Abstract
Background
The incremental value of regional left ventricular function (LVF) over coronary assessment to detect acute coronary syndrome (ACS) is uncertain.
Methods and Results
We analyzed 356 patients (mean age 53±12 years, 62% male) with acute chest pain and inconclusive initial ED evaluation. Patients underwent 64-slice contrast-enhanced cardiac CT prior to hospital admission. Caregivers and patients remained blinded to the results. Regional LVF and presence of coronary atherosclerotic plaque and significant stenosis (>50%) were separately assessed by two independent readers. Incremental value of regional LVF to predict ACS was determined in the entire cohort and in subgroups of patients with nonobstructive CAD, inconclusive assessment for stenosis (defined as inability to exclude significant stenosis due to calcium or motion), and significant stenosis. During their index hospitalization, 31 patients were ultimately diagnosed with ACS (8 myocardial infarction, 22 unstable angina), of which 74% (23 patients) had regional LV dysfunction. Adding regional LVF resulted in a 10% increase in sensitivity to detect ACS by cardiac CT (87%, 95%-confidence interval [CI]: 70–96%) and significantly improved the overall accuracy (c-statistic: 0.88 vs. 0.94 and 0.79 vs. 0.88, for extent of plaque and presence of stenosis; respectively; both p<0.03). The diagnostic accuracy of regional LVF for detection of ACS has 89% sensitivity and 86% specificity in patients with significant stenosis (n=33) and 60% sensitivity and 86% specificity in patients with inconclusive coronary CTA (n=33).
Conclusions
Regional LVF assessment at rest improves diagnostic accuracy for ACS in patients with acute chest pain, especially in those with coronary artery disease and thus may be helpful to guide further management in patients at intermediate risk for ACS.
Clinical Trial Registration
URL: http://clinicaltrials.gov/ct2/show/NCT00990262. Unique Identifier: NCT00990262
Keywords: computed tomography, left ventricular function, acute coronary syndrome, emergency department
INTRODUCTION
Patients with acute chest pain represent a global health and economical challenge for the healthcare systems worldwide. The more than eight million annual emergency department (ED) visits in United States alone impart a significant economic burden in excess of $8 billion annually, particularly as 80% of these patients are admitted to the hospital 1–3. One major problem is the inability to accurately triage patients early on because neither a single set of biochemical markers for myocardial necrosis (Troponin I, Troponin T, Creatine Kinase, MB-type [CK-MB]) 4–5, nor initial 12-lead electrocardiography (ECG) alone or in combination identifies a group of subjects that can be safely discharged home 6–8. While subsequent stress testing such as exercise treadmill testing, single photon emission computed tomography or stress echocardiography provides valuable information for risk stratification they may be limited in their utilization for early triage and their diagnostic accuracy for the detection of significant coronary artery disease (CAD) 9–13. Acute imaging techniques such as rest echocardiography and rest myocardial perfusion imaging have been shown to improve the triage process although their availability is limited in most facilities 14–15.
Several studies suggest that cardiac computed tomography angiography (CTA) with its unique ability to non-invasively visualize coronary atherosclerotic plaque and stenosis may improve management of patients with acute chest pain 16–18. These studies have established the high negative predictive value of the absence of CAD for ACS. However, they also noted the inability to exclude significant stenosis in the presence of severe calcification or motion artifacts in about 10% of patients and the difficulty to determine whether a detected significant stenotic lesion is actually the underlying reason for the current chest pain presentation. Resting regional left ventricular (LV) function is an accurate predictor of ACS in the setting of acute chest pain 19, and cardiac CT assessment of resting regional left ventricular (LV) function is highly concordant with cardiac magnetic resonance imaging and echocardiography 20–25.
Thus, the goal of our study was to determine if assessment of LV function may provide incremental diagnostic value to the assessment of coronary morphology for the prediction of ACS in patients with acute chest pain and therefore potentially aid in clinical decision-making specifically in those patients who have CAD.
METHODS
Patient Population
This is a subanalysis of the ROMICAT trial 17, which included patients who had a chief complaint of acute chest pain lasting >5 minutes during the previous 24 hours, normal initial troponin and an initial ECG without evidence of myocardial ischemia. In all patients, experienced ED physicians had sufficient clinical suspicion for an ischemic origin of chest pain and admitted these patients to the hospital to rule out ACS. Most notably, patients with prior history of coronary stents or bypass surgery were excluded due to limitation of stenosis assessment caused by either metal, calcium, and/or beam hardening artifacts from either the stents or severely diseased native vessels. Detailed inclusion and exclusion criteria are provided in Table 1.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
| Age >18 years |
| Chest pain for >5 minutes within the previous 24 hours |
| Admitted for rule out myocardial infarction through standard care protocols |
| Sinus rhythm |
| Ability to perform a breath hold of 10–15 seconds |
| Negative pregnancy test or post menopause in female subjects |
| Exclusion Criteria |
| Elevated Troponin I or CK-MB levels in the initial blood sample obtained in the ED |
| New diagnostic ECG changes (ST-segment elevation or depression >1 mm or T-wave inversion >4 mm in >2 anatomically contiguous leads |
| History of CAD defined as stent implantation or coronary artery bypass graft |
| Hemodynamic or clinical instability (systolic blood pressure <80 mmHg, clinically significant atrial or ventricular arrhythmias, persistent chest pain despite therapy) |
| Perceived interference with standard clinical care of patients |
| Known allergy to iodinated contrast agent |
| Serum creatinine >1.3 mg/dL |
| Metformin treatment, hyperthyroidism |
| Inability to provide informed consent |
We screened patients who presented with a chief complaint of chest pain to the ED on weekdays from 7am to 7pm (May 2005 to May 2007). All eligible patients who agreed to participate underwent contrast-enhanced cardiac CT prior to admission to the hospital floor. Patients had to be chest pain free at the time of CT. All physicians, including those in the ED, who were involved in the standard clinical care of the patients, remained blinded to the result of cardiac CT. The institutional review board approved the study protocol and all patients provided written informed consent. Study protocol conformed to the Declaration of Helsinki.
