Summary
Immunization with vaccinia virus (VACV), the virus comprising the smallpox vaccine, induces memory CD8+ T cells that protect from subsequent infections with smallpox in humans or the related ectromelia virus (ECTV) in mice. Memory CD8+ T cells largely mediate these effects by expanding into secondary effectors that secrete the antiviral cytokine interferon-γ (IFNγ) and induce cytolysis via releasing factors such as perforin, which permeabilizes target cells. We show that protection from ECTV infection after VACV immunization depends on the initial memory cell frequency and ability of expanded secondary effectors to kill infected targets in a perforin-dependent manner. Although IFNγ is essential for anti-viral protection, it can be produced either by secondary effectors or concomitant primary effector CD8+ T cells recruited to the response. Thus, during lethal virus challenge, memory CD8+ T cells are required for cytolytic killing of infected cells but primary effectors can play important roles by producing IFNγ.
The severity of a viral infection, from asymptomatic to lethal, depends on the balance between the swiftness and strength of the innate and adaptive immune responses and the speed of virus replication and spread in the permissive host. Vaccination expands the pool of anti-viral lymphocytes and/or generates circulating antibodies altering this balance in favor of the host. This paradigm becomes vivid following footpad infection of different mouse strains with the Orthopoxvirus (OPV) ectromelia virus (ECTV). ECTV is a natural mouse pathogen that causes a disease known as mousepox. It is genetically and antigenically very similar to the virus of human smallpox and also to the virus in the smallpox vaccine, vaccinia virus (VACV) (Fenner et al., 1988). Following footpad infection of all laboratory mouse strains, ECTV spreads lympho-hematogenously (LHY) to seed the visceral organs, mainly the liver and spleen. However, the outcome of the infection varies depending on the mouse strain. C57BL/6 (B6) mice mount an effective innate natural killer cell (NKC) response in the draining lymph node (D-LN) at 2 days post infection (dpi) followed by an adaptive CD8+ T cell response that peaks in the D-LNs at 5 dpi and in the liver and spleen at 7 dpi (Fang et al., 2008; Fang et al., 2011; Fang and Sigal, 2005; Fang and Sigal, 2006; Parker et al., 2007). As a consequence, B6 mice suffer a relatively mild infection without major clinical symptoms of disease. On the other hand, mice of the strains BALB/c, A/J, DBA/2J, and B6 congenic B6.D2-(D6Mit149-D6Mit15)/LusJ (B6.D2-D6)(Davis et al., 2005; Fang et al., 2011), generally succumb at 7–10 dpi most likely due to the high virus titers and consequential massive necrosis of the liver (Wallace et al., 1985). In the case of the DBA/2J strain, a susceptibility gene has been mapped to the distal region of chromosome 6. This region is known as the NK complex (Delano and Brownstein, 1995) because it houses many NKC receptors genes including Klrd1, which encodes CD94 and is not expressed in DBA2/J mice (Vance et al., 2002). Notably, Klrd1−/− B6 mice and B6.D2-D6 mice which are congenic B6 mice with the NKC complex from DBA2/J mice are susceptible to mousepox and both can be rescued by transgenic expression of CD94 (Fang et al., 2011).
As with humans and smallpox, susceptible mice can be protected from mousepox by immunization with VACV (Fenner, 1994). Thus, ECTV can be used as an invaluable experimental model to understand how the smallpox vaccine protects. In addition to its importance as a smallpox model, ECTV is a textbook model for the many human and animal viruses that become systemic and cause disease by disseminating LHY (Flint et al., 2009). Hence, ECTV is uniquely suited to study the mechanisms of acquired protection against the many viruses that spread using this route.
CD8+ T cells play a major role in anti-viral immunity. Anti-viral CD8+ T cells become effectors and proliferate when their T cell receptor specifically recognizes viral peptide determinants bound to Major Histocompatibility Class I molecules (MHC I) at the surface of antigen presenting cells. Effector CD8+ T cells contribute to reduce the severity of disease by killing infected cells and by producing anti-viral cytokines (Harty et al., 2000). The major antiviral cytokine produced by effector CD8+ T cells is IFN-γ and the main cytolytic mechanism of effector CD8+ T cells is granule exocytosis whereby they release pro-apoptotic enzymes, prominently granzyme B (GzB), into the cytosol of the target cell through pores formed by perforin (Prf) (Trapani and Smyth, 2002). However, IFN-γ production and granule exocytosis mediated-killing are hallmarks but not the exclusive domain of CD8+ T cells. NKC, which recognize targets through germline encoded receptors, use these exact same mechanisms to control viruses during the early stages of infection (Cerwenka and Lanier, 2001). Moreover, IFN-γ production and granule exocytosis-mediated killing are also functions of NK T cells and CD4+ T cells (Billiau and Matthys, 2009; Fang et al., 2012; Marshall and Swain, 2011; Tupin et al., 2007).
If a virus is eliminated, most of the effector CD8+ T cells die but an expanded pool of virus-specific CD8+ T cells remains. These memory CD8+ T cells coexist with a naïve pool of anti-viral CD8+ T cells that may have been present during the primary infection but did not encounter antigen or may be newly produced (Martin et al., 2011). When a subsequent infection with a pathogen that carries the appropriate viral peptide determinants occurs, the memory CD8+ T cells rapidly become secondary effectors and expand. Similar to the primary effectors, these secondary effectors can kill infected cells and produce IFN-γ diminishing the seriousness of the infection (Welsh et al., 2004). Of note, by reducing virus loads, killing antigen presenting cells and/or advantageously competing for access to them, memory CD8+ T cells can also dampen the response of the coexisting anti-viral naïve cells (Guarda et al., 2007; Kedl et al., 2000). Yet, concomitant primary and secondary responses can occur, as demonstrated in other infection models (Badovinac et al., 2003; Martin et al., 2012; Martin et al., 2011; Turner et al., 2001). However, whether these concomitant primary responses can help combat the infection is unknown.
After primary infection, mousepox susceptible mice such as Klrd1−/− B6 mice and B6.D2-D6 fail to mount CD8+ T cell responses (Fang et al., 2006; Fang et al., 2011). However, they mount strong CD8+ T cell responses to VACV. Due to their genetic similarity, most CD8+ T cell determinants from VACV and ECTV are identical (Remakus et al., 2012; Tscharke et al., 2005; Tscharke et al., 2006). As a consequence, memory CD8+ T cells from VACV immune mice protect susceptible BALB/c mice from mousepox (Xu et al., 2007). Hence, the pairing of VACV immunization and challenge with ECTV serves as a unique model to study how memory CD8+ T cells protect from a lethal disease that spreads LHY within the context of a highly successful human vaccine. Using this model, we have previously shown that a major mechanism whereby memory CD8+ T cells protect from mousepox is by curbing lympho-hematogenous (LH) spread (Xu et al., 2007). They do this by rapidly becoming secondary effectors and proliferating in the D-LN. However, given that other cells have similar functions, it is still unknown whether for this or any other infection protection from disease requires the secondary effectors to perform both, kill infected cells and produce IFN-γ. Moreover, it is also unknown whether concomitant primary and secondary responses can occur during infections with highly virulent viruses that normally curtail the primary CD8+ T cell response of the naïve host. If so, it is possible that the concomitant primary response could play a role in protection, particularly if the secondary effectors are suboptimal.
