Abstract
Infections and inflammatory conditions during pregnancy can dysregulate neural development and increase the risk for developing autism and schizophrenia. The following research utilized a nonhuman primate model to investigate the potential impact of a mild endotoxemia during pregnancy on brain maturation and behavioral reactivity as well as the infants’ hormone and immune physiology. Nine pregnant female rhesus monkeys (Macaca mulatta) were administered nanogram concentrations of lipopolysaccharide (LPS) on two consecutive days, six weeks before term, and their offspring were compared to nine control animals. When tested under arousing challenge conditions, infants from the LPS pregnancies were more behaviorally disturbed, including a failure to show a normal attenuation of startle responses on tests of prepulse inhibition. Examination of their brains at one year of age with magnetic resonance imaging (MRI) revealed the unexpected finding of a significant 8.8% increase in global white matter volume distributed across many cortical regions compared to controls. More selective changes in regional gray matter volume and cortical thickness were noted in parietal, medial temporal, and frontal areas. While inhibited neural growth has been described previously after prenatal infection and LPS administration at higher doses in rodents, this low dose endotoxemia in the monkey is the first paradigm to produce a neural phenotype associated with augmented gray and white matter growth.
Keywords: endotoxin, prenatal, emotionality, interleukin-6, MRI, cortical thickness, infant, development
1. Introduction
Prenatal infections and inflammatory responses during pregnancy can result in adverse effects on brain development that contribute to the etiology of affective disorders or neurodevelopmental pathologies like autism and schizophrenia [1-3]. One behavioral feature common to these disorders is a heightened reactivity to visually or acoustically arousing stimuli, often manifest by greater anxiousness and impaired sensory-motor integration, which is evident on prepulse inhibition (PPI) tests of attention and startle [3-5]. In addition, many prenatal challenge studies in rodents and nonhuman primates have found that the offspring exhibit abnormal neuroendocrine and immune responses [6-8].
Developmental abnormalities caused by prenatal infections can be mimicked by the administration of noninfectious agents that evoke inflammatory responses, such as polyriboinosinic-polyribocytidilic acid (Poly I:C) or lipopolysaccharide (LPS) [9-12]. Therefore, it appears that one common mediating pathway initiating this pathogenesis is a disruption of placental functioning by proinflammatory cytokines [13,14]. In turn, cascading effects on the developing brain impact postnatal behavior in ways that resemble dysfunctional features seen in autism and schizophrenia [2,11,15]. Teratogenic disruptions of synapse formation, cellular proliferation, and myelination may promote these abnormal neural phenotypes [16,17]. For example, prenatal LPS treatment in rodents induces white matter (WM) abnormalities and reduced myelin density in the corpus callosum, as well as in cortical and subcortical regions [15-18,19]. Poly I:C administration also reduces gray matter (GM) in medial temporal areas such as entorhinal cortex and hippocampus [9,20]. In addition, influenza virus infections in pregnant mice result in pups with reduced cortical thickness in the frontal lobe [6], and juvenile rhesus monkeys exposed to influenza prenatally have smaller GM volumes in several cortical regions, including frontal, temporal and parietal cortices [21]. Yet, while prenatal infection typically inhibits neural growth and proliferation, increased GM and WM in certain regions has been found in many individuals with autism--at least early in development [22,23].
To replicate and extend behavioral and neural findings from rodents and nonhuman primates, we sought to model a moderate, self-limiting bacterial infection during pregnancy by administering LPS to gravid female monkeys. Because monkeys and humans are comparatively sensitive to LPS, a low dose protocol was employed to minimize the overt pathology that occurs when high concentrations damage the placenta and fetal brain [24]. Prior research in juvenile monkeys has shown that 4 ng/kg of LPS induces transient increases in IL-6 and cortisol for up to 24 h [25]. The offspring from the prenatal LPS condition were then assessed longitudinally across the first 1.5 years of life. The a priori prediction was that infants from an endotoxin-challenged pregnancy would: 1) be more behaviorally reactive; 2) manifest signs of neuroendocrine and immune dysregulation; 3) show changes in cortical volume and thickness in sensitive brain areas related to emotional regulation and attention. Although we found the expected effects on behavior, the brains of monkeys from LPS pregnancies showed robust increases in WM volumes and selective GM changes in parietal and temporal regions [22]. This novel brain phenotype may be useful for investigating certain neurodevelopmental disorders like autism.
2. Methods
2.1. Animals and prenatal treatments
Details of the LPS administration to gravid females and effects on leukocyte demargination and circulating IL-6 levels are provided in Supplement Table 1. Briefly, nine pregnant monkeys (Macaca mulatta) received two intravenous injections of LPS each morning on Days 125 and 126 of their 169-day pregnancy (2 ng/kg, n=1; 4 ng/kg, n=8). Viral infection during this prenatal period had been found to induce neurodevelopmental and behavioral alterations [21]. The lower 2 ng/kg dose was used just once to assess toxicity and possible risk of miscarriage. Two other gravid females were piloted at the 4 ng/kg dose before the remaining 6 females in this condition were administered with LPS. The nine control mothers were either administered identical volumes of saline (n=2) or not handled (n=7), neither of which significantly affected maternal IL-6 or leukocyte counts. Gestational length, neonatal weight and subsequent growth after birth were determined. Both prenatal conditions included similar numbers of male and female offspring (n= 8 and 10, respectively). The schedule of postnatal testing is summarized in Supplement Table 2. After it was determined that growth patterns and health among 3 initial LPS offspring were not greatly perturbed, additional testing incorporating PPI, cortisol assessment, and in vitro cellular stimulation assays was completed on the remaining cohort of subjects (LPS=6, Control=9). Researchers who collected behavioral and MRI data, as well as assayed physiological samples, were blind to the monkeys’ prenatal conditions. All experimental procedures were approved by the Institutional Animal Care and Use Committee and the Office of Biological Safety at the University of Wisconsin—Madison.
