Abstract
Purpose
Lymphatic disorders are poorly understood with few animal models. We designed a novel assay to measure lymphatic development using transgenic zebrafish with fluorescently labeled endothelial cells. Two major branches of the vascular endothelial growth factor receptor (VEGFR) signaling pathway were examined: the MAPK and PI3K pathways.
Methods
Direct visualization of lymphatic development was performed in control embryos or under chemical inhibition. Treatment involved a 6-hour pulse of inhibitor at 3 days post fertilization (dpf). Fish were analyzed for the presence of the TD at 4 dpf (n > 100 specimens).
Results
TD formation was prevented using selective inhibitors against kinases (MAPK, PI3K/TOR, or VEGFR). These kinases were important for TD formation, as the lymphatic vessel failed to form in a majority of treated animals. Remarkably, MAPK pathway inhibition most robustly reduced lymphangiogenesis, demonstrated by a lack of lymphatic endothelial cells.
Conclusion
We conclude that MAPK pathway function downstream of the VEGFRs is crucial at the early stages of TD development. This study provides a novel animal model and a potential target pathway for further investigation. We suggest further examination of MAPK pathway deregulation as a potential mechanism underlying lymphatic disease in humans.
Keywords: lymphatic disorders, primary lymphedema, VEGF, VEGF receptors, MEK inhibitor, lymphangiogenesis
The lymphatic system normally functions in the transport of fluids and facilitating the return of extravasated cells and macromolecules back into the blood circulation. Disorders of the lymphatic vasculature can lead to a variety of debilitating conditions, including disfigurement, bone overgrowth, bleeding, infection, pleural effusions and ascites. Lymphatic disease and its complications frequently hinder a child's normal development and induce added emotional distress. Despite centuries of clinical experience, little is known regarding the underlying mechanisms responsible for lymphatic disease. By employing a novel zebrafish model, we sought a basic understanding of how this unique unidirectional vascular system develops.
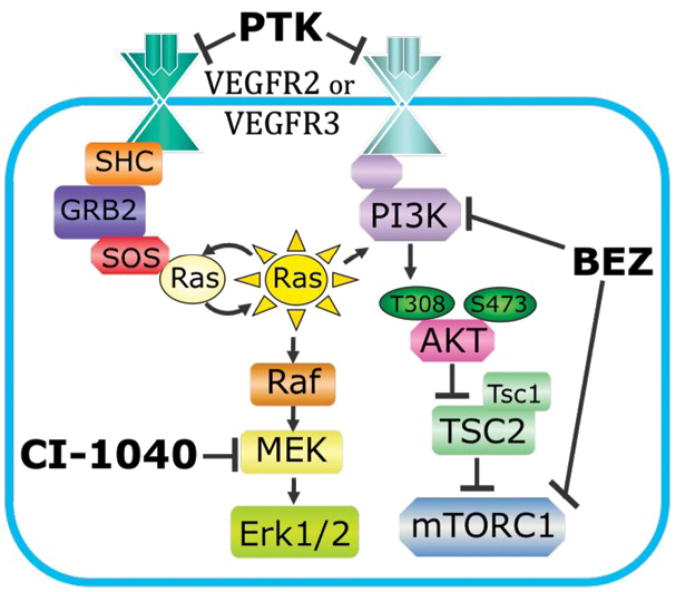
The VEGFs (vascular endothelial growth factors; VEGF-A, VEGF-C, and VEGF-D) and their receptor tyrosine kinases (VEGFR1, 2 and 3) are master regulators for the development of blood and lymphatic vessels in vertebrates . In developmental lymphangiogenesis, VEGF-C stimulation of VEGFR3 is essential for the proliferation, growth, and survival of lymphatic endothelial cells (LECs), while a role for VEGF-D appears to be to linked to pathological lymphangiogenesis . Evidence for overlapping roles for VEGFR2 and VEGFR3 function in angiogenesis and lymphangiogenesis has also been demonstrated, suggesting the expression of heterodimeric receptors consisting of VEGFR2 and VEGFR3 during development . Despite understanding of the endothelial functions of these receptors, the mechanism by which ligand stimulation is converted into an intracellular signal in vivo is largely unknown. Most of the data on VEGFR downstream signaling has been derived from in vitro blood or lymphatic endothelial cell (LEC) studies . From the first report of isolated LECs, VEGF-C stimulation of VEGFR3 was shown to lead to the downstream phosphorylation of both MAPK and AKT (Fig. 1) . The MAPK and PI3K-AKT-TOR pathways appear to act in parallel to each other. However, the stage-dependent need for these intracellular signaling pathways during lymphangiogenesis has not been clearly defined in vivo.