Here we demonstrate that in the absence of other sources, the IFN-γ produced by secondary effectors is necessary and sufficient for protection from mousepox. However, when other sources of IFN-γ are available, the secondary effectors need to be capable of granule exocytosis-mediated killing but not of IFN-γ production because this function can be complemented by a concomitant primary response. Our findings help understand the mechanisms whereby memory CD8+ T cells protect from viral diseases and unveil a hitherto unknown role for concomitant primary CD8+ T cell responses during the course of a secondary CD8+ T cell response.
Results
In the absence of other sources, IFN-γ produced by memory CD8+ T cells is necessary and sufficient for protection from mousepox
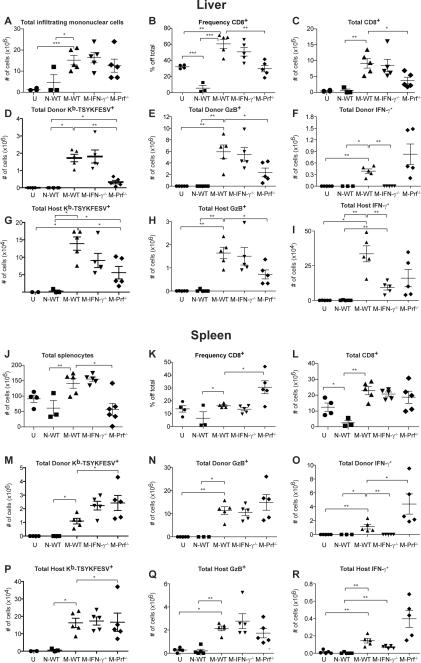
To investigate the need for IFN-γ during protection by memory CD8+ T cells, we took advantage that IFN-γ−/− mice (B6.129S7-Ifngtm1Ts/J, IFN-γ−/−) survive VACV infection and maintain a large memory CD8+ T cell pool that is highly cross-reactive with ECTV (Remakus et al., 2012; Remakus and Sigal, 2011). We transferred 5×106 purified CD8+ T cells obtained from naïve wild type (WT) B6 mice (N-WT), or VACV immune WT B6 (M-WT) or IFN-γ−/− (M-IFN-γ−/−) B6 mice into naïve IFN-γ−/− mice. One day post transfer, the mice were challenged with ECTV. All mice recipient of N-WT cells succumbed while all those that received M-WT survived the infection (Figure 1A) with minimal weight loss (Figure 1B). On the other hand, most M-IFN-γ−/− recipients succumbed (Figure 1A) and the few that survived became very sick as indicated by major weight loss (Figure 1B). Consistent with the survival data, the virus loads in the livers and spleens of M-WT recipients were several orders of magnitude lower than in those that received M-IFN-γ−/− or N-WT CD8+ T cells (Figure 1C and D). Histological analysis at 7 dpi showed that the livers of IFN-γ−/− mice recipient of N-WT or M-IFN-γ−/− CD8+ T cells were necrotic, devoid of marked mononuclear cell infiltrates localized in the lesions, had extensive hepatocellular necrosis, and a large number of foci that stained with rabbit antisera to the structural ECTV protein EVM-135. In contrast, the livers of IFN-γ−/− mice that received M-WT CD8+ T cells had very few necrotic areas, low tissue damage, high number of infiltrating lymphocytes in lesions, and significantly less EVM135+ foci than those that received N-WT or M-IFN-γ−/− CD8+ T cells (Figure S1). We also analyzed the CD8+ T cell responses at 7 dpi in the livers and spleens of IFN-γ−/− mice recipient of N-WT, M-WT and M-IFN-γ−/− CD8+ T cells. Liver infiltrating mononuclear cell (LIMC) counts in the livers were comparable in all infected IFN-γ−/− mice and significantly higher than in uninfected mice (Figure 2A, representative flow cytometry plots in Figure S2). Splenocyte counts (Figure 2G) were higher in M-WT and M-IFN-γ−/− recipients than in uninfected mice (because some N-WT recipients succumbed before the analysis, we were unable to statistically compare them with the other groups for these parameters). However, in both liver and spleen, the absolute number of total CD8+ T cells (Figure 2B and H), total CD8+ T cells that constitutively expressed GzB, (Figure 2C and I) and total CD8+ T cells specific for the Kb-restricted VACV/ECTV immunodominant determinant TSYKFESV+ as detected with Kb- TSYKFESV dimers (Dimer X, BD) (Figure 2D and J) were significantly higher in M-WT and M-IFN-γ−/− recipients than in N-WT recipients (note that GzB expression does not require ex vivo restimulation during acute ECTV infection and serves as a marker of total anti-viral effector CD8+ T cells (Fang and Sigal, 2005)). Also, following ex vivo restimulation with TSYKFESV, there was significantly more CD107a positive LIMC and splenic CD8+ T cells from M-WT and M-IFN-γ−/− recipients than from N-WT recipients (Figure 2E and K), which is a marker of cytotoxic CD8+ T cell degranulation (Betts et al., 2003). As expected, only those from M-WT recipients produced IFN-γ (Figure 2F and L). These experiments demonstrate that the presence of IFN-γ is essential during protection by memory CD8+ T cells and that the memory CD8+ T cells can produce all the IFN-γ required for protection. These experiments also show that IFN-γ deficient memory cells respond but do not protect in an IFN-γ deficient environment.
Figure 1. M-WT but not M-IFN-γ−/− CD8+ T cells efficiently protect IFN-γ−/− mice from mousepox.
A) IFN-γ−/− mice received 5×106 N-WT, M-WT or M-IFN-γ−/− CD8+ T cells and infected with ECTV. Survival was monitored. The experiment is representative of three, where n=5 for every group except M-IFN-γ−/− where n=6. B) The mice in A were weighed daily. C) IFN-γ−/− mice that received 5×106 N-WT, M-WT or M-IFN-γ−/− CD8+ T cells were infected with ECTV. Seven days p.i., mice were killed and virus titers determined in liver. Data corresponds to five mice per group ± SEM and is representative of two independent experiments. D) As in C but the virus titers were determined in spleen. Also see Figure S1 for liver pathology.
Figure 2. M-WT and M-IFN-γ−/− CD8+ T cells respond strongly in the liver and spleen of IFN-γ−/− mice.