2.2. Behavioral Assessments
2.2.1. Neonatal, mother-Infant, and peer behavior
Temperament, neuromotor reflexes, and attentional responses were assessed at two weeks of age using the Infant Behavioral Assessment Scale (IBAS), adapted from Brazelton [26] for use in monkeys [27,28]. Factor loadings of 4 IBAS categories, which are based on 29 test items, are described in detail in a recent report from our group [29]. Social interactions were observed between each infant and its mother from 1-4 months and with peers from 6-7 months during twelve non-contiguous 5-min periods per month.
2.2.2. Human Intruder Paradigm
Stress reactivity was assessed at 8-9 months of age using a modified version of the Human Intruder Paradigm [25,30]. Briefly, hostile and fearful behaviors defined in Supplement Table 3 were assessed during 5 stare (SC) and no eye contact (NEC) trials, each of 5 min. duration.
2.2.3. Prepulse Inhibition
Using a PPI protocol for monkeys [31], responses to acoustical startle and the extent of adaptation after pairing the stimulus probe with a softer prepulse sound were assessed at 10-12 mo. of age. Each infant was tested in a sound-attenuating booth while freely moving in a small cage. Shifts in movement were recorded along 3 dimensions and computed as x2+y2+z2 to remove direction effects. Startle sounds were played at either 105 or 115 dB for 40 msec. The prepulse was an 80 dB broadcast for 20 msec at intervals of 45, 120, or 500 msec before the startle probe. After a 15 min acclimation, each set of prepulse and startle probes was repeated four times across the 1 h test. Acceleration was used to index startle to remove the influence of body weight. Percent reduction in startle after prepulses, as compared to the trials with the startle probe alone, was computed using the following formula: (100 × [(mean Probe alone trials - mean Prepulse & Probe trials) / mean Probe alone trials]).
2.3. Physiological assessments
2.3.1. Interleukin-6 measures
At 2, 4, and 7 months of age, whole blood was collected from undisturbed animals, diluted 1:1 with IMD buffer, and separately stimulated with phytohemagglutinin (PHA; 5 μg/mL) for 48 h, LPS (10 ng/mL) for 24 h, or with saline. IL-6 levels in the supernatant were then quantified by ELISA. Blood samples were also cultured immediately after the HIP challenge test at 8-9 mo of age. Baseline blood levels of IL-6 in vivo were determined at 1 year of age and after administration of 4 ng/kg LPS i.v. (at 1.5 years of age) to evaluate if the monkeys showed signs of a possible endotoxin tolerance or sensitization [32]. Plasma IL-6 levels were determined at 1 and 3 h after this injection of LPS.
2.3.2. Cortisol Measures
Adrenal hormone levels were determined under basal and challenge conditions using a week-long protocol [21]. Briefly, cortisol was assessed at baseline, after transfer to a novel cage, 2 days later following acclimation, and on the day after overnight dexamethasone treatment.
2.4. Neuroimaging
At approximately one year of age, T1 and T2-weighted neural images were acquired using a GE Signa 3-T scanner (General Electric Systems, Milwaukee, WI). One animal was re-scanned at 1.5 years of age due to a positioning error of its head in the stereotax platform, which lead to inadequate normalization to the brain template; the new scan acquisition and the variation in age at scan did not bias group differences. For the neuroimaging scan acquisition, the animals were initially anesthetized by administration of ketamine hydrochloride (10 mg/kg, i.m.) followed by medetomidine (50 μg/kg, i.m.) and placed into an 18 cm quadrature extremity coil (IGC Medical Advances, Milwaukee, WI). Details of the scan acquisition and data analysis have been described previously [21, 33]. For the T1-weighted scan, a high resolution axial Inversion Recovery-prepped 3D-SPGR sequence was used: inversion time=600 msec; TR=8.6 msec; TE=2.0 msec; FOV=160 mm; flip angle=10°; matrix=256×256×124; slice thickness=1.5 mm; slice gap=−0.5 mm; bandwidth=15.63; voxel resolution of 0.234 × 0.234 × 0.498 mm. The T2-weighted scan had the following parameters: TR=12,000 msec; TE=92.8 msec; FOV=160 mm; flip angle=90°; matrix=512 × 512; slice thickness=1.5 mm; slice gap=0 mm; bandwidth=31.25; voxel resolution of 0.27 × 0.27 × 1.5 mm. T1 and T2 weighted scans were aligned using a 3-point localizer during the scanning session. For image preprocessing, an automated method was first used to skull-strip a given brain, followed by affine coregistration and then non-linear 12 parameter normalization to a customized juvenile rhesus monkey template [34]. T1 and T2 probability maps were jointly used for segmentation of GM, WM, and cerebrospinal fluid (CSF). Figure 1 depicts the segmentation of tissue classes for regional parcellation and the derivation of cortical thickness [34,35]. Volume was derived for each parcellated region of interest for GM and WM segments.
Figure 1.

Representative segmentation and parcellation of the brain into regional GM and WM volumes, respectively (top row). Cortical thickness was also determined in these hemispheric regions by examining the distance between the pial and WM surfaces (bottom row) [32].
2.5. Statistical analyses
Analyses were conducted using SPSS 15.0 (Chicago, IL). Effects of Prenatal Condition (LPS vs. Control) on behavior or physiology across Age or Trials were analyzed with either mixed repeated measures analysis of variance or covariance (ANOVA, ANCOVA) or by an omnibus F-test. Multiple scores in behavioral tests were collapsed together to improve homoscedasticity of variance and normality. Square root or base log 10 transformations corrected variables that deviated from normality, homoscedasticity, or sphericity. Two-tailed t-tests were used in post hoc analyses of individual variables. Omnibus repeated measures tests were used to guard against experiment-wise error for neural region of interest analyses. Intracranial volume (ICV)-corrected values were also examined. Alpha level was set at .05.