Cardiac CT Imaging
CT imaging was performed using a 64-slice CT scanner (Sensation 64; Siemens Medical Solutions, Forchheim, Germany). In preparation for the scan, patients with a heart rate >60 bpm received an intravenous beta-blocker (metoprolol, 5–20 mg) unless their systolic blood pressure was <100 mmHg, or other contraindications were present. In addition, patients received 0.6 mg of sublingual nitroglycerin. All image acquisitions were performed during a single breath hold in inspiration.
Per standard protocol, a test bolus of 20 mL contrast agent was administered with a flow rate of 5 mL/s to determine the optimal timing of contrast injection. cardiac CT data sets were acquired with 64 × 0.6 mm slice collimation, a gantry rotation time of 330 ms, tube voltage of 120 kV, and an effective tube current of 850 mAs using retrospective gating and ECG-correlated tube current modulation when appropriate 26. Contrast agent (80–100 ml, Iodixanol 320 mg/cm3, Visipaque, General Electrics Healthcare, Princeton, NJ, USA) was injected intravenously at a rate of 5 mL/s to ensure homogeneous enhancement of the entire coronary artery tree.
Assessment of Regional LV Function
CT Image Reconstruction
Ten datasets of axial images of the entire heart with a temporal resolution of 83–165 ms (automatically chosen depending on the heart rate) and a slice thickness of 1.5 mm were reconstructed at increments of 10% of the R-R interval increment, starting at 5% throughout the cardiac cycle (5%–95%) using a retrospective electrocardiogram-gated half-scan algorithm and a special cardiac reconstruction kernel (B25f). The reconstructed data set permitted the visualization of the entire cardiac cycle in a cine mode and all cardiac standard orientations used in echocardiography (4 chamber, 2 chamber and LV short axis) were reconstructed.
Image Analysis
All assessments were performed as consensus reading by two independent and experienced observers (S.K.S., M.D.S) blinded to the clinical course of the patient and the CTA findings on coronary artery stenosis and plaque. Disagreement was resolved by consensus. Regional LV dysfunction including wall motion and wall thickening of the myocardium were assessed qualitatively based on the AHA/ACC 17-segment model 27. Regional LV dysfunction had to be present in at least two contiguous myocardial segments or in one segment visualized in two different views to be considered a true positive finding. Each LV segment was graded as normal, hypokinetic (impaired contraction), akinetic (absent contraction), dyskinetic (paradoxical outward wall motion during systole without aneurysmal formation in diastole) or aneurysmal 28. However for the purpose of subsequent analysis each segment was classified as having either normal or abnormal (hypokinetic, akinetic, dyskinetic or aneurismal) function. “In Space” software was used for post processing (Siemens Medical Solutions).
Assessment of Presence and Extent of CAD by Cardiac CT
Assessment of the CT data sets for the presence of significant coronary stenosis (>50% luminal narrowing) and the presence of coronary atherosclerotic plaque was performed as a consensus reading by two experienced investigators blinded to the subject’s clinical presentation and history using a modified 17-segment model of the coronary artery tree 29. A CT scan was labeled inconclusive, if the presence of significant stenosis could not be excluded. If a consensus could not be reached, a third expert reader made the final diagnosis. This method has been demonstrated to be highly reproducible; details of this analysis have been previously published 30.
Clinical Covariates
We prospectively collected data on demographics, risk factor profile, and clinical course in all patients. Medical records were reviewed to obtain results of all diagnostic tests performed during index hospitalization and a period of one week post discharge. Presence of risk factors (i.e. hypertension, hypercholesterolemia, and diabetes mellitus) and TIMI risk score were established from actual measurements obtained during the hospitalization or related medication use.
Clinical Endpoints
ACS during Index Hospitalization
To establish a diagnosis of ACS, two experienced board certified physicians with more than 10 years experience (one ED physician [J.T.N] and one cardiologist [S.K.S.]) reviewed patient data forms containing prospectively collected information on the history and nature of chest pain, risk factors and medical history, as well as medical records pertaining to the hospital admission. The reviewers were blinded to the findings of cardiac CT. Disagreement was resolved by consensus, which included an additional senior cardiologist (C.C.) 31. ACS was defined as either an acute myocardial infarction (MI; i.e. patients developed a positive troponin (>0.09 ng/dL during serial testing) or unstable angina pectoris (UAP) according to the AHA/ACC/ESC guidelines 32–34. UAP was defined as clinical symptoms suggestive of ACS (unstable pattern of chest pain - at rest, new onset, or crescendo angina) optimally with objective evidence of myocardial ischemia 35.
Statistical Analysis
Univariate analysis was performed to determine the crude association between regional LV function and ACS. The significance of differences of the prevalence of ACS between subjects with normal vs. abnormal LV function was tested using Fishers Exact Test.
For the entire cohort, we calculated sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) with 95%-confidence intervals (95%-CI) of regional LV dysfunction for ACS as well as the incremental diagnostic accuracy of regional LV function for the inability to rule out significant stenosis (significant stenosis or non diagnostic scan).
We also calculated sensitivity, specificity, NPV, and PPV with 95%-CI of regional LV dysfunction for ACS in four groups of patients: 1) definitive coronary assessment, 2) inconclusive coronary assessment (indeterminate lesions or inability to exclude significant stenosis due to motion artifact or calcification), 3) presence of plaque but no stenosis, and 4) presence of significant stenosis.
Area under the receiver operating curve (AUC) was calculated using c-statistic of logistic regression. Baseline models included the CT finding for the extent of plaque, defined as the number of coronary artery segments with plaque based on the 17-segment model, and presence of stenosis separately and subsequent models included these individual CT findings with the addition of regional LV. To determine the incremental discriminatory power of regional LV function to coronary assessment by CT, we compared the c-statistic of nested models using the likelihood ratio test (no effect modification - interaction terms for LV dysfunction with extent of plaque and presence of stenosis did not reach statistical significance (p=0.66 and p=0.29; respectively). All analyses were performed using SAS (Version 9.2, SAS Institute Inc., Cary, NC, USA). A two-sided p-value <0.05 was considered to indicate statistical significance.