IFN-γ−/− mice received 5×106 N-WT, M-WT or M-IFN-γ−/− CD8+ T cells. One day later, the mice were infected with ECTV and at 7 dpi the mononuclear cells infiltrating the livers (A-F) and splenocytes (G-L) were incubated for 5 h with TSYKFESV or without peptide and the indicated parameters were determined. Data corresponds to an experiment with five mice per group ± SEM, with exception of N-WT that had 2 mice/group because three mice died at 7 dpi. Data are representative of two experiments. Data are represented as a mean ± SEM. Representative flow cytometry plots are shown in Figure S2.
IFN-γ−/− but not Prf−/− memory CD8+ T cells protect susceptible IFN-γ+ mice from lethal mousepox
Given that M-IFN-γ−/− cells did not protect IFN-γ−/− mice but were able to respond to ECTV, we next tested whether they could protect mousepox susceptible IFN-γ-sufficient B6.D2-D6 mice. Because Prf is another major CD8+ T cells effector molecule required for granule exocytosis-mediated killing, we also tested whether memory CD8+ T cells obtained from Prf−/− deficient mice (M-Prf−/−) could protect from lethal mousepox. Graded numbers of N-WT, M-WT, M-IFN-γ−/− or M-Prf−/− CD8+ T cells were adoptively transferred into B6.D2-D6 mice. The proportion of Kb-TSYKFESV specific cells in the transferred populations was determined by flow cytometry (Figure 3A) and used to calculate the approximate number of Kb-TSYKFESV specific cells transferred. Upon ECTV challenge, all B6.D2-D6 mice that received N-WT or Prf−/− cells died although the death of the M-Prf−/− recipients was slightly but significantly delayed (Figure 3B and C). All the mice that received ~60,000 or more Kb-TSYKFESV specific M-WT or M-IFN-γ−/− CD8+ T cells were significantly protected from death and did not show symptoms of disease except for relatively minor weight loss. Protection by the memory cells was dose dependent because all the mice succumbed when adoptively transferred with only ~25,000 Kb-TSYKFESV specific M-WT CD8+ T cells (reported as text). Virus loads in liver (Figure 3D) and spleen (Figure 3E) at 7 dpi, were significantly lower in M-WT and M-IFN-γ−/− than in M-Prf−/− and N-WT B6.D2-D6 recipients. While there was a tendency for higher virus loads in mice recipient of M-IFN-γ−/− than in M-WT, it was not statistically significant. Compared to N-WT recipients, the virus titers in the liver of M-Prf−/− recipients were moderately but significantly decreased indicating a low level of protection which could explain their delayed death. Thus, contrary to what occurs with IFN-γ−/− mice, M-IFN-γ−/− cells can protect susceptible B6.D2-D6 mice from lethal mousepox. This suggests that the memory CD8+ T cells can outsource the necessary IFN-γ production to other cells to protect from lethal mousepox. On the other hand, the expression of Prf cannot be outsourced.
Figure 3. Memory CD8+ T cells deficient in IFN-γ but not in Prf protect susceptible mice from lethal mousepox.
CD8+ cells were magnetically purified from pooled LNs and spleens from donor naïve or VACV immune B6, IFN-γ−/− or Prf−/− mice. 106, 2.5×106 or 5×106 CD8+ purified CD8+ T cells from each type of donor cell were transferred i.v. into groups of 5 B6.D2-D6 mice. One day later the mice were infected with ECTV and survival was monitored. A) The frequency of CD8+ T cells specific for the immunodominant determinant TSYKFESV was determined by staining with Kb- TSYKFESV dimers. B) Kaplan-Meier survival curve for mice transferred with ~65,000 Kb- TSYKFESV+ cells as determined from the results in A. C) Kaplan-Meier survival curve for mice transferred with ~130,000 Kb- TSYKFESV+ cells as determined from the results in A. All mice transferred with 106 M-WT or M-Prf−/− (~25,000 Kb-TSYKFESV+ cells) succumbed to the infection, all the mice transferred with 5×106 M-IFN-γ−/− (~270,000 Kb-TSYKFESV+ cells) survived and are not displayed graphically. D,E) Virus titers at 7 dpi in livers (D) and spleens (E) from B6.D2-D6-Thy1.1+ mice that received 5×106 N-WT CD8+ T cells, or enough M-WT, M-IFN-γ−/− or M-Prf−/− CD8+ T cells to contain ~75,000 Kb-TSYKFESV+ cells. Data are represented as a mean ± SEM and are representative of two independent experiments.
Endogenous Prf but not IFN-γ in memory CD8+ T cells is required for the early control of ECTV LH spread
We have previously shown in BALB/c mice that that memory CD8+ T cells curb LH spread to the liver and spleen. Similarly, at 4 dpi, the virus loads in the liver (Figure 4A) and spleen (Figure 4B) of M-WT and M-IFN-γ−/− B6.D2-D6 recipients were significantly lower than in N-WT and M-Prf−/− B6.D2-D6 recipients. While there was some significant protection of the spleen in M-Prf−/− recipients, it was much less pronounced than in M-WT or M-IFN-γ−/− recipients. To identify the mechanism of the early protection of the liver and spleen, we transferred CFSE labeled N-WT, M-WT, M-IFN-γ−/− or M-Prf−/− CD8+ T cells into double congenic B6.D2-D6 mice carrying the Thy1.1 allele (B6.D2-D6-Thy1.1). Consistent with our previous experiments in BALB/c mice, there were no significant donor or host derived CD8+ T responses in the liver or spleen at 4 dpi (reported as text) indicating that the protection of the liver and spleen at 4 dpi was not due to in situ responses. However, at 4 dpi, the D-LN of M-WT and M-IFN-γ−/− but not M-Prf−/− recipient mice were enlarged with more than twice the total number of cells than uninfected mice or N-WT recipients (Figure 4C). In addition, compared to uninfected mice, the total numbers of CD8+ T cells in the D-LN of M-WT and M-IFN-γ−/− were significantly increased but not different in Prf−/− recipients. On the other hand, the relative (reported as text) and absolute numbers of CD8+ T cells were significantly decreased in N-WT recipients (Figure 4D) The number of donor (Thy1.2+) CD8+ T cells in M-WT and M-IFN-γ−/− recipients was also significantly higher than in N-WT or M-Prf−/− recipients (Figure 4E and F). Most M-WT and M-IFN-γ−/− but not M-Prf−/− or N-WT cells in the D-LN were effectors because they had divided extensively as indicated by CFSE dilution (Figure 4E and G) and expressed GzB (Figure 4H). Thus, the early protection of the liver and spleen in M-WT and M-IFN-γ−/− recipient mice was due to a rapid response in the D-LN that curbed LH spread. The data also suggests that the inability of M-Prf−/− cells to control the virus (probably due to their failure to rapidly kill infected cells) curtailed their own response in the D-LN (likely because they became infected and died).