Figure 1. MAPK and PI3K Signaling Pathways.
VEGFR2 and R3 can stimulate MAPK and PI3K signaling pathways in vitro. Inhibitors CI-1040, BEZ235 (BEZ), and PTK787/ZK2222584 (PTK) were employed to target the MAPK pathway, the PI3K pathway, or both pathways, respectively.
Currently, the zebrafish is the simplest vertebrate organism system for investigation of lymphatic development. Zebrafish offer the advantages of rapid external development, transparency, high fecundity, homologous genes and conserved cellular processes with humans and mice . Like the mouse model, zebrafish lymphangioblasts initially sprout from venous endothelial cells (VECs) by about 2 days post fertilization (dpf) . A distinct subpopulation of endothelial cells (ECs) in the posterior cardinal vein commits to a lymphatic linage through expression of a lymphatic cell fate regulator Prox-1 . In zebrafish, as in mammals, VEGF-C and VEGFR3 signaling are essential in the early formation and migration of lymphangioblasts derived from VECs . In fish, these lymphangioblasts travel a defined path along the intersegmental and parachordal vessels to eventually reside just ventral to the aorta, in isolated patches . Over the next couple of days, these LECs coalesce to form the thoracic duct (TD). This central lymphatic duct is complete and functional by the first week of development . Using the presence or absence of a continuous TD over 6 somites as a measurement for lymphatic development, we used selective chemical kinase inhibitors to define signaling cascades activated during lymphangiogenesis in vivo.
Materials and Methods
Animals
The Institutional Animal Care and Use Committee of Children's Hospital Boston approved all animal protocols. Zebrafish (Danio rerio) were maintained at 28.5°C on a 14-h-light/10-h-dark cycle. Embryos were collected by natural spawning and raised in 10% Hanks' buffered saline solution at 32°C.
Chemical Treatment with Inhibitors
Small molecule inhibitors were added to the embryo medium at 3 dpf for a period of 6 h and kept at 32°C. Inhibitors used consisted of CI-1040 (1.5 mM), BEZ235 (500 nM), and PTK787/ZK222584 (5 mM). Inhibitor was then washed out with three washes of embryo medium. All inhibitors were purchased commercially from Axon Medchem.
Quantitation of Thoracic Duct Formation
Embryos were anesthetized with tricaine (Sigma) and mounted in 4% methylcellulose. TD formation in a transgenic endothelially-driven GFP line [15] was evaluated at 4 dpf via fluorescence microscopy. The presence of the dorsal aorta and posterior cardinal vein was identified anatomically. In addition, blood flow was ensured by microscopic evaluation in embryos at this time. The TD was identified as a separate tubular structure just ventral to the aorta over a length of the first six intersegmental vessels (ISVs), i.e. over 6 somites. This was the only lymphatic vessel directly and clearly observed in the embryo. The duct was counted as present only if it spanned this entire length. Partial formation was rarely observed, and scored as absence of TD.
Statistics Analysis
Statistical analysis was performed using Chi squared analysis. A total of eight separate experiments were combined according to identical treatment conditions. Error bars indicate standard deviation between experiments.