RESULTS
Patient Population
A total of 1,869 patients with a primary complaint of chest pain lasting >5 minutes were screened. Exclusion criteria were present in 1270 patients (impaired renal function (n=454), history of CAD defined as previous stent placement or coronary bypass (n=231), ECG diagnostic for myocardial ischemia or positive initial biomarkers (n=209), arrhythmia (n=97), inability to pause metformin (n=68), who enrolled in different research study or were previously included in this study (n=63), history of allergy to iodine (n=58), inability to administer beta blocker due to asthma (n=37), clinically unstable (n=31), or lack of pregnancy testing (n=20)). In addition, we excluded 231 patients who were ineligible because of interference with standard clinical care (n=100), who refused participation (n=124), or did not complete the CT exam (n=7). We further excluded 12 patients who had an incomplete LV function study. The baseline characteristics of the 356 study patients are shown in Table 2. On average, patients were 53 years old, predominantly male (62%), and had two cardiovascular risk factors (median); the majority of patients had a TIMI risk score between 0–2 (n=336, 95%). There were 16 patients (4.5%) with a self-report history of MI but no documented CAD in the medical records and no stent or coronary artery bypass graft on CTA. Overall, 8.7% of patients (n=31) developed ACS (MI: n=8, UAP: n=23) during the index admission, while in the remaining 325 patients (91.3%) ACS was ruled out. There were no cardiac events during a follow up period of six months.
Table 2.
Baseline characteristics
| Age (years, mean ± SD) | 52.7 ± 11.8 |
| Male Gender (n, %) | 221 (62%) |
| Race (n, %) | |
| African American | 34 (10%) |
| Caucasian | 304 (85%) |
| Asians | 4 (1%) |
| Others | 14 (4%) |
| Ethnicity (n, %) | |
| Hispanic | 41 (12%) |
| Non-Hispanic | 315 (88%) |
| BMI (kg/m2, mean ± SD) | 29.1 ± 6.0 |
| Diabetes (n, %) | 39 (11%) |
| Hypertension (n, %) | 141 (40%) |
| Hyperlipidemia or statin use (n, %) | 131 (37%) |
| Current or former smoking (n, %) | 178 (50%) |
| Number of risk factors (median; IQR) | 2; 1–3 |
| TIMI risk score (0–2/3–4/5–7) in % | 95% / 5% / 0% |
| ACS during index hospitalization (n, %) | 31 (9%) |
| Unstable angina pectoris (n, %) | 23 (74%) |
| Myocardial infarction (n, %) | 8 (36%) |
Baseline characteristics of 356 patients who presented to the emergency department (ED) with acute chest pain but negative initial biomarkers and non-ischemic ECG.
as determined the ED caregiver at the time of ED triage,
ACS: acute coronary syndrome, BMI: body mass index, IQR: Interquartile Range,
The average time to perform a cardiac CTA including patient preparation was 16±7 minutes. The mean actual scan time to obtain the CT data set was 14±2 seconds. The mean dose length product was 984±258 milliGray (mGy) × centimeter (cm), range 574–1380 mGy×cm, which when multiplied by the chest-weighting factor of 0.014, corresponded to a mean estimated radiation dose of 13.8±3.6 millisievert (mSv), range of 8.0–19.3 mSv. Average time for the interpretation of CT images (coronary anatomy and LV function) was 17±9 minutes (range: 6–30) with 73% of the exams being classified as having excellent image quality for both coronary and LV function assessment. Disagreement as to whether regional LV function was normal was solved by consensus in six patients (1.6%).
Diagnostic Accuracy of LV Function for ACS in the overall Cohort
Regional LV function was impaired in 46 patients (12.9%) with overall 241 abnormal segments. Of these, 205 were hypokinetic, 32 akinetic and 4 dyskinetic. There were no aneurysmal segments. The most common segments with abnormal function were mid-anterior and mid antero-lateral walls (30/46). Overall, 8/310 patients with normal regional LV function had ACS (NPV: 97%, 95%-CI: 95–98%), 23/325 patients without ACS had regional LV dysfunction (specificity: 93%, 95%-CI: 90–96%), 23/31 patients with ACS had regional LV dysfunction (sensitivity: 74%, 95%-CI: 55–88%), and 23/46 patients with regional LV dysfunction had ACS (PPV: 50%, 95%-CI: 35–65%). Of the 46 patients with regional LV dysfunction, 10 patients (21.7%) had regional LV dysfunction and co-existing self-report history of MI. Of this subgroup of patients, 4 patients (17.4%) had ACS during index hospitalization while 6 patients (26.1%) did not have ACS (p=0.72).
Symptom Onset and Assessment of LV function
Time from onset of most recent chest pain symptoms and the assessment of LV function was 10.3 hours (median, interquartile range 6.0–21.3). There was no association between the presence LV dysfunction and characteristics of chest pain such as time from onset of chest pain to cardiac CTA (p=0.13), duration of chest pain (p=0.50), or presence of chest pain on ED arrival (p=0.55). In patients with regional LV dysfunction, the median time from onset of chest pain symptoms to CT evaluation of LV function was not different between those with and without a self-reported history of MI (7.4 vs. 8.7 hours, p=0.91).
Stratification by presence of Plaque and Stenosis
By coronary CTA, 49.4% (n=176) were free of CAD, 32% (n=114) had plaque but no stenosis, 9.1% (n=33) had a significant stenosis, and 9.1% (n=33) had plaque and a significant stenosis could not be ruled out. Among 180 patients with any plaque, 47 (26%) had calcified, 116 (64%) mixed and 17 (10%) exclusively non-calcified plaque. None of the patients without CAD developed ACS during the index admission or follow up. Only 1 patient with exclusively non-calcified plaque developed ACS.
Incremental Diagnostic Value of Regional LV Function to Coronary Assessment
Overall cohort (n=356)
The sensitivity of CT to detect ACS improved by 10% from 77% based on significant stenosis to 87% based on combined assessment (Table 3).
Table 3.