3. Results
3.1. Maternal response to LPS and neonatal health
Compared to pre-injection blood levels, administration of LPS resulted in a significant increase in IL-6 levels in the gravid females [F(1,8)=25.10, p=.001]. An acute increase in neutrophils [F(1,8)=16.64, p<.01] and decrease in lymphocytes [F(1,8)=40.99, p<.001] were evident in all LPS-injected animals. Post hoc tests confirmed these changes for Day 1 and 2 (see Supplement Table 1). Physiological values in control animals remained similar to baseline across the 2 days. All infants were born normally without delivery complications. Prenatal LPS treatment did not affect gestation length, neonatal weight, and subsequent growth patterns. No significant differences were noted in maternal care or rate of infant maturation.
3.2. Neuroimaging
When scanned at one year of age, the intracranial volume (ICV) of monkeys from the LPS-treated pregnancies was marginally 5.9% larger than for the control monkeys (Tables 1 and 2). More dramatically, LPS monkeys had a significant 8.8% increase in mean global WM volume. Global GM, total CSF, and ventricular size did not differ statistically from controls. Variations in age at scan did not account for these results. Omnibus tests were conducted to guard against type 1 error and justify volumetric analyses for regions of interest (see Supplement Text 1). Nearly all WM regions were significantly larger in LPS-exposed monkeys, whereas selective GM changes were seen in parietal and frontal areas (Tables 1 and 2; Figure 2), as well as in hippocampus and putamen. The findings for cortical thickness supported the GM results (Supplement Table 4), with marginally thicker GM in the right parietal and frontal lobes, but thinner GM in medial temporal lobe. To assess the relative magnitude of the regional changes, the volumes and cortical thickness were divided by the monkey’s ICV or its cube root, respectively. The statistical significance of volumetric and cortical thickness results remained largely unchanged.
Table 1.
White matter volumes, both globally and in cortical regions and select neural structures, for control (n=9) and LPS (n=9) progeny.
| Neural Area | Hemisphere | Control Mean (voxels) |
LPS Mean (voxels) |
p-value ICV uncorrected |
p-value ICV corrected |
|---|---|---|---|---|---|
| Intracranial volume | 88 512 ± 1194 | 93 726 ± 2,625 | p ≤ .09 | N/A | |
| Global WM | 20 068 ± 285 | 21 839 ± 738 | p ≤ .05 | p ≤ .06 | |
|
| |||||
| Cortical Regions | |||||
| Prefrontal | Left | 712 ± 18 | 829 ± 30 | p ≤ .01 | p ≤ .001 |
| Right | 708 ± 18 | 812 ± 29 | p ≤ .01 | p ≤ .01 | |
| Frontal | Left | 1 260 ± 21 | 1 430 ± 52 | p ≤ .01 | p ≤ .01 |
| Right | 1 263 ± 21 | 1 419 ± 55 | p ≤ .05 | p ≤ .05 | |
| Cingulate | Left | 139 ± 3 | 160 ± 6 | p ≤ .01 | p ≤ .07 |
| Right | 129 ± 4 | 151 ± 6 | p ≤ .01 | p ≤ .09 | |
| Temporal Auditory | Left | 382 ± 9 | 447 ± 18 | p ≤ .01 | p ≤ .01 |
| Right | 388 ± 8 | 448 ± 19 | p ≤ .01 | p ≤ .05 | |
| Temporal Visual | Left | 1 336 ± 23 | 1 414 ± 39 | ||
| Right | 1 344 ± 22 | 1 415 ± 41 | |||
| Medial Temporal | Left | 129 ± 6 | 161 ± 9 | p ≤ .01 | p ≤ .05 |
| Right | 161 ± 6 | 195 ± 11 | p ≤ .05 | p ≤ .05 | |
| Parietal | Left | 1 574 ± 31 | 1 730 ± 67 | p ≤ .05 | |
| Right | 1 605 ± 28 | 1 761 ± 61 | p ≤ .05 | p ≤ .09 | |
| Occipital | Left | 1 315 ± 39 | 1 362 ± 33 | ||
| Right | 1 397 ± 44 | 1 452 ± 30 | |||
|
| |||||
| Subcortical Regions | |||||
| Corpus Callosum | 577 ± 23 | 649 ± 30 | p ≤ .09 | ||
| Cerebellum | Left | 1 071 ± 22 | 1 094 ± 37 | ||
| Right | 1 082 ± 23 | 1 115 ± 36 | |||
| Brainstem | 1 360 ± 35 | 1 453 ± 59 | |||
Mean±SEM is reported for raw WM volume estimates of monkeys from control and LPS pregnancies. The absolute value of each region of interest was divided by subject’s ICV to assess differential effects on each region relative to total brain effects. Blank spaces signify non-significant results.
Table 2.
Gray matter volumes, both globally and in cortical regions and select subcortical structures, for control (n=9) and LPS (n=9) progeny at one year of age.
| Neural Area | Control Mean (voxels) |
LPS Mean (voxels) |
p-value ICV uncorrected |
p-value ICV corrected |
|---|---|---|---|---|
| Intracranial volume | 88 512 ± 1 194 | 93 727 ± 2 625 | p ≤ .09 | N/A |
| Global GM | 50 648 ± 792 | 53 432 ± 895 | ||
|
| ||||
| Cortical Regions | ||||
| Prefrontal | 5 055 ± 127 | 5 501 ± 242 | ||
| Frontal | 5 436 ± 129 | 5 977 ± 191 | p ≤ .05 | p ≤ .05 |
| Cingulate | 1 628 ± 23 | 1 696 ± 63 | ||
| Temporal Auditory | 3 745 ± 78 | 3 911 ± 128 | ||
| Temporal Visual | 5 605 ± 99 | 5 863 ± 192 | ||
| Medial Temporal | 2 217 ± 43 | 2 286 ± 57 | ||
| Parietal | 5 812 ± 133 | 6 385 ± 234 | p ≤ .05 | p ≤ .07 |
| Occipital | 8 066 ± 156 | 8 211 ± 252 | p ≤ .06 | |
|
| ||||
| Sub-Cortical Regions | ||||
| Caudate | 606 ± 12 | 624 ± 16 | ||
| Putamen | 860 ± 11 | 921 ± 24 | p ≤ .05 | |
| Hippocampus | 400 ± 5 | 429 ± 11 | p ≤ .05 | |
| Amygdala | 381 ± 6 | 402 ± 11 | ||
| Cerebellum | 4 212 ± 98 | 4 536 ± 157 | ||
| Brainstem | 226 ± 8 | 230 ± 9 | ||
Mean±SEM is reported for raw GM volume estimates of monkeys from control and LPS pregnancies. The absolute value of each region of interest was divided by subject’s ICV to assess differential effects on GM of each region relative to global GM. The small size of the insula precluded accurate estimation and analysis. Blank spaces signify non-significant results.