Results
Effects of MAPK Inhibition on Thoracic Duct Development
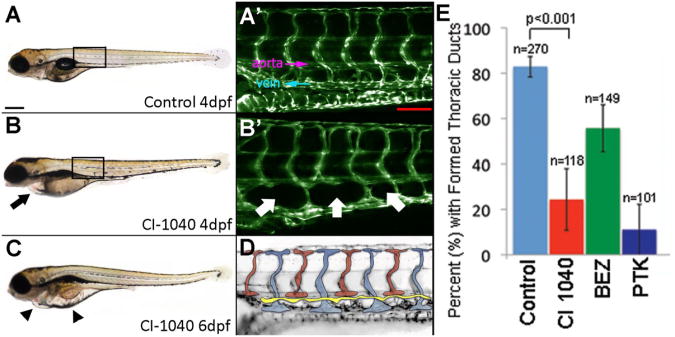
In order to directly visualize the vasculature, we used transgenic zebrafish lines with fluorescently labeled endothelial cells . Zebrafish embryos were allowed to develop normally so that angiogenesis and functional blood flow was clearly established by from 1-3 dpf . The development of the TD was monitored in untreated embryos over time and formation was nearly complete in 80% of animals by 4 dpf (unpublished data). This allowed for a standard time-sensitive phenotype with which to compare our treated embryos (corresponding to E10-11 in mice) . Our experimental design involved a short-term 6 h pulse of chemical inhibition, followed by 18 h of recovery, then quantitation of lymphatic vessel over 6 somites. As VEGFR3 activates both the MAPK and PI3K/TOR signaling cascades, we sought to determine the extent to which each pathway contributed to TD formation. Prior studies in mice suggested a role for MAPK in lymphatic vessel development. However, its precise spatiotemporal function could not be determined owing to embryonic lethality . We have previously shown that CI-1040, a highly selective and noncompetitive inhibitor for MEK, induced specific vascular effects in the zebrafish model . Our unpublished observations also suggested that short-term inhibition of the MAPK pathway effectively blocked lymphatic vessel formation. To investigate this process further, we targeted the MAPK pathway using CI-1040. Zebrafish were treated with a pulse of inhibitor at 3 dpf for 6 h, followed by triple washout with embryo medium and incubation without inhibitor for an additional 18 h, The following day (4 dpf), we measured TD formation over the first 6 somites in individual animals and scored for its absence or presence in an all-or-none manner (Figs. 2A′,D). A majority of larval fish lacked a TD; an average of only 24% of chemically treated animals displayed a connected lymphatic vessel over this region (Figs.2B′,E). By comparison, over 82% of untreated, wild type larvae had developed a TD by this time (CI 8.8-25.3). A range of CI-1040 doses were used to determine that treatment at 1.5 µM was most effective (unpublished data). Interestingly, our data suggest a precise spatiotemporal need for MEK-MAPK/ERK activation during this 6-h period because TD formation could not be recovered in the next 48 h, up to 6 dpf (data not shown), resulting in massive edema (Fig. 2C). This underscores the importance of investigating lymphatic vascular formation using an in vivo model. These data were highly reproducible over eight separate experiments (n >100 individual animals per condition; p<0.0001).
Figure 2. Formation of Thoracic Duct in Treated and Untreated Zebrafish Embryos.
Embryos treated with MAPK (Mek1/2) pathway inhibitor CI-1040 developed progressive pericardial (black arrow) and diffuse (black arrowhead) edema over time (panels A-C). Microscopic magnification of boxed areas in A and B are noted. TD development was evaluated over a length of 6 ISVs and located between the dorsal aorta and posterior cardinal vein (labeled) (panel A′). Panel D shows a schematic indicating the thoracic duct in yellow. In fish treated with CI-1040, loss of TD formation and lack of presumed LECs (white arrows) were noted (panel B′). Compared with controls, all treatment groups exhibited statistical prevention of formation of TD (p<0.0001, Chi squared test). In addition, CI-1040 more strongly prevented formation compared with BEZ (p<0.0001, OR=3.9) Error bars indicate standard deviation between experiments (panel E). Scale bar A 300 μm; A′ 100 μm.