Diagnostic Accuracy of CTA for the Detection of ACS using Coronary Assessment only and Coronary with Regional LV Function.
| CTA Evaluation | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Coronary assessment only | 24/31 77% (59–90) |
283/325 87% (83–91) |
24/66 36% (25–49) |
283/290 98% (95–99) |
| Coronary + Regional LV Function | 27/31 87% (70–96) |
266/325 82% (77–86) |
27/86 31% (22–42) |
266/270 99% (96–100) |
An abnormal CTA is defined as the presence of significant >50% stenosis or non-diagnostic CT scan for coronary assessment and/or presence of regional LV dysfunction, 95% confidence intervals are provided in parenthesis.
PPV: positive predictive value; NPV: negative predictive value.
Patients without CAD (n=176)
There was no incremental value of LV function assessment as none of these patients had ACS.
Patients with definitive coronary stenosis assessment by CT (n=323, Table 4)
Table 4.
Accuracy of Regional LV Functional Analysis for Detecting ACS in Subgroups
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| CT with definitive coronary stenosis assessment (n=323) | 20/26 77% (56–91) |
278/297 94% (90–96) |
20/39 51% (35–68) |
278/284 98% (96–99) |
| CT with inconclusive coronary stenosis assessment (n=33) | 3/5 60% (15 – 95) |
24/28 86% (67–96) |
3/7 43% (10–82) |
24/26 92% (75–99) |
| CT with non-obstructive plaque (n=114) | 3/7 43% (10–82) |
99/107 93% (86–97) |
3/11 27% (6–61) |
99/103 96% (90–99) |
| CT with significant stenosis (n=33) | 17/19 89% (67–99) |
12/14 86% (57–98) |
17/19 89% (67–99) |
12/14 86% (57–98) |
LV: left ventricular; 95%-confidence intervals are provided in parenthesis. PPV: positive predictive value; NPV: negative predictive value.
The specificity of combined stenosis and LV function assessment by CT for ACS was excellent with 94%, the PPV was 51%, while a high NPV was maintained (98%).
Patients with inconclusive coronary stenosis assessment by CT (n=33, Table 4)
In this group five patients had ACS and 7 had regional LV dysfunction (21%). In these patients, LV function assessment was helpful to rule out ACS as the absence of LV dysfunction correctly ruled out ACS in 24/26 patients (NPV: 92%). PPV and sensitivity were limited as only 3/7 patients with LV dysfunction had ACS and LV dysfunction was present in only 3/5 patients with ACS. In addition, LV function was normal in 24/28 subjects without ACS (specificity: 86%).
Patients with nonobstructive CAD (n=114; Table 4)
In this group seven patients had ACS and 11 had regional LV dysfunction (10%). Among the seven patients with ACS, three had regional LV dysfunction (sensitivity: 43%). A clinical example of a patient with nonobstructive CAD, ACS, and regional LV dysfunction is shown in Figure 1. LV dysfunction was present in all patients who had troponin elevation in subsequent measurements (2nd or 3rd serial measurements 6 and 12 hours after presentation) and were diagnosed with non-ST segment MI (n=3), while LV function was normal in four patients with UAP (only one underwent invasive angiography to demonstrate a significant stenosis, which was missed by coronary CTA).
Figure 1. Case of Nonobstructive Coronary Artery Disease of Left Anterior Descending Artery (LAD) and Regional LV dysfunction detected on Cardiac CT with Subsequent Diagnosis of Acute Coronary Syndrome (ACS).

52-year old man with no cardiovascular risk factors presented to the emergency department with exertional chest pain and admitted for a rule out myocardial initial negative troponin, and nonischemic electrocardiogram. Cardiac CT on admission showed nonobstructive coronary atherosclerotic plaque (noncalcified) in the LAD but with mid-distal anterior wall hypokinesis (Movie 1). The following troponin drawn 6 hours later became positive. Invasive coronary angiography revealed proximal LAD with 30% stenosis which was partially improved with intracoronary nitroglycerin. The patient was discharge with a final diagnosis of ACS due to coronary vasospasm. Curved multiplanar reformation CT image of the LAD (A) and invasive coronary angiography (B) showing mild nonobstructive coronary atherosclerosis. Mid-ventricular short-axis and two-chamber views obtained during end-systole (C, D) and end-diastole (E, F) demonstrating the anterior wall hypokinesis.
Patients in whom significant stenosis was detected (n=33; Table 4)
In this group, 19 patients had ACS and 19 had regional LV dysfunction (58%). In these patients, LV function assessment was helpful to assess hemodynamic significance of stenosis as the presence of LV dysfunction correctly predicted ACS in 17/19 patients (PPV: 89%). A clinical case is demonstrated in Figure 2. This finding was substantiated as territory of LV dysfunction matched the location of stenosis in 15 of those patients (88%). In addition, absence of regional LVF was highly predictive for the absence of ACS (NPV: 86%).
Figure 2. Case of Left Anterior Descending Artery (LAD) Stenosis and Regional LV dysfunction detected on Cardiac CT with Subsequent Diagnosis of Acute Coronary Syndrome (ACS).

41-year old man without any cardiovascular risk factors presented to the emergency department with intermittent substernal chest pain, initial negative troponin, and normal electrocardiogram. Cardiac CT on admission demonstrated a severe LAD stenosis and corresponding regional LV dysfunction (anterior wall hypokinesia; Movie 2). Patient was subsequently diagnosed as ACS after his third troponin test became elevated 12 hours after ED presentation. Invasive coronary angiography confirmed a severe lesion of the left anterior descending coronary artery which was treated percutaneously. Curved multiplanar reformation (A) of the LAD demonstrating significant coronary stenosis in the mid portion of the vessel (white arrow). Volume rendered 3-dimensional CT image (B) showing an anterior view of the heart depicting the significant coronary stenosis in the mid portion of the LAD (black arrow). Corresponding invasive angiography (C) of the left coronary artery demonstrating the high-grade stenosis (black arrow).