Figure 2.

The percentage change in grey matter and white matter cortical volumes induced by prenatal LPS treatment (n=9) relative to controls (n=9). *=p<.05, **=p<.01.
3.3. IL-6 and Cortisol levels
As detailed in Supplement Figures 1 and 2, there was a relatively mild impact on pituitary-adrenal activity and a complex bidirectional effect on IL-6 responses in LPS-exposed offspring over time. When monkeys from the LPS-treated pregnancies were moved to a new cage, their cortisol levels were higher after 2 days relative to baseline, as compared to hormonal adaptation of control animals. Following overnight dexamethasone treatment, the morning cortisol levels of the LPS-exposed monkeys were initially more suppressed, but by afternoon the cortisol levels were elevated when compared to controls. While with their mothers at 2 and 4 months of age, infants from the LPS condition initially appeared to show more cellular reactivity when their blood was stimulated in vitro with PHA. However, one month after being weaned from the mother, their cellular response to PHA as reflected by IL-6 in the supernatant was significantly lower than those of control offspring.
3.4. Behavior
3.4.1. IBAS at 2 weeks of age
Some behavioral differences were already evident at 2 weeks of age, when infants from LPS-treated pregnancies received higher Emotionality ratings during the IBAS test [t(15)=3.17, p<.01]. One LPS offspring was excluded from this analysis because it appeared to become weak and fatigued by the testing. This exclusion did not influence the significance of results. A repeated measures omnibus on all variables constituting this Emotionality factor [F(1,15)=6.88, p<.05], followed by t-tests, showed that LPS offspring were also significantly hyperresponsive for the constituent test items and related measures such as vocalizations (see Supplement Table 5). No other differences in behavioral maturation or activity were evident at this age.
3.4.2. Social interactions during first 7 months of age
No overt effect of the prenatal LPS treatment was seen on infants’ social and exploratory behavior while observed undisturbed with the mother or after weaning into small peer groups.
3.4.3. HIP behavior and post-HIP IL-6 response at 8-9 months of age
As detailed in Figure 3, 8-9 month old LPS offspring exhibited a pattern of marked behavioral reticence in contrast to their earlier reactions during the IBAS. While this test typically evokes a range of anxious and hostility behaviors that are listed in Supplement Table 3, the LPS progeny showed these behaviors less frequently than controls (e.g., vocalizations), both during the NEC and SC phases despite more exploratory activity during the baseline phase. Specifically, at baseline, LPS animals initially engaged in more tactile and oral exploration of their environment: 10% of the time compared to 4.3% for controls [t(16)=2.81, p<.05]. Both groups spent the remainder of this period predominantly moving and did not differ significantly. Following entrance of the experimenter into the room, LPS offspring became more behaviorally reticent during the SC and NEC phases. During the SC phase, a Stress Behavior × Prenatal Condition interaction [F(1,16)=3.09, p<.05], followed by post hoc testing, showed that the LPS animals spent less time or performed fewer bouts of freezing [t(16)=2.23, p<.05], self-contact [t(16)=3.05, p<.01], and experimenter-oriented fixation [t(16)=2.19, p<.05]. They also engaged in less hostile behavior toward the observer [t(16)=2.41, p<.05], represented as a mean percentage of time spent during a 300s trial (Figure 3A). As compared to controls, the mean frequency of vocalizations was less during the SC phase [t(16)=2.41, p<.05] (Figure 3B). Monkeys from LPS-treated pregnancies also appeared less responsive during the NEC phase, during which they froze less often [t(16)=2.75, p<.05] and engaged in less visual fixation toward the experimenter [t(16)=2.75, p<.05]. The mean frequency of fearful non-vocalization behaviors is depicted in Figure 3C. Immediately after the HIP, blood was drawn and stimulated with phytohemagglutinin (PHA) to examine IL-6 levels. When blood was collected immediately after the test and stimulated in vitro with PHA, there was a significantly greater stress-related suppression of IL-6 release in the blood taken from LPS-exposed monkeys [t(13)=2.16, p<.05] (Figure 3D).
Figure 3.

Frequency and percentage duration of HIP behavior during the stare challenge (SC) and no eye contact (NEC), as well as the post-HIP in vitro assessment of IL-6 production. Monkeys from the control (n=9) and LPS (n=9) pregnancies were assessed. See Supplementary Table 3 for the list of behaviors recorded. Data are depicted as mean ± SEM. *=Student’s t-test, p<.05.
3.4.4. Acoustical startle and PPI at 10-12 months of age
When PPI tests were conducted at 10-12 months of age, monkeys from the LPS-treated pregnancies were found to freeze and startle less than controls across the four initial 115 dB pulses [F(1,13)=11.56, p<.01]. Mean pulse-induced startles were reduced by 55% across all subsequent pulse trials [F(8,104)=2.35, p<.05] (Figure 4A). For %PPI at 115 dB, a repeated measures omnibus indicated that juvenile monkeys from LPS pregnancies had a dysregulated response following the prepulse across the 3 inter-stimulus intervals (ISI) [F(1,13)=9.99, p<.05]. Specifically, for these monkeys, the prepulse generally did not suppress but rather augmented the startle—a facilitative event noted by others after some prenatal challenge paradigms [18]. Post hoc analyses indicated that this change in %PPI was evident at the ISI of 45 msec [t(13)=2.46, p<.05], 120 msec [t(13)=3.45, p<.01], and 500 msec [t(13)=2.73, p<.05]. Similarly, at 105 dB, LPS animals had a potentiated startle [F(1,13) = 7.01, p<.05]. This effect occurred at 45 msec [t(13)=2.45, p<.05] and 120 msec [t(13)=2.94, p<.05]. The startle data for 105 and 115 dB probes were also analyzed altogether. The joint analysis indicated that the LPS-induced change in %PPI was significant for all 3 ISIs at 45 msec [t(13)=2.72, p<.05], 120 msec [t(13) = 3.02, p<.01], and 500 msec [t(13)=2.60, p<.05]) (Figure 4B). Three LPS monkeys manifested high reactivity to prepulse-pulse trials in the 45ms and 500ms ISI trials, producing wide variance.