In addition to the lack of formation of the TD, more subtle differences between treated and untreated embryos were encountered. In wild type fish, presumptive lymphangioblasts accumulate in the space between the dorsal aorta and posterior cardinal vein . As the TD forms, a lymphatic vascular plexus can be seen in this region, which remodels into lymphatic intersegmental vessels guided by intersomitic arteries . By 4 dpf, a large proportion (∼80-90%) of zebrafish developed a lymphatic plexus (data not shown). However, in larval fish treated with the 6 h pulse of CI-1040, large gaps were frequently observed (Fig. 2B′). The lack of GFP-positive endothelial cells suggests a reduction in proliferation and/or migration of lymphangioblasts into this region. In addition, CI-1040-treated fish began to develop pericardial edema by this time point (4 dpf), as an early sign of an inability to clear extracellular fluid (Fig. 2B). The presence of pericardial edema was frequently directly correlated with the absence of the TD in the same animal.
Inhibition of PI3K/TOR on Thoracic Duct Assay
In addition to the MAPK pathway, involvement of the PI3K-AKT-mTOR signaling axis in lymphatic vessel formation, particularly at later stages of lymphatic valve and collecting duct formation, has been implicated . To investigate a direct stage-specific role in vivo for this pathway, we used a dual specificity inhibitor, BEZ235, which blocks the function of both PI3K and TOR . In addition, we also examined upstream receptor inhibitors: PTK787/ZK222584 (5 µM); which blocks all 3 VEGFRs . Each chemical was introduced at 3 dpf for a pulse of 6 h, followed by triple washout and 18 h of recovery, as above. Evaluation of TD formation with each inhibitor demonstrated reduced lymphangiogenesis, as compared with controls (Fig. 2E). Curiously, in embryos treated with PTK787 (targeting VEGFRs; thus both PI3K and MAPK pathways), lymphatic duct development was similarly affected as in the MEK/MAPK treated group (11% for PTK787, CI19.7-80.4; 24% for CI-1040). In contrast, the use of BEZ235 was less effective, allowing 55% of treated animals to score positive in our assay (CI 2.5-6.1; Fig. 2D).
Discussion
Understanding the basic developmental mechanisms of lymphangiogenesis provides important insights into how its deregulation may contribute to lymphatic disorders. By applying current understanding of the roles for VEGFR3 activation of MAPK and PI3K-AKT-TOR signaling pathways, we used selective small molecule inhibitors to determine how functional blockade for a defined period of 6 h at 3 dpf would affect lymphatic vessel development (Fig. 1). Interestingly, our targeted treatment revealed a narrow window for lymphangiogenesis that appears to depend strongly on MAPK signaling in vivo. This observation is supported by the lack of recovery in TD development over the next 2.5 d (54 h), correlated with excessive edema, suggesting functional impediment of fluid clearance.
Over the last 30 years, study of the function of the VEGF-A ligand and VEGFR2 signaling pathway has led to the first clinical use of anti-angiogenic therapies for the treatment of cancer and the vascularized form of macular degeneration . However, little is known about the intracellular signaling components downstream of receptor stimulation in vivo. Recessive VEGFR3 or VEGF-C null mice are embryonically lethal, while VEGFR3 heterozygous adults exhibit haploinsufficiency, affecting lymphatic vessel function . In humans, an autosomal dominant mutation in VEGFR3 results in a rare lymphatic disease known as Milroy disease . Affected individuals develop progressive lower extremity edema. Reduced function of the VEGFR3 signaling pathway and hypo-proliferation of LECs have been proposed as a cause of this disease . However, it is important to note that disorganized lymphatic vessels and poor function are also found in other forms of lymphatic disorders . We hypothesized that small molecule inhibitors targeting the receptor or its effector kinases might also be effective in regulating the overall signaling level of these pathways during distinct phases of lymphangiogenesis. Formation of the lymphatic system likely involves a specific stepwise activation of external receptors and intracellular signaling pathways. Examination of an early in vitro report of VEGF-C versus VEGF-A signaling in isolated human LECs demonstrated a qualitatively distinct mode of MAPK activation . VEGF-A stimulation led to rapid and robust phosphorylation that peaked at 10-20 min and then returned to basal levels. In contrast, VEGF-C activation triggered a sustained but lower level increase in MAPK phosphorylation for 6 h . Using chemical modulation of VEGFR receptor signaling pathways established in our laboratory, we have demonstrated that TD formation is severely and similarly impaired by either VEGFR or MAPK inhibition in vivo. Therefore, intracellular activation of the MAPK signaling pathway promotes lymphangiogenesis. In contrast, the limited effects on lymphatics of inhibiting the PI3K-AKT-TOR signaling pathway suggest a minor role during this narrow time window. This is supported by the need for AKT function in lymphatic valve formation in the mouse model .