Incremental Value of LV Function over Coronary CTA for Risk Stratification
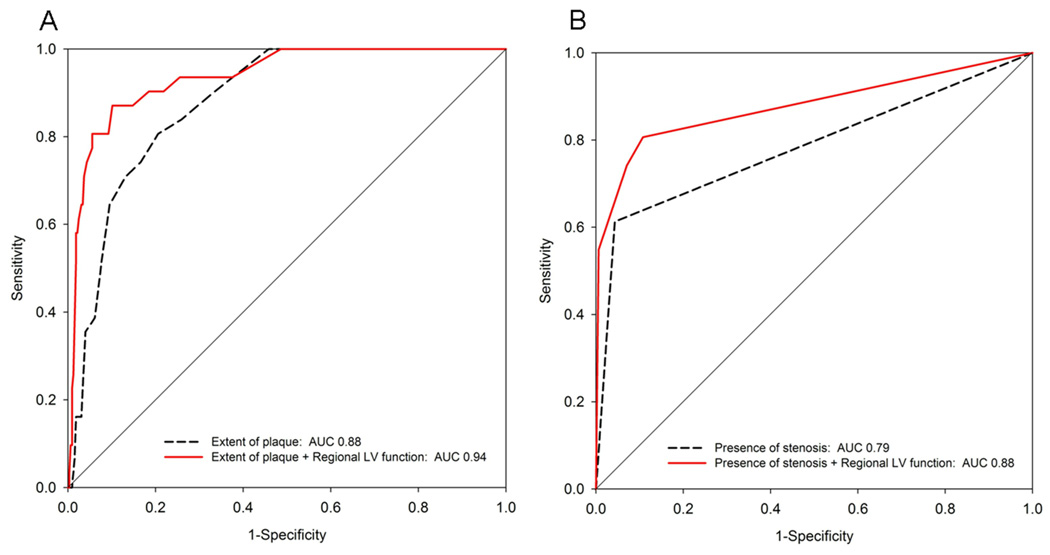
When regional LVF was added to baseline models of the extent of plaque and presence of stenosis for the detection of ACS, it was independently associated with a 25-fold increased risk in the model with plaque (OR: 25.3, 95%-CI: 9.4–68.6, p<0.0001) and a 20-fold increased risk in the model with stenosis (OR: 20.1, 95%-CI: 7.2–55.4, p<0.0001). Moreover, the area under the receiver operating characteristics curve (Figure 3) improved significantly after the addition of regional LVF to both the extent of plaque (AUC improved from 0.88 to 0.94) and the presence of stenosis (AUC improved from 0.79 to 0.88), both p<0.03.
Figure 3. Incremental diagnostic value of Regional LV function above extent of plaque (A) and presence of stenosis (B).
The assessment of regional LV function provided incremental diagnostic value to the assessment of the extent of plaque and the presence of stenosis. AUC: area under the receiver operating curve. Extent of plaque is defined as the number of coronary segments with plaque based on a 17 segment model.
DISCUSSION
In the present study we demonstrate that cardiac CT derived regional LV function has incremental value over the assessment of coronary morphology for the diagnosis and prediction of ACS in patients with acute chest pain but inconclusive initial ED evaluation. Specifically, we demonstrate: 1) that in the overall population (8% ACS) regional LVF resulted in a modest 10% increase in sensitivity to detect ACS (87%, 95%-CI: 70–96%), 2) however, that regional LVF particularly adds diagnostic value and may enable effective management in patients with plaque or stenosis detected by CT. This is suggested by a PPV of 89% when adding regional LVF to the assessment of patients with significant stenosis and by a NPV of 92% when added to the evaluation of patients with non-diagnostic CT scan which cannot exclude significant stenosis. These results suggest that regional LVF may aid clinical decision making of patients with acute chest pain specifically in those with CAD.
Our study confirms the results of previous studies using echocardiography and cardiac magnetic resonance imaging that resting regional LV function has excellent diagnostic accuracy for ACS using contrast enhanced cardiac CT 19, 36. LV dysfunction in this setting can be a result of either myocardial infarction (before a rise of troponin) but could also be an expression of unstable angina pectoris as well as myocardial stunning which renders wall motion abnormalities observable for several hours even after the resolution of acute chest pain in an ischemic rather than infarct setting 37. While we did not find a relationship between the time of onset of chest pain and presence of regional LV dysfunction, potential explanations include the intrinsic challenges of establishing accurate temporal history from patients as well as the prolonged median time symptom onset until CT LV function evaluation of 10.3 hours in our study. The strengths of this study include a large well characterized and prospectively enrolled cohort, the observational design (clinicians and patients blinded to the CT findings; CT readers blinded to the clinical findings), the systematic and standardized review of CT findings and the adjudication of clinical events by independent expert readers.
Overall, the diagnostic characteristics of adding LV function to coronary assessments are excellent but not perfect. While the evaluation of regional LVF resulted in only a modest sensitivity of 43% for ACS detection in the 114 patients with nonobstructive CAD, these are 3 extra patients (out of 7) who were accurately identified to have ACS with subsequent troponin elevation that would have otherwise been a “missed” diagnosis if coronary assessments were solely performed.
It is well known that the presence of severe calcification often defined as an Agatston Score of >400 significantly impairs the accuracy of coronary CTA to detect significant stenosis with a specificity dropping to 48% 16–18, 38. In our study, 90% of patients with coronary plaques had some degree of calcification (26% had exclusively calcified plaques and an additional 64% with mixed plaques). Although, we did not obtain a calcium score as part of the ROMICAT trial and thus could not assess the incremental value of LV function in various patient groups with increasing calcium scores, our stratified analysis was performed to highlight the benefit of LV function where a significant stenosis could not be excluded due to severe calcification or motion artifacts and found the assessment of regional LV function to improve the specificity to 86% and the NPV to 92% for detection of ACS. While this less than perfect NPV at first glance appears subpar, this effect is driven by the high prevalence of disease in our subgroup analysis leading to a resultant lower NPV than anticipated if all-comers were included (NPV of 99%) and should be interpreted within this context of high disease prevalence.