Figure 4. Endogenous Prf but not IFN-γ in memory CD8+ T cells is required for the early control of ECTV LH spread.
A-B) Virus titers at 7 dpi in livers (A) and spleens (B) from B6.D2-D6 mice that received 5×106 N-WT CD8+ T cells, or enough MWT, M-IFN-γ−/− or M-Prf−/− CD8+ T cells to contain ~75,000 Kb-TSYKFESV+ cells. Data are representative of three independent experiments. C-J) N-WT, M-WT, M-IFN-γ−/− or M-Prf−/− CD8+ T cells were labeled with CFSE to identify divided donor cells. 5×106 N-WT CD8+ T cells or a number of M-WT, M-IFN-γ−/− or M-Prf−/− CD8+ T cells that contained ~75,000 Kb-TSYKFESV+ cells were transferred into B6.D2-D6-Thy1.1+ mice. One day later, the mice were infected with ECTV and at 4 dpi D-LN cells were counted and analyzed by flow cytometry. The indicated parameters were analyzed. Data are represented as a mean ± SEM and are representative of two similar experiments. Representative flow cytometry plots are shown in (E). Also see Figure S3 for Cidofovir treated mice and confocal microscopy, respectively.
We have previously shown that B6.D2-D6 mice infected with WT ECTV do not mount primary CD8+ T cell responses to ECTV and succumb to mousepox (Fang et al., 2011). However, treatment of B6.D2-D6 mice with the antiviral drug Cidofovir at 2 dpi, prevented mousepox (reported as text) and enabled a strong primary CD8+ T cell response (Figure S3 A). This demonstrates that the failure of B6.D2-D6 mice to generate potent primary CD8+ T cell responses during ECTV infection is not due to intrinsic defects of the CD8+ T cells or accessory cells but, most likely, to excessive viral replication. Thus, we studied whether similar to Cidofovir, protective memory CD8+ T cells enable primary CD8+ T cell responses to ECTV by looking for a primary anti-ECTV CD8+ T cell response (Thy1.2−) in the D-LN of the B6.D2-D6-Thy1.1 recipient mice. Interestingly, the absolute number of total Thy1.2− CD8+ T cells (Figure 4I) and of Thy1.2− GzB+ CD8+ T cells (Figure 4J) was significantly higher in M-WT and M-IFN-γ−/− recipients than in N-WT recipients. Thus, protective M-WT and M-IFN-γ−/− CD8+ T cells enabled a primary CD8+ T cell response in the D-LN. Given the small size of the D-LNs a more detailed analysis of the primary response was not possible in these mice. In a related experiment, naïve (N-EGFP) or VACV immune (M-EGFP) CD8+ T cells from C57BL/6-Tg(CAG-EGFP)1Osb/J (B6-EGFP) mice that express enhanced green fluorescence protein (EGFP) ubiquitously, were transferred into B6.D2-D6 mice that were infected or not with ECTV expressing the red fluorescence protein mCherry (ECTV-mCherry). The D-LNs were collected at 5 dpi and analyzed by confocal microscopy. The results suggested that M-EGFP decreased the spread of the infection from the paracortical areas into the medulla of the D-LN providing some insights into how memory cells could prevent virus spread (Figure S3 B).
Endogenous Prf but not IFN-γ in memory CD8+ T cells is required for the late control of ECTV in the liver and spleen
Given that naïve B6.D2-D6 mice succumb to ECTV starting at 7 dpi, we also analyzed the response and effects of the different memory CD8+ T cells at this time point in the liver and spleen. M-WT, M-IFN-γ−/− and M-Prf−/− recipients had significantly higher numbers of LIMC as compared to N-WT recipients and uninfected controls (Figure 5A) (Representative flow cytometry plots for Figure 5 can be found in Figure S4 A–B). More LIMC were CD8+ T cells in M-WT and M-IFN-γ−/− as compared to M-Prf−/− recipients in relative and absolute numbers (Figure 5B and C). Analysis of the donor (Thy1.2+) CD8+ T cell response showed that Kb-TSYKFESV-specific (Figure 5D) and GzB+ LIMC (Figure 5E) were present in M-WT, M-IFN-γ−/− and M-Prf−/− recipients but not in N-WT recipients. However, their absolute numbers were significantly higher in M-WT and MIFN-γ−/− than in M-Prf−/− recipients. After ex vivo restimulation with TSYKFESV, many LIMC expressed IFN-γ in M-WT and M-Prf−/− recipients but not in M-IFN-γ−/− recipients (Figure 5F). Analysis of the host-derived (Thy1.2−) primary response showed significantly higher numbers of Kb-TSYKFESV-specific (Figure 5G) and GzB+ (Figure 5H) LIMC in M-WT and M-IFN-γ−/− than in M-Prf−/−. After ex vivo restimulation with TSYKFESV, a significant number of host-derived CD8+ T LIMC expressed IFN-γ in M-WT, M-Prf−/− and, importantly, also in M-IFN-γ−/− recipients (Figure 5I). Spleens were lymphopenic in most N-WT and M-Prf−/− recipients while those in M-WT and M-IFN-γ−/− recipients had increased cellularity (Figure 5J). The frequency of splenic CD8+ T cells was unchanged in M-WT and M-IFN-γ−/− recipients, decreased or unchanged in N-WT recipients and significantly increased in M-Prf−/− recipients suggesting that activated cells were not killed by the virus but resting cells were (Figure 5K). As compared to uninfected mice, the absolute numbers of CD8+ T cells in the spleens of N-WT recipients were significantly decreased but they were not significantly affected in recipients of memory CD8+ T cells (Figure 5L). Analysis of Thy1.2+ donor-derived CD8+ T cells showed significantly higher numbers of Kb-TSYKFESV-specific (Figure 5M) and GzB+ cells (Figure 5N) in M-WT, M-IFN-γ−/− and M-Prf−/− as compared to N-WT recipients. Following ex vivo restimulation with TSYKFESV, a significant number of donor-derived CD8+ T cells expressed IFN-γ in MWT and M-Prf−/− but not in M-IFN-γ−/− recipients (Figure 5O). While smaller compared to the donor-derived, significant numbers of host-derived (Thy1.2−) Kb-TSYKFESV-specific (Figure 5P) and GzB+ cells (Figure 5Q) were present in M-WT, M-IFN-γ−/− and M-Prf−/− but not in N-WT recipients. Following ex vivo restimulation with TSYKFESV, a small but significant number of host-derived CD8+ T cells expressed IFN-γ in M-WT, M-Prf−/− and also in M-IFN-γ−/− but not in N-WT recipients (Figure 5R). Thus, M-IFN-γ−/− cells were at least as effective as M-WT cells at mounting a response in the liver and spleen, at enabling a primary response that produced IFN-γ, and at protecting from splenocyte loss. On the other hand, M-Prf−/− CD8+ T cells were less able to respond or enable a primary response in the liver; and while they responded strongly in the spleen, they were unable to protect from splenocyte loss.