Despite these promising results, limitations of our model mandate additional experimentation. For example, chemical treatment of the whole organism affects every cell in the body. Thus, we have been careful in restricting our inhibitor treatment to a pulse of 6 h, followed by extensive washout and recovery as in our reported studies . Our previous experience suggests that this controlled treatment period can produce a more selective targeting of essential steps in vascular development. Our data is consistent with other groups in measuring the spatiotemporal development of the lymphatic vascular system in the zebrafish model . Furthermore, our assays are defined to target clinically relevant signaling pathways that have been shown to be significantly upregulated in human cancers . Current cancer studies suggest that identifying the deregulated pathway driving cancer growth can facilitate treatment options . Consistent with this idea, we suggest that identification of aberrantly activated pathways in vascular anomalies may also be useful in the development of potential drug therapy.
Further characterization of lymphatic vascular development is required in order to obtain insights into deregulation in lymphatic disorders. Since our study defines an in vivo role for MAPK signaling during lymphangiogenesis, it is possible to consider that deregulation, such as overactivity, may contribute to the progression of lymphatic diseases. This possibility is supported by malformation and dysfunction of the mouse lymphatic system upon loss of MAPK pathway regulators, SPRED1/2 . Further examination of this pathway in patient lesional samples of lymphatic disorders may provide support for the development of an anti-MAPK therapy using selective MEK inhibitors currently under preclinical development for cancer treatment.
Acknowledgments
We thank David Zurakowski, PhD, Dario Fauza, MD, and Karen Pepper, PhD for helpful discussions and/or critical reading of the manuscript. This work was supported in part by grants from the NIH (CA111564), the Department of Defense (TS093079), and the Weitzman Family Vascular Anomalies Fund. Dr. Fevurly is supported by the Stuart and Jane Weitzman Fellowship in Vascular Anomalies.
Footnotes
The authors disclose no financial conflict of interest that might be construed to influence the results or interpretation of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bahram F, Claesson-Welsh L. VEGF-mediated signal transduction in lymphatic endothelial cells. Pathophysiology. 2010;17:253–261. doi: 10.1016/j.pathophys.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Bahram F, Claesson-Welsh L. VEGF-mediated signal transduction in lymphatic endothelial cells. Pathophysiology. 2010;17:253–261. doi: 10.1016/j.pathophys.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010;24:2115–2126. doi: 10.1101/gad.1955910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams Ra, A K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Yamaguchi S, Chida K, et al. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isogai S, Hitomi J, Yaniv K, et al. Zebrafish as a new animal model to study lymphangiogenesis. Anatomical science international/Japanese Association of Anatomists. 2009;84:102–111. doi: 10.1007/s12565-009-0024-3. [DOI] [PubMed] [Google Scholar]
- 9.Butler MG, Isogai S, Weinstein BM. Lymphatic development. Birth Defects Res C Embryo Today. 2009;87:222–231. doi: 10.1002/bdrc.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaniv K, Isogai S, Castranova D, et al. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 11.Kuchler AM, Gjini E, Peterson-Maduro J, et al. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol. 2006;16:1244–1248. doi: 10.1016/j.cub.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan RS, Geng X, Yang Y, et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24:696–707. doi: 10.1101/gad.1859310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geudens I, Herpers R, Hermans K, et al. Role of Delta-like-4/Notch in the Formation and Wiring of the Lymphatic Network in Zebrafish. Arterioscler Thromb Vasc Biol. 2010;30:1695–1702. doi: 10.1161/ATVBAHA.110.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan BM, Herpers R, Witte M, et al. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development. 2009;136:4001–4009. doi: 10.1242/dev.039990. [DOI] [PubMed] [Google Scholar]