Figure 4.
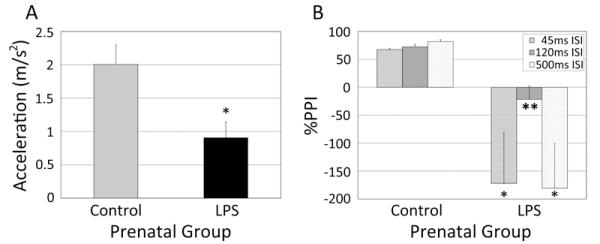
Behavioral responses during the Prepulse Inhibition (PPI) test among LPS treatment (n=6) and control (n=9) monkeys. (A) Mean startle reaction to a pulse sound probe during the PPI. (B) Change in startle response following an acoustic prepulse (Percentage PPI) calculated from the prepulse trials combined for the 105 and 115 dB pulses. Data are depicted as mean ± SEM. *=p<.05.;**=p<.01 (Student’s t-test).
4. Discussion
Our study has generated novel findings on the neural effects of a mild endotoxemia paradigm in pregnant monkeys using nanogram amounts of LPS. Specifically, this 2-day treatment expanded WM volume in many regions and selectively enlarged GM in the infants from LPS-treated pregnancies. This neurodevelopmental profile bears some similarity to the early brain overgrowth described in many individuals with autism [22,23]. In contrast, endotoxin and viral infection models in rodents typically result in reduced neural growth or no effect depending on the gestational timing and species [2]. Even though the brain effects in monkeys were opposite from the typically reported direction, the behavioral profile of these offspring from the LPS-treated pregnancies appeared comparable to several rodent models of prenatal infection and stress [36,37].
A similar experiment by our laboratory involving influenza virus infection during this period of gestation induced the more commonly observed reduction in the neural parenchyma [21]. Thus, it does not seem that differences in gestational timing and fetal brain maturation between monkeys and rats account for the differential outcome [38-41], but rather the degree of maternal inflammation induced by the various paradigms. Most mouse and rat models use concentrations of endotoxin in the microgram to milligram range, which can cause widespread physiological activation, including a marked retardation of fetal growth and some fetal death [42]. By contrast, the IL-6 levels post-LPS were only modestly upregulated in gravid monkeys over 2 days. Nevertheless, the elevated IL-6 likely crossed into the amniotic fluid and fetal compartment and was sufficient to induce the observed neurodevelopmental changes [12,43]. Indeed, IL-6 knockout mice are less likely to show neurobehavioral effects after prenatal inflammatory challenges [12]. It is not clear if the LPS-induced brain differences observed in our monkeys when they were approximately one year of age reflect an early overgrowth or an accelerated maturation that might persist into adulthood as seen in some rodent and monkey models [9,22,44,45]. The overall brain size and WM volumes of the LPS-exposed monkeys were roughly 6 months ahead of comparably aged infants from normal pregnancies [33].
The most striking finding in the offspring from the prenatal LPS condition was the significant 8.8% increase in global WM volume and a trend toward whole brain enlargement. As noted above, these results differed from the commonly reported decrements in brain size and neural development in rodents and sheep following E. coli, and other teratogenic and inflammatory stimulation [19,46-48]. Regional WM was increased throughout the hemispheres, especially in the more rostral and temporal areas. The effects on absolute GM and WM volume were not evident in the occipital lobe, however, which matures early in the fetal monkey and thus may have been spared [49]. This enlargement may be due to interference with the dendritic and synaptic pruning that normally occurs during gestation [50,51]. Alternatively, prenatal LPS may have accelerated the rate of myelination postnatally. Pro-oligodendrocyte precursors can be stimulated by certain mitogens and inflammatory stimuli [52,53] and could have resulted in enhanced myelin development.
Circumscribed increases in GM volume and, to a lesser extent, in cortical thickness were also seen in the parietal and frontal lobes, to approximately 4% more than controls. Alcohol or stress exposures typically reduce volume in these regions [54,55]. Our previous study on influenza virus infections [21] also found a decreased volume of approximately 6% and 10% for frontal and parietal lobes, respectively. Increased subcortical volumes were also seen in hippocampus and putamen in offspring from the prenatal LPS condition. It has been shown that LPS exposure to mice on gestational day 17 can increase cell density within the CA fields [56], and enlargement of the hippocampus is noted in some children with autism [57,58]--although others have noted no changes [59] or decreased GM relative to total brain volume [60]. In contrast to the general enlargement, the decreased cortical thickness in the medial temporal lobe of the LPS-exposed monkeys has been observed in human paradigms [55]. Damage to medial temporal lobe including entorhinal and perirhinal cortices can disrupt sensorimotor gating [61] and also sensitize rhesus monkeys to aversive stimuli [62]. LPS-induced disruption in the neurocircuitry or GM of this area could underlie the changes in behavioral reactivity observed in the LPS-exposed offspring under arousing conditions.