Figure 5. Endogenous Prf but not IFN-γ in memory CD8+ T cells is required for the late control of ECTV in the liver and spleen.
B6.D2-D6-Thy1.1+ mice received 5×106 N-WT CD8+ T cells, or enough M-WT, M-IFN-γ−/− or M-Prf−/− CD8+ T cells to contain ~75,000 Kb-TSYKFESV+ cells. One day later, the mice were infected with ECTV and at 7 dpi: A-I) Liver infiltrating mononuclear cells (J-R) and splenocytes were incubated for 5 h with TSYKFESV or without peptide and the indicated parameters were determined. Data correspond to five mice per group with exception of uninfected mice that had 4 mice/group and N-WT that had 3 mice/group because two mice died at 7 dpi. Data are represented as a mean ± SEM and are representative of two experiments using B6.D2-D6 Thy1.1+ recipients (shown) and a third experiment using B6.D2-D6 mice as recipients. See Figure S4 for representative flow cytometry plots and for higher ECTV doses and VACV and LCMV infection.
It was of interest to determine whether concomitant primary responses to ECTV can also occur in resistant B6 mice that normally mount a strong primary response to ECTV and whether very high numbers of memory CD8+ T cells could inhibit the concomitant primary response. For this purpose, B6 mice were transferred with 5×106 (M-WTLOW) or 30×106 (M-WTHIGH) CD8+ T cells from VACV immune mice and their CD8+ T cell responses determined in the spleen at 7 dpi (Figure S4 C). We found that albeit reduced in comparison to un-transferred controls, concomitant primary responses occurred in the presence of both, M-WTHIGH and M-WTLOW cells. While the presence of concomitant primary and secondary responses have been described for other pathogens infected through the intravenous or respiratory routes (Badovinac et al., 2003; Martin et al., 2012; Martin et al., 2011; Turner et al., 2001), whether they are unique to ECTV or also occur for other pathogens when introduced via the footpad has not been explored. We found concomitant primary and secondary responses not only to VACV (which is non-pathogenic but antigenically almost identical to ECTV) but also to lymphocytic choriomeningitis virus (LCMV) Armstrong, a non-lytic RNA virus that naturally infects mice but does not cause disease following footpad inoculation (Figure S4 D–E). In the presence of the memory cells, primary responses to VACV were partially inhibited but those to LCMV were unchanged. Thus, following footpad infection, concomitant primary and secondary responses occur not only to ECTV in mousepox susceptible mice but also to ECTV in mousepox resistant mice, to non-pathogenic VACV, and to non-cytolytic LCMV.
N-WT T cells can complement M-IFN-γ−/− CD8+ T cells to protect IFN-γ−/− mice from mousepox
Not only CD8+ T, but also CD4+ T and NK cells can produce IFN-γ. We therefore investigated whether any of these populations from naïve B6 mice could complement the ability of the IFN-γ−/− memory CD8+ T cells to protect from mousepox. In a preliminary experiment, we found that most IFN-γ−/− mice that received M-IFN-γ−/− CD8+ T cells combined with a large number (4×107) of total leukocytes pooled from spleens, LNs and livers of naïve mice survived (N-WT leukocytes). Reducing the dose of N-WT leukocytes resulted in decreased survival with all the mice receiving M-IFN-γ−/− CD8+ T cells combined with only 107 N-WT leukocytes succumbing to mousepox. All IFN-γ−/− mice that had been transferred with 4×107 N-WT leukocytes alone also succumbed to mousepox indicating that this large number of IFN-γ-sufficient naïve cells in the absence of memory CD8+ T cells was not protective. As before, M-IFN-γ−/− cells alone did not protect the IFN-γ−/− mice (Figure S5). Thus, a sufficient number of N-WT leukocytes can complement MIFN-γ−/− CD8+ T cells to protect from mousepox. To identify which leukocyte populations were capable of complementing the M-IFN-γ−/− CD8+ T cells, we performed adoptive transfer experiments where the donors of N-WT leukocytes had been depleted of NK cells, CD4+ T cells, CD8+ T cells or CD4+ and CD8+ T cells one day before harvest and transfer (Figure 6). All mice that received M-IFN-γ−/− CD8+ T cells combined with total N-WT leukocytes, or M-IFN-γ−/− CD8+ T cells combined with N-WT leukocytes from NK cell depleted mice survived the infection without major weight loss. This indicated that production of IFN-γ by NK cells is not essential to complement protection by M-IFN-γ−/− CD8+ T cells. On the other hand, most mice transferred with M-IFN-γ−/− CD8+ T cells together with N-WT leukocytes from mice depleted of CD4+ and CD8+ T cells succumbed to mousepox indicating that N-WT T cells can complement M-IFN-γ−/− CD8+ T cell protection from mousepox. Most mice that received M-IFN-γ−/− CD8+ T cells together with N-WT leukocytes from either CD4+ T cell or CD8+ T cell depleted mice survived ECTV challenge indicating that either CD4+ or CD8+ T cells can complement the protective capacity of M-IFN-γ−/− CD8+ T cells. Control mice that received either M-IFN-γ−/− CD8+ T cells or total N-WT leukocytes alone succumbed to mousepox. Thus, WT CD4+ or CD8+ T cells recruited from the naïve pool can complement IFN-γ deficient memory CD8+ T cells to protect from an acute viral disease.
Figure 6. N-WT T cells can complement M-IFN-γ−/− CD8+ T cells to protect IFN-γ−/− mice from mousepox.
IFN-γ−/− mice were transferred with 2.5×106 M-IFN-γ−/− CD8+ T cells and/or with 5×107 leukocytes (pooled splenocytes, LN cells and liver mononuclear cells) from naïve B6 mice that had been depleted or not of CD4+, CD8+, CD4+ and CD8+ T cells, or NK cells as indicated. One day after transfer, the mice were challenged with ECTV in the footpad. A) Survival. B) Weight loss. Data correspond to the mean of five mice per group ± SEM and is representative of two independent experiments. Also see Figure S5.
Discussion
Based on correlation, Panchanathan et al. have argued that only antibodies can protect from mousepox (Panchanathan et al., 2010). Contrary to this view, we have previously shown that memory CD8+ T cells are effective at protecting susceptible mice from lethal mousepox and visible symptoms of disease (Remakus et al., 2012; Xu et al., 2007). Furthermore, we have shown that circulating antibodies and memory CD8+ T cells fulfill a similar role; they subdue the spread of the virus, tipping the balance in favor of the host and allowing for the development of a full immune response (Xu et al., 2007). Here we investigated whether intrinsic or extrinsic IFN-γ and granule exocytotic killing are required during memory CD8+ T cells protection from mousepox.