Our results also suggest that assessment of regional LV function may be able to provide information on the hemodynamic significance of lesions deemed to be stenotic by coronary CTA as almost 90% of patients with a significant stenosis, who also had impaired regional LV function developed ACS (PPV: 89%). This finding emphasizes that cardiac CT may be highly effective in guiding appropriate further testing and specifically limiting unnecessary referrals to invasive coronary angiography. An additional benefit of LV function assessment is the improved ability to identify patients with ACS due to stenosis of small vessels or side branches, when the spatial resolution of coronary CTA is limited and may result in a false negative scan18. Regional LVF could also aid in the detection of ACS due to coronary vasospasm, which may occur on top of nonobstructive atherosclerotic plaque.39 In our study we identified three additional patients with ACS, in whom stenosis assessment was negative resulting in an improvement in sensitivity of 10% from 77% to 87%. These results are further substantiated by demonstrating that regional LV dysfunction is an excellent predictor of ACS independent (20- to 25-fold increase in risk) and incremental to coronary assessment (significant increase in c-statistic). Thus, if regional LV dysfunction is present, then these patients are at higher risk for ACS and a conservative approach with hospitalization and further diagnostic testing may be warranted.
Notably, assessment of LV function from retrospectively ECG-gated protocols can be performed without additional administration of contrast or radiation. In our study, the mean radiation exposure was 13.8 mSv, which is comparable to the median 12 mSv (range 8–18 mSv) reported in the Prospective Multicenter Study On Radiation Dose Estimates of Cardiac CT Angiography in Daily Practice I (PROTECTION I) multicenter, observational study of 50 international sites.40 In contrast, sequential scanning or prospectively triggered CT scans permit a significant reduction of radiation, with up to a 78% reduction in radiation dose, even dose <2.5 mSv.40–42 However, this algorithm requires the patient to have a regular and slow heart rate for diagnostic image quality and the benefit of the radiation-dose reduction must be balanced against the loss of incremental LV functional data. Further studies are required to determine whether the potential to avoid subsequent testing justifies the increased radiation exposure.
Limitations
The study was performed in a single center. However, this is the first study performing a combined assessment of LV function and coronary morphology and the results may justify larger multicenter studies. The relatively small number of events limits the statistical power of this study, especially for sensitivity and positive predictive values in subgroup analysis except for patients with significant stenosis where the majority of clinical events (ACS) occurred. Unfortunately, tight 95% confidence intervals within subgroups would require a study of about 10000 patients using our entry criteria. In addition, our study has substantially higher event rates compared to other larger CT studies 43–44, and we have recently shown that this may be the population, in which CT may be effective 45. The generalizability of the results and thus the applicability of cardiac CT imaging in the ED may be limited to 1/3 of ED patients as 2/3 of patients who presented with acute chest pain were not enrolled in the study due to contraindications for contrast enhanced CT imaging or due to limitations of current technology (i.e.: arrhythmias). Although the radiation exposure in our study is similar to that of published multicenter registries, the relatively high radiation exposure remains a limitation especially once techniques such as prospective triggering with a medium radiation exposure below <10mSv become clinical standard. The current study does not provide a comparison with cardiac magnetic resonance imaging, the gold standard of LV function assessment or more widely available echocardiography. However a number of studies demonstrate an excellent agreement between cardiac CT and cardiac magnetic resonance tomography for the assessment of both global and regional LV dysfunction 20–23, 46–48.
Clinical Implications
Our results demonstrate that assessment of LV function has incremental value to assessment of coronary artery morphology by cardiac CT in patients with acute chest pain, specifically in those who have detectable coronary artery disease by CT. Because the presence of regional LV dysfunction has excellent PPV for ACS in patients with a significant stenosis detected by cardiac CT, it may guide appropriate and early referral for invasive coronary angiography without the need for additional stress testing. In addition, the high specificity in patients with an inconclusive coronary CTA may also alter the need for subsequent stress testing or coronary interventions.
Conclusion
Regional LVF assessment at rest improves diagnostic accuracy for ACS in patients with acute chest pain, especially in those with coronary artery disease. Thus, combined assessment of coronary morphology and regional LVF by cardiac CT may be helpful to guide further management in patients at intermediate risk for ACS.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge the enthusiastic support in patient enrollment of the team of faculty, residents, nursing and administrative staff of the Emergency Department of the Massachusetts General Hospital.
SOURCES OF FUNDING
This work was supported by the NIH (R01 HL080053). Dr Seneviratne received support from the National Heart Foundation of New Zealand grant 1152. Drs. Rogers, Truong, and Shapiro, were supported by the National Institutes of Health grant T32HL076136.