Changes in the monkeys’ temperament were evident throughout development, which corresponded to our periodic finding of physiological differences in IL-6 levels. LPS infants showed heightened responsiveness (e.g., more vocalizations) during the IBAS testing at 2 weeks of age, whereas they later became behaviorally reticent (e.g., fewer vocalizations) during the HIP at 8-9 months. Following weaning from the mother, and also immediately after the HIP test, there was a greater stress-induced inhibition of PHA-stimulated IL-6 production in vitro. They also startled less to PPI test pulses and failed to manifest the typical adaptation after prepulse sounds. These behavioral effects are similar to deficits in rodents prenatally treated with proinflammatory cytokines or endotoxin [12,18]. These effects are also reminiscent of the poorer behavioral modulation seen in inhibited children at risk for affective psychopathology [63], although this animal model did not replicate several of the key features of autism, such as social and communication deficits. The impact of our prenatal treatment on HPA activity was also less than described for most rodent endotoxemia models [64,65], but the LPS-exposed animals did take longer to adapt to transfer into a novel cage and had a differential response to negative glucocorticoid feedback after overnight Dexamethasone treatment.
5. Conclusion
In summary, a 2-day endotoxin provocation during pregnancy had a striking impact on many brain regions, increasing GM and WM volumes, and altering cortical thickness. Notwithstanding these marked changes in the brain phenotype, the LPS- exposed monkeys were healthy and appeared behaviorally and physiologically normal until examined under arousing and challenging conditions. This new primate model may afford an opportunity to examine processes that mediate the neural overgrowth seen in some neurodevelopment disorders such as autism, where problems with attention and reactivity are sometimes associated with regional increases in either cortical GM or WM [66-68], as well as in subcortical structures including hippocampus and putamen [22,58,66].
Supplementary Material
Supplement Figure 1. IL-6 levels derived from PHA-stimulated cell cultures of monkeys from control (n=9) and LPS-treated pregnancies (n=6). Supernatants were collected and analyzed at 2, 4, and 7 mo. of age. Because in vitro stimulation of the cell cultures with LPS maximally elevated IL-6 release for all animals, statistical analyses of the IL-6 in supernatants focused on the PHA-stimulated blood cultures. A significant interaction between Prenatal Condition and Age indicated that IL-6 release was initially higher in the LPS animals at 2 and 4 months of age, but then declined sharply below the cellular responses of the controls at 7 months of age [F(1,13)=9.82, p<.05]. This time point was one month after separation from their mothers. Post hoc testing confirmed this larger decrease in the cellular release of IL-6 for monkeys from the LPS pregnancy condition after re-housing into the social group with peers [t(13)=3.13, p<. 01]. By contrast, when IL-6 levels in vivo were measured unchallenged later in life, systemic levels did not significantly differ at one year of age (LPS—.34±.13 pg/mL, Control—2.25±1.33 pg/mL). Both groups of monkeys also reacted similarly to i.v. administration of LPS at 1.5 years of age with comparable increases in circulating IL-6 and neutrophil counts. Thus, the prenatal treatment did not appear to either induce toleration or sensitization of the monkeys to endotoxin as has been found in some rodent studies. Data are mean ± SEM. *= independent samples t-test, p<.05. @=repeated measures ANOVA across time, p<.05.
Supplement Figure 2. Plasma cortisol levels for monkeys from the control (n=9) and LPS pregnancy conditions (n=6). Cortisol values were generated by radioimmunoassay (Gammacoat, Stillwater, MN). (A) Change from baseline cortisol at 2 days after transfer to a novel cage. (B) Cortisol levels at 0800 and 1500 on the day after an overnight dexamethasone treatment (.25 mg/kg). Both groups of animals responded to the six experimental conditions for assessing cortisol secretion across time [F(1,13)=5.54, p<.05]. Basal cortisol at one year of age was similar between groups, as well as at 1 h after transfer to a novel cage. However, when examined 2 days later to monitor acclimation, monkeys from the LPS condition still had higher cortisol levels above baseline values [t(1,13)=2.30, p<.05]. In addition, following overnight dexamethasone treatment, monkeys from the LPS pregnancies had lower morning cortisol levels than controls, and then appeared to ‘break through’ the dexamethasone suppression to reach significantly higher levels at 1500 [F(1,13)=4.83, p<.05]. Data are mean ± SEM. *= independent samples t-test, p<.05. @ = repeated measures ANOVA across assessment times.
Acknowledgements
This research was supported by grants to CLC from the NIAID (AI067518), to JG from the NIMH (Conte Center, MH064065), and to MS from NICHD (UNC Neurodevelopmental Disorders Research Center (HD03110). Cortisol assays were conducted through the Endocrine Services unit of a NCRR-supported CTSA (IUL1RR025011). The assistance of A. Slukvina, H. Crispen, and other HPL staff with sample collection and assays is greatly appreciated. R. Bernstein provided valuable assistance in collecting peer behavior data.
Footnotes
Disclosure/Conflict of Interest: The authors have no conflicts of interest regarding this manuscript.