Naïve IFN-γ−/− and IFN-γ receptor deficient (IFN-γR−/−) mice succumb to footpad ECTV infection within 7–9 dpi (Chaudhri et al., 2004; Karupiah et al., 1993). However, IFN-γR−/− mice resist a secondary challenge with virulent ECTV if previously immunized with an attenuated ECTV strain indicating that IFN-γ is not essential for resistance to secondary ECTV infection. However, this protection was mediated by antibodies (Panchanathan et al., 2005). Here we have shown that IFN-γ is not essential for strong memory CD8+ T cell responses to ECTV, because M-IFN-γ−/− CD8+ T cells responded swiftly in IFN-γ−/− mice. Indeed, they responded stronger than M-WT CD8+ T cells most likely because in these experiments we transferred equal number of total CD8+ T cells which have higher frequency of memory cells in VACV immunized IFN-γ−/− than WT B6 mice (Remakus and Sigal, 2011) or, less likely, because a higher rate of expansion of the M-IFN-γ−/− cells. Still, despite their strong responses, M-IFN-γ−/− CD8+ T cells did not protect M-IFN-γ−/− mice from mousepox. This indicates that, distinct from Ab mediated protection, the presence of IFN-γ is crucial during memory CD8+ T cell protection. On the other hand, M-WT CD8+ T cells fully protected IFN-γ−/− mice from mousepox demonstrating that the responding memory cells can be the sole source of the necessary IFN-γ.
Despite the need for IFN-γ during protection by memory CD8+ T cells, we also found that WT and IFN-γ−/− memory CD8+ T cells controlled early ECTV LH spread and protected susceptible B6.D2-D6 mice from mousepox. This was the direct result of their ability to kill infected cells in the D-LN because Prf−/− memory CD8+ T cells did not prevent early LH spread or protect from mousepox. Consequently, memory CD8+ T cells have to be capable of Prf-dependent killing but not IFN-γ production to prevent mousepox in susceptible B6.D2-D6 mice.
We also found that protection by IFN-γ sufficient and deficient memory CD8+ T cells was highly dependent on their initial frequency. Hence, while ~25,000 M-WT CD8+ T cells did not protect any mouse from mousepox, ~60,000 or more of these cells were highly protective. Accordingly, the requirement for a high frequency of memory CD8+ T cells may be due to the need for direct contact between infected cells and the memory CD8+ cells to exert killing. Hence, we suggest it may be necessary to revise how physiologically relevant CD8+ T cell memory is currently measured. While poly-functionality is a correlate of disease progression in elite HIV controllers, the absence of poly-functional memory T cells after vaccination (Betts et al., 2006; Kannanganat et al., 2007; Precopio et al., 2007) may not necessarily indicate suboptimal protection. Thus, instead of assessing T cell functional superiority by their ability to co-produce multiple cytokines, it may be important to focus on memory CD8+ T cell frequency or their ability to protect from disease.
Given that IFN-γ is absolutely required during protection by memory CD8+ T cells it was important to identify the source of IFN-γ during protection by M-IFN-γ−/− cells. Following virulent ECTV infection, naïve B6 mice mount very strong primary CD8+ T cell responses while naïve B6.D2-D6 mice do not (Fang et al., 2011). Our experiments show that in the presence of memory CD8+ T cells, the primary CD8+ T cell responses of B6.D2-D6 mice to virulent ECTV were enabled in the D-LN, liver and spleen. Moreover, we showed that protection of IFN-γ−/− mice by M-IFN-γ−/− could be achieved if adequate numbers of IFN-γ+ naïve T cells were present. While some reports have demonstrated that memory CD8+ T cells can outcompete naïve CD8+ T cells by limiting their access to or by killing antigen presenting cells (Guarda et al., 2007; Kedl et al., 2000), our finding of concomitant primary and secondary responses following footpad infection is not unique to B6.D2-D6 mice or to ECTV infection but also occurs after infection of resistant B6-CD45.1 mice with virulent ECTV even after transfer of very large numbers of memory CD8+ T cells, and following infection with non-virulent VACV and non-cytolytic LCMV. Simultaneous primary and secondary responses have also been shown for other pathogens (Badovinac et al., 2003; Martin et al., 2012; Martin et al., 2011; Turner et al., 2001). However, in most of these cases and in our experiments with VACV there was partial inhibition of the primary response probably because the pathogen is well controlled in the naïve host. On the other hand, the primary response to virulent ECTV was enabled.
How do the memory CD8+ T cells enable the primary response to virulent ECTV? Several lines of evidence suggest that the inability of naïve B6.D2-D6 mice to mount primary CD8+ T cell responses to virulent ECTV is due to the excessive replication of the virus and not to intrinsic deficiencies in the CD8+ T cells or the antigen presenting cells: 1) Klrd1−/− mice, which have the exact same genetic deficiency as B6.D2-D6 mice, mount perfectly normal CD8+ T cell responses to VACV which shares the same determinants with ECTV but replicates poorly in mice (Fang et al., 2011). 2) CD8+ T cells from B6.D2-D6 and Klrd1−/− mice mount similar responses when adoptively transferred to B6 mice (Fang et al., 2011). 3) Inhibition of viral replication with the antiviral drug Cidofovir at 2 dpi enabled the primary CD8+ T cell response to virulent ECTV in B6.D2-D6 mice (Figure S3 A). Hence, the enablement of a concomitant naïve response to virulent ECTV by the memory CD8+ T cells resulted from their ability to reduce virus replication. This reduction in virus replication was mostly the consequence of cytolytic killing because Prf−/− M-CD8+ T cells did not reduce virus replication or efficiently enable a primary response.
In summary, our experiments demonstrate that during protection by memory CD8+ T cell from a lethal OPV disease that spreads LHY, IFN-γ and cytolytic killing are both essential. However, while the production of IFN-γ can be outsourced to concomitant primary effectors, the ability of the responding memory CD8+ T cells to kill infected cells cannot be replaced. This data is important for our understanding of anti-viral immunity and should be instrumental for designing and evaluating antiviral vaccines.
Methods
Viruses
Stocks were produced and titers determined as previously described (Fang et al., 2011; Fang and Sigal, 2010; Fang and Sigal, 2006; Xu et al., 2007). ECTV-OVA197-386-IRES-EGFP was produced by homologous recombination as previously described (Xu et al., 2008) by introducing a C-terminal fragment of chicken ovalbumin coding for amino acids 197–386 (preceded by a Kozac sequence and an ATG codon) followed by the internal ribosomal entry site from Encephalomyocarditis in front of EGFP.