ABBREVIATIONS
- AUC
area under the receiver operating curve
- CAD
coronary artery disease
- CTA
computed tomography angiography
- ECG
electrocardiography
- ED
emergency department
- LV
left ventricular
- MI
myocardial infarction
- NPV
negative predictive value
- OR
odds ratio
- PPV
positive predictive value
- UAP
unstable angina pectoris
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Pozen MW, D'Agostino RB, Selker HP, Sytkowski PA, Hood WB., Jr A predictive instrument to improve coronary-care-unit admission practices in acute ischemic heart disease. A prospective multicenter clinical trial. N Engl J Med. 1984;310:1273–1278. doi: 10.1056/NEJM198405173102001. [DOI] [PubMed] [Google Scholar]
- 2.Storrow AB, Gibler WB. Chest pain centers: diagnosis of acute coronary syndromes. Ann Emerg Med. 2000;35:449–461. [PubMed] [Google Scholar]
- 3.Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Exercise testing in chest pain units: rationale, implementation, and results. Cardiol Clin. 2005;23:503–516. doi: 10.1016/j.ccl.2005.08.016. vii. [DOI] [PubMed] [Google Scholar]
- 4.Limkakeng A, Jr, Gibler WB, Pollack C, Hoekstra JW, Sites F, Shofer FS, Tiffany B, Wilke E, Hollander JE. Combination of Goldman risk and initial cardiac troponin I for emergency department chest pain patient risk stratification. Acad Emerg Med. 2001;8:696–702. doi: 10.1111/j.1553-2712.2001.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman J, Fromm R, Meyer D, Boudreaux A, Wun CC, Smalling R, Davis B, Habib G, Roberts R. Diagnostic marker cooperative study for the diagnosis of myocardial infarction. Circulation. 1999;99:1671–1677. doi: 10.1161/01.cir.99.13.1671. [DOI] [PubMed] [Google Scholar]
- 6.Fesmire FM, Hughes AD, Fody EP, Jackson AP, Fesmire CE, Gilbert MA, Stout PK, Wojcik JF, Wharton DR, Creel JH. The Erlanger chest pain evaluation protocol: a one-year experience with serial 12-lead ECG monitoring, two-hour delta serum marker measurements, and selective nuclear stress testing to identify and exclude acute coronary syndromes. Ann Emerg Med. 2002;40:584–594. doi: 10.1067/mem.2002.129506. [DOI] [PubMed] [Google Scholar]
- 7.Hedges JR, Young GP, Henkel GF, Gibler WB, Green TR, Swanson JR. Serial ECGs are less accurate than serial CK-MB results for emergency department diagnosis of myocardial infarction. Ann Emerg Med. 1992;21:1445–1450. doi: 10.1016/s0196-0644(05)80057-5. [DOI] [PubMed] [Google Scholar]
- 8.Selker HP, Beshansky JR, Griffith JL, Aufderheide TP, Ballin DS, Bernard SA, Crespo SG, Feldman JA, Fish SS, Gibler WB, Kiez DA, McNutt RA, Moulton AW, Ornato JP, Podrid PJ, Pope JH, Salem DN, Sayre MR, Woolard RH. Use of the acute cardiac ischemia time-insensitive predictive instrument (ACI-TIPI) to assist with triage of patients with chest pain or other symptoms suggestive of acute cardiac ischemia. A multicenter, controlled clinical trial. Ann Intern Med. 1998;129:845–855. doi: 10.7326/0003-4819-129-11_part_1-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Immediate exercise testing to evaluate low-risk patients presenting to the emergency department with chest pain. J Am Coll Cardiol. 2002;40:251–256. doi: 10.1016/s0735-1097(02)01968-x. [DOI] [PubMed] [Google Scholar]
- 10.Jeetley P, Burden L, Greaves K, Senior R. Prognostic value of myocardial contrast echocardiography in patients presenting to hospital with acute chest pain and negative troponin. Am J Cardiol. 2007;99:1369–1373. doi: 10.1016/j.amjcard.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 11.Gani F, Jain D, Lahiri A. The role of cardiovascular imaging techniques in the assessment of patients with acute chest pain. Nucl Med Commun. 2007;28:441–449. doi: 10.1097/MNM.0b013e3281744491. [DOI] [PubMed] [Google Scholar]
- 12.Kim SC, Adams SL, Hendel RC. Role of nuclear cardiology in the evaluation of acute coronary syndromes. Ann Emerg Med. 1997;30:210–218. doi: 10.1016/s0196-0644(97)70143-4. [DOI] [PubMed] [Google Scholar]
- 13.Udelson JE, Flint EJ. Radionuclide imaging in risk assessment after acute coronary syndromes. Heart. 2004;90(Suppl 5):v16–v25. doi: 10.1136/hrt.2004.041194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udelson JE, Beshansky JR, Ballin DS, Feldman JA, Griffith JL, Handler J, Heller GV, Hendel RC, Pope JH, Ruthazer R, Spiegler EJ, Woolard RH, Selker HP. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial. Jama. 2002;288:2693–2700. doi: 10.1001/jama.288.21.2693. [DOI] [PubMed] [Google Scholar]
- 15.Kontos MC, Arrowood JA, Jesse RL, Ornato JP, Paulsen WH, Tatum JL, Nixon JV. Comparison between 2-dimensional echocardiography and myocardial perfusion imaging in the emergency department in patients with possible myocardial ischemia. Am Heart J. 1998;136:724–733. doi: 10.1016/s0002-8703(98)70022-5. [DOI] [PubMed] [Google Scholar]
- 16.Rubinshtein R, Halon DA, Gaspar T, Jaffe R, Karkabi B, Flugelman MY, Kogan A, Shapira R, Peled N, Lewis BS. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation. 2007;115:1762–1768. doi: 10.1161/CIRCULATIONAHA.106.618389. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raff GL, Gallagher MJ, O'Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–557. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 19.Kwong RY, Schussheim AE, Rekhraj S, Aletras AH, Geller N, Davis J, Christian TF, Balaban RS, Arai AE. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531–537. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- 20.Annuar BR, Liew CK, Chin SP, Ong TK, Seyfarth MT, Chan WL, Fong YY, Ang CK, Lin N, Liew HB, Sim KH. Assessment of global and regional left ventricular function using 64-slice multislice computed tomography and 2D echocardiography: a comparison with cardiac magnetic resonance. Eur J Radiol. 2008;65:112–119. doi: 10.1016/j.ejrad.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Sarwar A, Shapiro MD, Nasir K, Nieman K, Nomura CH, Brady TJ, Cury RC. Evaluating global and regional left ventricular function in patients with reperfused acute myocardial infarction by 64-slice multidetector CT: a comparison to magnetic resonance imaging. J Cardiovasc Comput Tomogr. 2009;3:170–177. doi: 10.1016/j.jcct.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Belge B, Coche E, Pasquet A, Vanoverschelde JL, Gerber BL. Accurate estimation of global and regional cardiac function by retrospectively gated multidetector row computed tomography: comparison with cine magnetic resonance imaging. Eur Radiol. 2006;16:1424–1433. doi: 10.1007/s00330-006-0169-6. [DOI] [PubMed] [Google Scholar]