- 15.Westerfield M. Guide for the laboratory use of zebrafish (Danio rerio) Eugene: Univ. of Oregon Press, Eugene; 1995. The zebrafish book. [Google Scholar]
- 16.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 17.Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- 18.Isogai S, Lawson ND, Torrealday S, et al. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- 19.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 20.Dumont DJ, Jussila L, Taipale J, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi K, Kohno R, Ayada T, et al. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Mol Cell Biol. 2007;27:4541–4550. doi: 10.1128/MCB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen LF, Sebolt-Leopold J, Meyer MB. CI-1040 (PD184352), a targeted signal transduction inhibitor of MEK (MAPKK) Semin Oncol. 2003;30:105–116. doi: 10.1053/j.seminoncol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Ohren JF, Chen H, Pavlovsky A, et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat Struct Mol Biol. 2004;11:1192–1197. doi: 10.1038/nsmb859. [DOI] [PubMed] [Google Scholar]
- 24.Bolcome RE, 3rd, Chan J. Constitutive MEK1 activation rescues anthrax lethal toxin-induced vascular effects in vivo. Infect Immun. 2010;78:5043–5053. doi: 10.1128/IAI.00604-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolcome RE, 3rd, Sullivan SE, Zeller R, et al. Anthrax lethal toxin induces cell death-independent permeability in zebrafish vasculature. Proc Natl Acad Sci U S A. 2008;105:2439–2444. doi: 10.1073/pnas.0712195105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F, Chang Z, Zhang L, et al. Akt/Protein Kinase B Is Required for Lymphatic Network Formation, Remodeling, and Valve Development. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnell CR, Stauffer F, Allegrini PR, et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Res. 2008;68:6598–6607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- 28.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 29.Wood JM, Bold G, Buchdunger E, et al. PTK788/ZK 222584, a novel and potent inhibitor of vasular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]
- 30.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 31.Dumont DJ, Jussila L, Taipale J, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 32.Haiko P, Makinen T, Keskitalo S, et al. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol Cell Biol. 2008;8:4843–4850. doi: 10.1128/MCB.02214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karkkainen MJ, Saaristo A, Jussila L, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghalamkarpour A, Holnthoner W, Saharinen P, et al. Recessive primary congenital lymphoedema caused by a VEGFR3 mutation. J Med Genet. 2009 doi: 10.1136/jmg.2008.064469. [DOI] [PubMed] [Google Scholar]
- 35.Mellor RH, Hubert CE, Stanton AW, et al. Lymphatic dysfunction, not aplasia, underlies Milroy disease. Microcirculation. 2010;17:281–296. doi: 10.1111/j.1549-8719.2010.00030.x. [DOI] [PubMed] [Google Scholar]
- 36.Cochran AJ, Binder S, Morton DL. The role of lymphatic mapping and sentinel node biopsy in the management of atypical and anomalous melanocytic lesions. J Cutan Pathol. 2010;37:54–59. doi: 10.1111/j.1600-0560.2010.01509.x. [DOI] [PubMed] [Google Scholar]
- 37.Chan J, Bayliss PE, Wood JM, et al. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell. 2002;1:257–267. doi: 10.1016/s1535-6108(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 38.Bayliss PE, Bellavance KL, Whitehead GG, et al. Chemical modulation of receptor signaling inhibits regenerative angiogenesis in adult zebrafish. Nat Chem Biol. 2006;2:265–273. doi: 10.1038/nchembio778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 40.Tokunaga E, Oki E, Egashira A, et al. Deregulation of the Akt pathway in human cancer. Current cancer drug targets. 2008;8:27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- 41.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]