Supplementary information is available at the Behavioral Brain Research website.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Machon RA, Mednick SA, Huttunen MO. Adult major affective disorder after prenatal exposure to an influenza epidemic. Archives of general psychiatry. 1997;54:322–8. doi: 10.1001/archpsyc.1997.01830160040006. [DOI] [PubMed] [Google Scholar]
- [2].Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neuroscience and biobehavioral reviews. 2009;33:1061–79. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- [3].Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. The American journal of psychiatry. 2010;167:261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grillon C, Warner V, Hille J, Merikangas KR, Bruder GE, Tenke CE, et al. Families at high and low risk for depression: a three-generation startle study. Biological psychiatry. 2005;57:953–60. doi: 10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]
- [5].Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biological psychiatry. 2007;61:482–6. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- [6].Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, et al. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Molecular psychiatry. 1999;4:145–54. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- [7].Craddock RM, Lockstone HE, Rider DA, Wayland MT, Harris LJ, McKenna PJ, et al. Altered T-cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PloS one. 2007;2:e692. doi: 10.1371/journal.pone.0000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coe CL, Lubach GR. Fetal programming: Prenatal origins of health and illness. Current Directions in Psychological Science. 2008;17:36–41. [Google Scholar]
- [9].Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–89. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- [10].Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hava G, Vered L, Yael M, Mordechai H, Mahoud H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Developmental psychobiology. 2006;48:162–8. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- [12].Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophrenia research. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- [14].Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Molecular psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- [15].Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatric research. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- [16].Lim KO, Rosenbloom MJ, Faustman WO, Sullivan EV, Pfefferbaum A. Cortical gray matter deficit in patients with bipolar disorder. Schizophrenia research. 1999;40:219–27. doi: 10.1016/s0920-9964(99)00063-8. [DOI] [PubMed] [Google Scholar]
- [17].Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. NeuroImage. 2004;23:364–9. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- [18].Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–15. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- [19].Pang Y, Rodts-Palenik S, Cai Z, Bennett WA, Rhodes PG. Suppression of glial activation is involved in the protection of IL-10 on maternal E. coli induced neonatal white matter injury. Brain research. 2005;157:141–9. doi: 10.1016/j.devbrainres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- [20].Piontkewitz Y, Arad M, Weiner I. Risperidone Administered During Asymptomatic Period of Adolescence Prevents the Emergence of Brain Structural Pathology and Behavioral Abnormalities in an Animal Model of Schizophrenia. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, et al. Maternal Influenza Infection During Pregnancy Impacts Postnatal Brain Development in the Rhesus Monkey. Biological psychiatry. 2010 doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- [23].Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [24].Saito M, Nameda S, Miura NN, Adachi Y, Ohno N. Effect of SPG/indomethacin treatment on sepsis, interleukin-6 production, and expression of hepatic cytochrome P450 isoforms in differing strains of mice. J Immunotoxicol. 2009;6:42–8. doi: 10.1080/15476910802604663. [DOI] [PubMed] [Google Scholar]
- [25].Willette AA, Lubach GR, Coe CL. Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain, behavior, and immunity. 2007;21:807–15. doi: 10.1016/j.bbi.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brazelton T. Assessment of the infant at risk. Clin Obstet Gynecol. 1973;16:361–75. doi: 10.1097/00003081-197303000-00020. [DOI] [PubMed] [Google Scholar]
- [27].Schneider M, Moore C, Suomi S, Champoux M. Laboratory assessment of temperament and environmental enrichment in rhesus monkey infants (Macaca mulatta) Am J Primatology. 1991;25:137–55. doi: 10.1002/ajp.1350250302. [DOI] [PubMed] [Google Scholar]
- [28].Schneider ML, Suomi SJ. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): Developmental changes, behavioral stability, and early experience. Infant Behavior and Development. 1992;15:155–177. [Google Scholar]
- [29].Coe CL, Lubach GR, Crispen HR, Shirtcliff EA, Schneider ML. Challenges to maternal wellbeing during pregnancy impact temperament, attention, and neuromotor responses in the infant rhesus monkey. Dev Psychobiol. 2010;52(7):625–37. doi: 10.1002/dev.20489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Annals of the New York Academy of Sciences. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- [31].Winslow JT, Parr LA, Davis M. Acoustic startle, prepulse inhibition, and fear-potentiated startle measured in rhesus monkeys. Biological psychiatry. 2002;51:859–66. doi: 10.1016/s0006-3223(02)01345-8. [DOI] [PubMed] [Google Scholar]
- [32].West M, Heagy W. Endotoxin tolerance: A review. Crit Care Med. 2002;30:S64–S73. [PubMed] [Google Scholar]
- [33].Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, et al. Maturational Trajectories of Cortical Brain Development through the Pubertal Transition: Unique Species and Sex Differences in the Monkey Revealed through Structural Magnetic Resonance Imaging. Cereb Cortex. 2010;20:1053–63. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Styner M, Knickmeyer RC, Joshi S, Coe CL, Short SJ, Gilmore JH. Automatic brain segmentation in rhesus monkeys. Spie PP 65122. 2007:L65121–L65128. [Google Scholar]
- [35].Kabani N, Le Goualher G, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. NeuroImage. 2001;13:375–80. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- [36].Hodyl NA, Krivanek KM, Clifton VL, Hodgson DM. Innate immune dysfunction in the neonatal rat following prenatal endotoxin exposure. Journal of neuroimmunology. 2008;204:126–30. doi: 10.1016/j.jneuroim.2008.06.041. [DOI] [PubMed] [Google Scholar]
- [37].Bernardi MM, Kirsten TB, Matsuoka SM, Teodorov E, Habr SF, Penteado SH, et al. Prenatal lipopolysaccharide exposure affects maternal behavior and male offspring sexual behavior in adulthood. Neuroimmunomodulation. 2010;17:47–55. doi: 10.1159/000243085. [DOI] [PubMed] [Google Scholar]
- [38].Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behavioural brain research. 2007;181:270–7. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- [39].Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–62. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–84. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–7. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wei YL, Li XH, Zhou JZ. Prenatal exposure to lipopolysaccharide results in increases in blood pressure and body weight in rats. Acta pharmacologica Sinica. 2007;28:651–6. doi: 10.1111/j.1745-7254.2007.00593.x. [DOI] [PubMed] [Google Scholar]