- 23.Raman SV, Shah M, McCarthy B, Garcia A, Ferketich AK. Multi-detector row cardiac computed tomography accurately quantifies right and left ventricular size and function compared with cardiac magnetic resonance. Am Heart J. 2006;151:736–744. doi: 10.1016/j.ahj.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Bastarrika G, Arraiza M, De Cecco CN, Mastrobuoni S, Ubilla M, Rabago G. Quantification of left ventricular function and mass in heart transplant recipients using dual-source CT and MRI: initial clinical experience. Eur Radiol. 2008;18:1784–1790. doi: 10.1007/s00330-008-0949-2. [DOI] [PubMed] [Google Scholar]
- 25.Cury RC, Nieman K, Shapiro MD, Butler J, Nomura CH, Ferencik M, Hoffmann U, Abbara S, Jassal DS, Yasuda T, Gold HK, Jang IK, Brady TJ. Comprehensive assessment of myocardial perfusion defects, regional wall motion, and left ventricular function by using 64-section multidetector CT. Radiology. 2008;248:466–475. doi: 10.1148/radiol.2482071478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hundt W, Rust F, Stabler A, Wolff H, Suess C, Reiser M. Dose reduction in multislice computed tomography. J Comput Assist Tomogr. 2005;29:140–147. doi: 10.1097/01.rct.0000151188.72850.0d. [DOI] [PubMed] [Google Scholar]
- 27.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 28.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 29.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann U, Nagurney JT, Moselewski F, Pena A, Ferencik M, Chae CU, Cury RC, Butler J, Abbara S, Brown DF, Manini A, Nichols JH, Achenbach S, Brady TJ. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation. 2006;114:2251–2260. doi: 10.1161/CIRCULATIONAHA.106.634808. [DOI] [PubMed] [Google Scholar]
- 31.Nagurney JT, Brown DF, Chae C, Chang Y, Chung WG, Cranmer H, Dan L, Fisher J, Grossman S, Tedrow U, Lewandrowski K, Jang IK. Disagreement between formal and medical record criteria for the diagnosis of acute coronary syndrome. Acad Emerg Med. 2005;12:446–452. doi: 10.1197/j.aem.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 32.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, 3rd, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Smith SC., Jr ACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction--2002: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina) Circulation. 2002;106:1893–1900. doi: 10.1161/01.cir.0000037106.76139.53. [DOI] [PubMed] [Google Scholar]
- 33.Gibler WB, Cannon CP, Blomkalns AL, Char DM, Drew BJ, Hollander JE, Jaffe AS, Jesse RL, Newby LK, Ohman EM, Peterson ED, Pollack CV. Practical implementation of the guidelines for unstable angina/non-ST-segment elevation myocardial infarction in the emergency department: a scientific statement from the American Heart Association Council on Clinical Cardiology (Subcommittee on Acute Cardiac Care), Council on Cardiovascular Nursing, and Quality of Care and Outcomes Research Interdisciplinary Working Group, in Collaboration With the Society of Chest Pain Centers. Circulation. 2005;111:2699–2710. doi: 10.1161/01.CIR.0000165556.44271.BE. [DOI] [PubMed] [Google Scholar]
- 34.Gibler WB, Cannon CP, Blomkalns AL, Char DM, Drew BJ, Hollander JE, Jaffe AS, Jesse RL, Newby LK, Ohman EM, Peterson ED, Pollack CV. Practical implementation of the Guidelines for Unstable Angina/Non-ST-Segment Elevation Myocardial Infarction in the emergency department. Ann Emerg Med. 2005;46:185–197. doi: 10.1016/j.annemergmed.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Braunwald E. Unstable angina. A classification. Circulation. 1989;80:410–414. doi: 10.1161/01.cir.80.2.410. [DOI] [PubMed] [Google Scholar]
- 36.Kontos MC, Arrowood JA, Paulsen WH, Nixon JV. Early echocardiography can predict cardiac events in emergency department patients with chest pain. Ann Emerg Med. 1998;31:550–557. doi: 10.1016/s0196-0644(98)70200-8. [DOI] [PubMed] [Google Scholar]
- 37.Braunwald E, Kloner RA. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982;66:1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- 38.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 39.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, McCollough C, Martinoff S, Kastrati A, Schomig A, Achenbach S. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 41.Husmann L, Valenta I, Gaemperli O, Adda O, Treyer V, Wyss CA, Veit-Haibach P, Tatsugami F, von Schulthess GK, Kaufmann PA. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008;29:191–197. doi: 10.1093/eurheartj/ehm613. [DOI] [PubMed] [Google Scholar]
- 42.Maruyama T, Takada M, Hasuike T, Yoshikawa A, Namimatsu E, Yoshizumi T. Radiation Dose Reduction and Coronary Assessability of Prospective Electrocardiogram-Gated Computed Tomography Coronary Angiography Comparison With Retrospective Electrocardiogram-Gated Helical Scan. J Am Coll Cardiol. 2008;52:1450–1455. doi: 10.1016/j.jacc.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein JA, Gallagher MJ, O'Neill WW, Ross MA, O'Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49:863–871. doi: 10.1016/j.jacc.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 44.Hollander JE, Chang AM, Shofer FS, McCusker CM, Baxt WG, Litt HI. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med. 2009;53:295–304. doi: 10.1016/j.annemergmed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 45.Ladapo JA, Hoffmann U, Bamberg F, Nagurney JT, Cutler DM, Weinstein MC, Gazelle GS. Cost-effectiveness of coronary MDCT in the triage of patients with acute chest pain. AJR Am J Roentgenol. 2008;191:455–463. doi: 10.2214/AJR.07.3611. [DOI] [PubMed] [Google Scholar]
- 46.Mahnken AH, Koos R, Katoh M, Spuentrup E, Busch P, Wildberger JE, Kuhl HP, Gunther RW. Sixteen-slice spiral CT versus MR imaging for the assessment of left ventricular function in acute myocardial infarction. Eur Radiol. 2005;15:714–720. doi: 10.1007/s00330-004-2592-x. [DOI] [PubMed] [Google Scholar]
- 47.Dirksen MS, Jukema JW, Bax JJ, Lamb HJ, Boersma E, Tuinenburg JC, Geleijns J, van der Wall EE, de Roos A. Cardiac multidetector-row computed tomography in patients with unstable angina. Am J Cardiol. 2005;95:457–461. doi: 10.1016/j.amjcard.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Salm LP, Schuijf JD, de Roos A, Lamb HJ, Vliegen HW, Jukema JW, Joemai R, van der Wall EE, Bax JJ. Global and regional left ventricular function assessment with 16-detector row CT: comparison with echocardiography and cardiovascular magnetic resonance. Eur J Echocardiogr. 2006;7:308–314. doi: 10.1016/j.euje.2005.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.