Mice and infections
The Fox Chase Cancer Center Institutional Animal Care and Use Committee approved the experimental protocols involving animals. C57BL/6 (B6) mice were purchased from Taconic when they were 8–10 weeks of age and rested at least a week before use in experiments. The B6.D2-D6, IFN-γ−/− and B6-EGFP mice were initially purchased from Jackson Laboratory, and bred in the Fox Chase Cancer Center Laboratory Animal Facility. B6.D2-D6 Thy1.1 mice were generated by crossing B6.D2-D6 with B6.PL-Thy1a/CyJ mice (Jackson) and genotyped by staining PBMCs for Thy1.1+, NK1.1-, and Ly49H. Unless indicated, mice were infected with ECTV in the left footpad with 30 μl PBS containing 3×103 pfu. VACV was inoculated via the i.p. route with 500 μl PBS containing 106 PFU and boosted similarly 4 weeks later. Following infections, mice were observed daily for signs of disease (lethargy, ruffled hair, weight loss, skin rash, eye secretions) and imminent death (unresponsiveness to touch, lack of voluntary movements). When required mice were treated with 400 μg Cidofovir as previously (Fang and Sigal, 2010)
Histopathology
Was as previously described (Xu et al., 2012).
Adoptive transfers
CD8+ cells were magnetically purified from LNs and spleens using an Automacs magnetic cell sorter (Miltenyi Biotechnology) at the normal setting as previously described (Fang and Sigal, 2006; Xu et al., 2007). The efficiency of the purification and percent of virus-specific cells was monitored by Kb-TSYKFESV Dimer-X staining (BD Pharmingen) and FACS analysis. Unless indicated, cells were resuspended in PBS (107/ml) and 500 μl were inoculated i.v. into recipient mice. For experiments in Figure 4, donor cells were labeled with 4 μm CFSE (Invitrogen) as we did previously (Xu et al., 2007). For the experiments in Figures 7 and S7, M-IFN-γ−/− cells were obtained as above and the complementing leukocytes were obtained from LNs, spleen and livers of naïve B6 mice. When indicated, the B6 donor mice had been depleted 24 h before transfer with 100 μg of depleting CD4 (GK1.5, ATCC # HB-191), 100 μg of CD8 (2.43), or 200 μg of NK (PK136) monoclonal antibodies in 0.5 ml of PBS i.p. Depletion was assessed by flow cytometry. The depleting monoclonal antibodies were produced by the FCCC Tissue Culture Facility in CELLine bioreactors (BD) in Cell Animal Free Medium (BD) as directed by the manufacturer. Before adoptive transfer, effective depletion was confirmed by flow cytometry using an aliquot of the leukocytes.
Flow cytometry
Was performed as previously described (Fang and Sigal, 2010; Fang and Sigal, 2005; Fang and Sigal, 2006; Xu et al., 2007). The following Ab were used: anti-CD3 (145-2C11, Biolegend), anti-CD4 (GK1.5, Biolegend) and anti-CD8a (53–6.7, Biolegend), anti-Thy1.1 (OX-7, Biolegend), anti-Thy1.2 (30-H12, Biolegend), anti-IFN-γ (clone XMG1.2, Biolegend), anti-CD14 (Sa14–2, Biolegend), anti-CD16 (93, Biolegend), anti-CD19 (6D5, Biolegend), anti-CD94 (18d3, Biolegend), anti-CD49b (DX5, BD), anti-CD107a (1D4B, Biolegend), anti-NK1.1 (PK136, BD), anti-Ly49C/F/I/H (14B11, BD), anti-NKp46 (29A1.4, eBioscience), and PECy5.5 labelled anti-human GzB (GzB, Caltag) that cross-reacts with mouse GzB (Wolint et al., 2004). The hybridoma producing mAb 25-D1.16 was grown in Cell Line bioreactors (BD) in animal protein free media as recommended by the manufacturer. The 25-D1.16 mAb in the supernatant was purified by ammonium sulphate precipitation following standard techniques and labelled with an APEX® Alexa 647 Antibody labeling Kit (Invitrogen) as recommended by the manufacturer. For TSYKFESV specific TCD8+, H-2Kb:Ig recombinant fusion protein (Dimer-X, BD) were incubated with synthetic TSYKFESV (GenScript) and used as recommended by the manufacturer. mAb 25.D-1Stained cells were analyzed by flow cytometry at the Fox Chase Cell Sorting Facility using a LSR II system (BD). At least 100,000 were analyzed.
Data display and statistical analysis
Unless indicated, all displayed data correspond to one representative experiment of at least two similar experiments with groups of three to six mice. Statistical analysis was performed using Graph Pad Prism software. For survival studies, P values were obtained using the Log-rank (Mantel-Cox) Test. All other statistical analyses were performed using unpaired two-tailed t test or the Mann-Whitney test as applicable. When applicable, data is displayed with mean ± standard error of the mean (SEM). P values were determined between M-WT recipients and all other groups. * = P<0.05; ** = P<0.01 and ***= P<0.001. When not marked, the differences were not statistically significant. Unless specifically indicated, all groups were compared to M-WT recipients.
