Abstract
Proteasome inhibition has emerged as a novel approach to anticancer therapy. Numerous natural compounds, such as gambogic acid, have been tested in vitro and in vivo as anticancer agents for cancer prevention and therapy. However, whether gambogic acid has chemosensitizing properties when combined with proteasome inhibitors in the treatment of malignant cells is still unknown. In an effort to investigate this effect, human leukemia K562 cells, mouse hepatocarcinoma H22 cells and H22 cell allografts were treated with gambogic acid, a proteasome inhibitor (MG132 or MG262) or the combination of both, followed by measurement of cellular viability, apoptosis induction and tumor growth inhibition. We report, for the first time, that: (i) the combination of natural product gambogic acid and the proteasome inhibitor MG132 or MG262 results in a synergistic inhibitory effect on growth of malignant cells and tumors in allograft animal models and (ii) there was no apparent systemic toxicity observed in the animals treated with the combination. Therefore, the findings presented in this study demonstrate that natural product gambogic acid is a valuable candidate to be used in combination with proteasome inhibitors, thus representing a compelling anticancer strategy.
Keywords: Gambogic acid, Proteasome inhibitors, Antitumor activity, Synergistic effect
1. Introduction
The ubiquitin–proteasome system controls the turnover of regulatory proteins involved in critical cellular processes including cell cycle progression, cell development and differentiation, apoptosis, angiogenesis and cell signaling pathways [1–4]. Since aberrant proteasome-dependent proteolysis appears to be associated with the pathophysiology of some malignancies, it was suggested that pharmacological inhibition of proteasome function may prove useful as a novel anticancer strategy [5–7]. Indeed, the first proteasome inhibitor bortezomib was approved by the US Food and Drug Administration (FDA) in 2003 for the treatment of multiple myeloma which provided “proof of concept” that targeting the ubiquitin–proteasome pathway is a viable route for the treatment of human cancer [8,9]. Although bortezomib has achieved significant clinical benefit for multiple myeloma in clinical trials, its effectiveness and administration have been limited by toxic side effects, including asthenic conditions (such as fatigue, generalized weakness), gastrointestinal events (nausea, diarrhea, vomiting, poor appetite, etc.), hematological toxicity (low platelet and erythrocytes counts), peripheral neuropathy and high rate of shingles [10,11]. Therefore, efforts are ongoing to discover adjuvant agents, especially from natural resources, to chemosensitize malignant cells to proteasome inhibitors. The ideal adjuvant should augment the effect of proteasome inhibitors to achieve optimal therapeutic effects at lower doses, while resulting in minimal toxicity.
Gambogic acid (GA, Fig. 1A) is a natural product isolated from gamboge, which is a dry resin secreted from the Garcinia hurburyi tree. The gamboge resin has been used as a coloring agent and in traditional Chinese medicine for the treatment of human diseases including indigestion, inflammation, and ulcers [12]. Early studies revealed that GA acts as a potent inducer of apoptosis in cancer cells [13,14]. Recent studies have demonstrated that GA has anticancer effects and inhibits the growth of several types of human cancer cells, including prostate, breast, gastric carcinoma, hepatocarcinoma, epithelial cervical cancer, lung cancer and leukemia in vitro and in vivo [13–21]. GA has been approved by the Chinese Food and Drug Administration for the treatment of various cancers in clinical trials [22]. However, whether GA possesses synergistic anticancer effects when combined with proteasome inhibitors in malignant cells has yet to be reported.
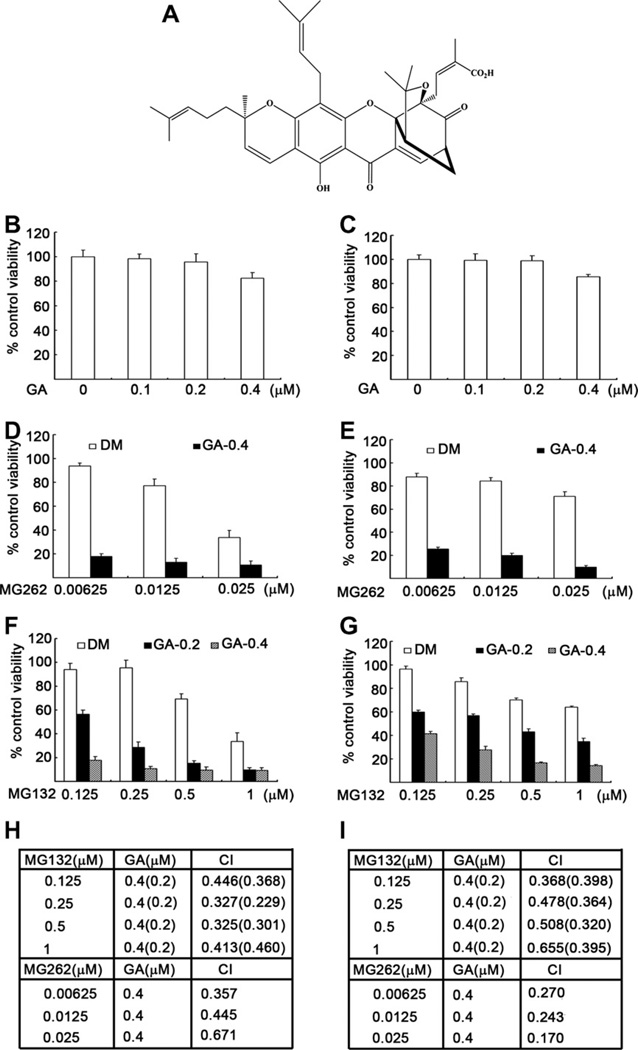
Fig. 1.
