermore, we used a modified Paffenbarger Physical Activity Questionnaire to assess self-reported physical activity, which has been shown to reasonably estimate historical physical activity.31 People who were cognitively impaired may also have been more likely to misreport physical activity levels, which may bias results. In addition, although a common criterion for cognitive impairment (score of 1.5 SD below the mean) was used, there was no clinical assessment for cognitive impairment so some women may have been misclassified and we cannot be sure of the etiology of the cognitive impairment. Furthermore, the mMMSE is a relatively simple test of cognitive function that may not be sensitive enough to distinguish subtle changes in cognition, especially at higher levels of cognitive functioning. This may account for the lack of dose response between physical activity and cognition in these analyses. Finally, most of the subjects in our sample were white women so we do not know if our findings are generalizable to men or people from other ethnic groups.
In this study, we found that women who reported being physical inactive at various points across the life course, and particularly at teenage, had poorer cognitive performance and higher prevalence of cognitive impairment in old age. Future research should examine the relationship between physical activity at various ages and cognitive decline in late life. However, our preliminary findings suggest that physical activity should be advocated from an early age and throughout life in order to optimize cognition and minimize the risk of cognitive impairment in old age.
Acknowledgements
The Study of Osteoporotic Fractures (SOF) is supported by the National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, and 2 R01 AG027574-22A1.
Dr. Laura Middleton is supported by a Canadian Institute of Health Research (CIHR) fellowship.
Dr. Deborah Barnes is supported by a career development award from the National Institutes of Health (K01 AG024069).
Dr. Kristine Yaffe is supported in part by NIA grant K24 AG 031155 and an Independent Investigator Award from the Alzheimer’s Association.
Footnotes
Conflict of Interest (as per form)
Ms. Li-Yung Lui reports no disclosures.
Author Contributions: The authors were fully responsible for the study concept and design, methods, data collections, analysis, interpretation, and manuscript preparation.
Sponsor’s Role: None - the sponsors (funding agencies) had no role in the study concept and design, methods, data collections, analysis, interpretation, and manuscript preparation.
References
- 1.Moore A. Older people. We can work it out. Health Serv J. 2007;117:24–26. [PubMed] [Google Scholar]
- 2.Middleton LE, Yaffe K. Promising Strategies for the Prevention of Dementia. Arch Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 4.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 5.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 7.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Dik M, Deeg DJ, Visser M, Jonker C. Early life physical activity and cognition at old age. J Clin Exp Neuropsychol. 2003;25:643–653. doi: 10.1076/jcen.25.5.643.14583. [DOI] [PubMed] [Google Scholar]
- 9.Richards M, Hardy R, Wadsworth ME. Does active leisure protect cognition? Evidence from a national birth cohort. Soc Sci Med. 2003;56:785–792. doi: 10.1016/s0277-9536(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 10.Sibley B, Etnier J. The Relationship Between Physical Activity and Cognition in Children: A Meta-Analysis. Ped Exerc Sci. 2003;15:243–256. [Google Scholar]
- 11.Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25:625–633. doi: 10.1076/jcen.25.5.625.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGavock J, Sellers E, Dean H. Physical activity for the prevention and management of youth-onset type 2 diabetes mellitus: focus on cardiovascular complications. Diab Vasc Dis Res. 2007;4:305–310. doi: 10.3132/dvdr.2007.057. [DOI] [PubMed] [Google Scholar]
- 13.Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. Eur J Pharmacol. 2008;585:119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 15.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 16.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 17.Yaffe K, Cauley J, Sands L, Browner W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch Neurol. 1997;54:1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Sheikh JI, Yesavage JA, Brooks JO, 3rd, et al. Proposed factor structure of the Geriatric Depression Scale. Int Psychogeriatr. 1991;3:23–28. doi: 10.1017/s1041610291000480. [DOI] [PubMed] [Google Scholar]
- 20.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 23.Kesaniemi YK, Danforth E, Jr, Jensen MD, Kopelman PG, Lefebvre P, Reeder BA. Dose-response issues concerning physical activity and health: an evidence-based symposium. Med Sci Sports Exerc. 2001;33:S351–S358. doi: 10.1097/00005768-200106001-00003. [DOI] [PubMed] [Google Scholar]
- 24.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 25.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 27.Fritsch T, McClendon MJ, Smyth KA, Lerner AJ, Friedland RP, Larsen JD. Cognitive functioning in healthy aging: the role of reserve and lifestyle factors early in life. Gerontologist. 2007;47:307–322. doi: 10.1093/geront/47.3.307. [DOI] [PubMed] [Google Scholar]
- 28.Fritsch T, Smyth KA, McClendon MJ, et al. Associations between dementia/mild cognitive impairment and cognitive performance and activity levels in youth. J Am Geriatr Soc. 2005;53:1191–1196. doi: 10.1111/j.1532-5415.2005.53361.x. [DOI] [PubMed] [Google Scholar]
- 29.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerterp KR. Assessment of physical activity: a critical appraisal. Eur J Appl Physiol. 2009;105:823–828. doi: 10.1007/s00421-009-1000-2. [DOI] [PubMed] [Google Scholar]
- 31.Winters-Hart CS, Brach JS, Storti KL, Trauth JM, Kriska AM. Validity of a questionnaire to assess historical physical activity in older women. Med Sci Sports Exerc. 2004;36:2082–2087. doi: 10.1249/01.mss.0000147592.20866.07. [DOI] [PubMed] [Google Scholar]