Supplementary Material
Highlights
T cell-mediated IFNγ secretion and cytolysis are required for ectromelia virus clearance
Memory CD8+ T cells can be the sole source of IFNγ required for antiviral protection
Memory CD8+ T cells enable a concomitant primary response to virus challenge
IFNγ but not cytolysis by memory CD8+ T cells can be outsourced to primary effectors
Acknowledgments
We thank Fox Chase Cancer Center Laboratory Animal, Flow Cytometry, Imaging, and Tissue Culture Facilities, Ms. Holly Gillin for assistance in the preparation of the manuscript, Dr. Andres Klein-Szanto for histopathology, Dr. E. John Wherry (University of Pennsylvania) for GP33 tetramers, Dr. Glenn Rall for LCMV, and Dr. Laurence Eisenlohr for critical reading of the manuscript. This work was supported by grants R01AI048849, R01AI065544 and 5U19AI083008 to LJS and P30CA006927 to FCCC. S.R. was partially supported by NIH T32 CA-009035036 to FCCC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. Journal of immunological methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A, Matthys P. Interferon-gamma: a historical perspective. Cytokine & growth factor reviews. 2009;20:97–113. doi: 10.1016/j.cytogfr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nature reviews. Immunology. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- Chaudhri G, Panchanathan V, Buller RM, van den Eertwegh AJ, Claassen E, Zhou J, de Chazal R, Laman JD, Karupiah G. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9057–9062. doi: 10.1073/pnas.0402949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RC, Schadt EE, Smith DJ, Hsieh EW, Cervino AC, van Nas A, Rosales M, Doss S, Meng H, Allayee H, Lusis AJ. A genome-wide set of congenic mouse strains derived from DBA/2J on a C57BL/6J background. Genomics. 2005;86:259–270. doi: 10.1016/j.ygeno.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Delano ML, Brownstein DG. Innate resistance to lethal mousepox is genetically linked to the NK gene complex on chromosome 6 and correlates with early restriction of virus replication by cells with an NK phenotype. Journal of virology. 1995;69:5875–5877. doi: 10.1128/jvi.69.9.5875-5877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Cheng H, Dai Z, Bu Z, Sigal LJ. Immunization with a single extracellular enveloped virus protein produced in bacteria provides partial protection from a lethal orthopoxvirus infection in a natural host. Virology. 2006;345:231–243. doi: 10.1016/j.virol.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Fang M, Lanier LL, Sigal LJ. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS Pathog. 2008;4:e30. doi: 10.1371/journal.ppat.0040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Orr MT, Spee P, Egebjerg T, Lanier LL, Sigal LJ. CD94 Is Essential for NK Cell-Mediated Resistance to a Lethal Viral Disease. Immunity. 2011;34:579–589. doi: 10.1016/j.immuni.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Siciliano NA, Hersperger AR, Roscoe F, Hu A, Ma X, Shamsedeen AR, Eisenlohr LC, Sigal LJ. Perforin-dependent CD4+ T-cell cytotoxicity contributes to control a murine poxvirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9983–9988. doi: 10.1073/pnas.1202143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Sigal L. Studying NK cell responses to ectromelia virus infections in mice. Methods Mol Biol. 2010;612:411–428. doi: 10.1007/978-1-60761-362-6_28. [DOI] [PubMed] [Google Scholar]
- Fang M, Sigal LJ. Antibodies and CD8+ T Cells Are Complementary and Essential for Natural Resistance to a Highly Lethal Cytopathic Virus. J Immunol. 2005;175:6829–6836. doi: 10.4049/jimmunol.175.10.6829. [DOI] [PubMed] [Google Scholar]
- Fang M, Sigal LJ. Direct CD28 Costimulation Is Required for CD8+ T Cell-Mediated Resistance to an Acute Viral Disease in a Natural Host. J Immunol. 2006;177:8027–8036. doi: 10.4049/jimmunol.177.11.8027. [DOI] [PubMed] [Google Scholar]
- Fenner F. Mousepox (ectromelia) In: Osterhaus A, editor. Virus infections of rodents and lagomorphs. Elsevier Science; Amsterdam; New York: 1994. p. 412. [Google Scholar]
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi D, Organization WH. Smallpox and its eradication. World Health Organization; Geneva: 1988. [Google Scholar]
- Flint SJ, Enquist LW, Racaniello VR, Skalka AM. Principles of virology. 3rd edn ASM Press; Washington, DC: 2009. [Google Scholar]
- Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martin-Fontecha A, Stein JV, Germain RN, Lanzavecchia A, Sallusto F. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. Journal of virology. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G, Fredrickson TN, Holmes KL, Khairallah LH, Buller RM. Importance of interferons in recovery from mousepox. Journal of virology. 1993;67:4214–4226. doi: 10.1128/jvi.67.7.4214-4226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. J Biomed Biotechnol. 2011;2011:954602. doi: 10.1155/2011/954602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MD, Condotta SA, Harty JT, Badovinac VP. Population dynamics of naive and memory CD8 T cell responses after antigen stimulations in vivo. J Immunol. 2012;188:1255–1265. doi: 10.4049/jimmunol.1101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MD, Wirth TC, Lauer P, Harty JT, Badovinac VP. The impact of pre-existing memory on differentiation of newly recruited naive CD8 T cells. J Immunol. 2011;187:2923–2931. doi: 10.4049/jimmunol.1100698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan V, Chaudhri G, Karupiah G. Interferon function is not required for recovery from a secondary poxvirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12921–12926. doi: 10.1073/pnas.0505180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan V, Chaudhri G, Karupiah G. Antiviral protection following immunization correlates with humoral but not cell-mediated immunity. Immunology and cell biology. 2010;88:461–467. doi: 10.1038/icb.2009.110. [DOI] [PubMed] [Google Scholar]
- Parker AK, Parker S, Yokoyama WM, Corbett JA, Buller RM. Induction of natural killer cell responses by ectromelia virus controls infection. Journal of virology. 2007;81:4070–4079. doi: 10.1128/JVI.02061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remakus S, Rubio D, Ma X, Sette A, Sigal LJ. Memory CD8+ T cells specific for a single immunodominant or subdominant determinant induced by peptide-dendritic cell immunization protect from an acute lethal viral disease. Journal of virology. 2012;86:9748–9759. doi: 10.1128/JVI.00981-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remakus S, Sigal LJ. Gamma interferon and perforin control the strength, but not the hierarchy, of immunodominance of an antiviral CD8+ T cell response. Journal of virology. 2011;85:12578–12584. doi: 10.1128/JVI.05334-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nature reviews. Immunology. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharke DC, Woo WP, Sakala IG, Sidney J, Sette A, Moss DJ, Bennink JR, Karupiah G, Yewdell JW. Poxvirus CD8+ T-cell determinants and cross-reactivity in BALB/c mice. Journal of virology. 2006;80:6318–6323. doi: 10.1128/JVI.00427-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nature reviews. Microbiology. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- Turner SJ, Cross R, Xie W, Doherty PC. Concurrent naive and memory CD8(+) T cell responses to an influenza A virus. J Immunol. 2001;167:2753–2758. doi: 10.4049/jimmunol.167.5.2753. [DOI] [PubMed] [Google Scholar]
- Vance RE, Jamieson AM, Cado D, Raulet DH. Implications of CD94 deficiency and monoallelic NKG2A expression for natural killer cell development and repertoire formation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:868–873. doi: 10.1073/pnas.022500599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GD, Buller RM, Morse HC., 3rd Genetic determinants of resistance to ectromelia (mousepox) virus-induced mortality. Journal of virology. 1985;55:890–891. doi: 10.1128/jvi.55.3.890-891.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Selin LK, Szomolanyi-Tsuda E. Immunological memory to viral infections. Annu Rev Immunol. 2004;22:711–743. doi: 10.1146/annurev.immunol.22.012703.104527. [DOI] [PubMed] [Google Scholar]
- Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J Exp Med. 2004;199:925–936. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RH, Cohen M, Tang Y, Lazear E, Whitbeck JC, Eisenberg RJ, Cohen GH, Sigal LJ. The orthopoxvirus type I IFN binding protein is essential for virulence and an effective target for vaccination. J Exp Med. 2008;205:981–992. doi: 10.1084/jem.20071854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RH, Fang M, Klein-Szanto A, Sigal LJ. Memory CD8+ T cells are gatekeepers of the lymph node draining the site of viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10992–10997. doi: 10.1073/pnas.0701822104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RH, Rubio D, Roscoe F, Krouse TE, Truckenmiller ME, Norbury CC, Hudson PN, Damon IK, Alcami A, Sigal LJ. Antibody inhibition of a viral type 1 interferon decoy receptor cures a viral disease by restoring interferon signaling in the liver. PLoS Pathog. 2012;8:e1002475. doi: 10.1371/journal.ppat.1002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.