- [43].Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain, behavior, and immunity. 2010;24:881–97. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- [44].Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biological psychiatry. 2003;54:1025–34. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- [45].Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. The European journal of neuroscience. 2006;24:1477–87. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- [46].Campbell IL, Powell HC. Role of Cytokines in Demyelinating Disease Studied in Transgenic Mice. Methods. 1996;10:462–77. doi: 10.1006/meth.1996.0124. [DOI] [PubMed] [Google Scholar]
- [47].Duncan JR, Cock ML, Scheerlinck JP, Westcott KT, McLean C, Harding R, et al. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatric research. 2002;52:941–9. doi: 10.1203/00006450-200212000-00021. [DOI] [PubMed] [Google Scholar]
- [48].Graham EM, Holcroft CJ, Rai KK, Donohue PK, Allen MC. Neonatal cerebral white matter injury in preterm infants is associated with culture positive infections and only rarely with metabolic acidosis. American journal of obstetrics and gynecology. 2004;191:1305–10. doi: 10.1016/j.ajog.2004.06.058. [DOI] [PubMed] [Google Scholar]
- [49].Kostovic I, Rakic P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci. 1984;4:25–42. doi: 10.1523/JNEUROSCI.04-01-00025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].LaMantia AS, Rakic P. Axon overproduction and elimination in the anterior commissure of the developing rhesus monkey. The Journal of comparative neurology. 1994;340:328–36. doi: 10.1002/cne.903400304. [DOI] [PubMed] [Google Scholar]
- [51].Rakic P. Less is more: progenitor death and cortical size. Nature neuroscience. 2005;8:981–2. doi: 10.1038/nn0805-981. [DOI] [PubMed] [Google Scholar]
- [52].Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends in cell biology. 1993;3:191–7. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- [53].Setzu A, Lathia JD, Zhao C, Wells K, Rao MS, Ffrench-Constant C, et al. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54:297–303. doi: 10.1002/glia.20371. [DOI] [PubMed] [Google Scholar]
- [54].Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental medicine and child neurology. 2001;43:148–54. [PubMed] [Google Scholar]
- [55].Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6-9-year-old children. Psychoneuroendocrinology. 2010;35:141–53. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–17. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- [57].Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–92. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- [58].Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mitchell SR, Reiss AL, Tatusko DH, Ikuta I, Kazmerski DB, Botti JA, et al. Neuroanatomic alterations and social and communication deficits in monozygotic twins discordant for autism disorder. The American journal of psychiatry. 2009;166:917–25. doi: 10.1176/appi.ajp.2009.08101538. [DOI] [PubMed] [Google Scholar]
- [60].Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–50. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- [61].Uehara T, Sumiyoshi T, Matsuoka T, Itoh H, Kurachi M. Effect of prefrontal cortex inactivation on behavioral and neurochemical abnormalities in rats with excitotoxic lesions of the entorhinal cortex. Synapse. 2007;61:391–400. doi: 10.1002/syn.20383. [DOI] [PubMed] [Google Scholar]
- [62].Meunier M, Cirilli L, Bachevalier J. Responses to affective stimuli in monkeys with entorhinal or perirhinal cortex lesions. J Neurosci. 2006;26:7718–7722. doi: 10.1523/JNEUROSCI.1949-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual review of psychology. 2005;56:235–62. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- [64].Reul JM, Stec I, Wiegers GJ, Labeur MS, Linthorst AC, Arzt E, et al. Prenatal immune challenge alters the hypothalamic-pituitary-adrenocortical axis in adult rats. The Journal of clinical investigation. 1994;93:2600–7. doi: 10.1172/JCI117272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hodgson DM, Knott B, Walker FR. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatric research. 2001;50:750–5. doi: 10.1203/00006450-200112000-00020. [DOI] [PubMed] [Google Scholar]
- [66].Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–92. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- [67].Hendry J, DeVito T, Gelman N, Densmore M, Rajakumar N, Pavlosky W, et al. White matter abnormalities in autism detected through transverse relaxation time imaging. NeuroImage. 2006;29:1049–57. doi: 10.1016/j.neuroimage.2005.08.039. [DOI] [PubMed] [Google Scholar]
- [68].Ke X, Hong S, Tang T, Zou B, Li H, Hang Y, et al. Voxel-based morphometry study on brain structure in children with high-functioning autism. Neuroreport. 2008;19:921–5. doi: 10.1097/WNR.0b013e328300edf3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1. IL-6 levels derived from PHA-stimulated cell cultures of monkeys from control (n=9) and LPS-treated pregnancies (n=6). Supernatants were collected and analyzed at 2, 4, and 7 mo. of age. Because in vitro stimulation of the cell cultures with LPS maximally elevated IL-6 release for all animals, statistical analyses of the IL-6 in supernatants focused on the PHA-stimulated blood cultures. A significant interaction between Prenatal Condition and Age indicated that IL-6 release was initially higher in the LPS animals at 2 and 4 months of age, but then declined sharply below the cellular responses of the controls at 7 months of age [F(1,13)=9.82, p<.05]. This time point was one month after separation from their mothers. Post hoc testing confirmed this larger decrease in the cellular release of IL-6 for monkeys from the LPS pregnancy condition after re-housing into the social group with peers [t(13)=3.13, p<. 01]. By contrast, when IL-6 levels in vivo were measured unchallenged later in life, systemic levels did not significantly differ at one year of age (LPS—.34±.13 pg/mL, Control—2.25±1.33 pg/mL). Both groups of monkeys also reacted similarly to i.v. administration of LPS at 1.5 years of age with comparable increases in circulating IL-6 and neutrophil counts. Thus, the prenatal treatment did not appear to either induce toleration or sensitization of the monkeys to endotoxin as has been found in some rodent studies. Data are mean ± SEM. *= independent samples t-test, p<.05. @=repeated measures ANOVA across time, p<.05.
Supplement Figure 2. Plasma cortisol levels for monkeys from the control (n=9) and LPS pregnancy conditions (n=6). Cortisol values were generated by radioimmunoassay (Gammacoat, Stillwater, MN). (A) Change from baseline cortisol at 2 days after transfer to a novel cage. (B) Cortisol levels at 0800 and 1500 on the day after an overnight dexamethasone treatment (.25 mg/kg). Both groups of animals responded to the six experimental conditions for assessing cortisol secretion across time [F(1,13)=5.54, p<.05]. Basal cortisol at one year of age was similar between groups, as well as at 1 h after transfer to a novel cage. However, when examined 2 days later to monitor acclimation, monkeys from the LPS condition still had higher cortisol levels above baseline values [t(1,13)=2.30, p<.05]. In addition, following overnight dexamethasone treatment, monkeys from the LPS pregnancies had lower morning cortisol levels than controls, and then appeared to ‘break through’ the dexamethasone suppression to reach significantly higher levels at 1500 [F(1,13)=4.83, p<.05]. Data are mean ± SEM. *= independent samples t-test, p<.05. @ = repeated measures ANOVA across assessment times.