Synergistic effect of GA and proteasome inhibitors on inhibition of cell viability/proliferation in malignant cells. (A) Chemical structure of GA. (B and C) K562 (B) or H22 cells (C) were treated with indicated doses of GA for 48 h, followed by MTS assay. (D and E) K562 (D) or H22 cells (E) were exposed to indicated concentrations of MG262 in the presence or absence of GA (0.4 µM) for 48 h, followed by MTS assay. (F, G) K562 (F) or H22 cells (G) were treated with indicated doses of MG132 in the presence or absence of GA (0.2 or 0.4 µM) for 48 h, followed by MTS assay. (H, I) Combination index (CI) of proteasome inhibitors plus GA in K562 cells (H) or H22 cells (I) was presented. CI < 1 indicates synergistic effect. Bars, SD, mean of four experiments.
In the present study, we report for the first time that the combination of natural product GA with two proteasome inhibitors (MG132, MG262) produces a significant synergistic effect in both malignant cells and tumors, resulting in reduced cell viability/proliferation and apoptotic cell death.
2. Materials and methods
2.1. Materials, reagents, and antibodies
MG132, MG262, z-VAD-fmk and Gambogic acid were purchased from BIOMOL International LP (Plymouth Meeting, PA, USA). MG132, MG262 and GA were dissolved in DMSO (Sigma; St. Louis, MO) at a stock concentration of 10 mM, aliquoted and stored at −80°C. Cremophor EL was purchased from Sigma (St. Louis, MO, USA). Fetal bovine serum (FBS), RPMI 1640, penicillin and streptomycin were purchased from Invitrogen by Life Technology (Carlsbad, CA, USA). Rabbit polyclonal antibody against GAPDH (FL-335) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit polyclonal antibody against nuclear poly(ADP-ribose) polymerase (PARP), rabbit monoclonal antibody against caspase-3 (8G10), and mouse monoclonal antibodies against caspase-8 (1C12) and caspase-9 (C9) were purchased from Cell Signaling (Beverly, MA, USA). Enhanced chemiluminescence (ECL) reagents were purchased from Amersham Biosciences (Piscataway, NJ, USA). Propidium idodide (PI) and Annexin V-FITC Apoptosis Detection Kit were purchased from Keygen Company (Nanjing, China).
2.2. Cell lines and cell culture
Murine hepatoma H22 and human leukemia K562 cells were purchased from American Type Culture Collection (Manassas, VA, USA) and grown in RPMI 1640 supplemented with 10% FBS, 100 units/mL of penicillin and 100 µg/mL of streptomycin. Cell cultures were maintained at 37°C and 5% CO2.
2.3. MTS assay
The effects of compounds on cell viability were determined by the MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation assay, Promega Corporation, Madison, WI, USA). The MTS tetrazolium compound is bioreduced by cells into a colored formazan product that is soluble in tissue culture medium. This conversion is presumably accomplished by NADPH or NADH produced by dehydrogenase enzymes in metabolically active cells. Exponentially growing cells were harvested and seeded at 2500 cells/well in a 96-well plate. After 6 h incubation, compounds or DMSO, as the untreated control, were added, followed by continuous incubation for 48 h. Twenty microliter of MTS were added to each well and the incubation was continued for an additional 3 h. The absorbance was measured with a microplate reader (Sunrise, Tecan) at 490 nm. The percent inhibition was calculated as follows: Inhibition rate (IR) (%) = [1 – (absorbance of experimental well – absorbance of blank)/(absorbance of untreated control well – absorbance of blank)] × 100%.
2.4. Cell death detection assay via flow cytometry
Apoptotic cell death was measured by Annexin V-FITC and propidium iodide (PI) double staining followed by flow cytometry as previously described [23]. Briefly, cultured K562 and H22 cells were harvested and washed with cold PBS and resuspended with the binding buffer, followed by Annexin V- FITC incubation for 15 min and PI staining for another 15 min at 4°C in dark. The stained cells were analyzed with flow cytometry within 30 min.
2.5. Morphological characterization of cell death
K562 or H22 cells were treated as described. To monitor temporal changes in the incidence of cell death in the live culture condition, propidium idodide (PI) was added to the cell culture medium and at the desired sequential time points, the cells in the culture dish were imaged with an inverted fluorescence microscope equipped with a digital camera (Axio Obsever Z1, Zeiss). PI is not able to enter the normal live cells but the dying or dead cells lose their membrane integrity and PI can enter their nucleus, bind to double-stranded DNA, and thereby positively stain the dying and dead cells.
2.6. Establishment and treatment of H22 allografts
H22 allograft model was established as previously described [24]. Briefly, murine hepatoma H22 cells (10 × 106) suspended in 0.2 ml of serum-free RPMI 1640 were inoculated s.c. in the left armpit of each mouse. After 24 h of inoculation, mice were randomly divided into four groups (10 mice per group) and treated with either vehicle (10% DMSO, 30% Cremophor, and 60% PBS), MG132 (2 mg/kg of body weight) and/or GA (1 mg/kg of body weight), respectively, via daily i.p. injection for seven consecutive days. Two days later after the treatment, the mice were sacrificed, and the tumor tissues were weighed.
2.7. Western blot analysis
Western blotting was performed as previously described [24,25]. In brief, an equal amount of total protein extracts from cultured cells were fractionated by 12% SDS–PAGE and electrically transferred onto polyvinylidene difluoride (PVDF) membranes. Mouse or rabbit primary antibodies and horseradish peroxidase (HRP)-conjugated appropriate secondary antibodies were used to detect the designated proteins. The bound secondary antibodies on the PVDF membrane were reacted with ECL detection reagents (Amersham Bioscience) and exposed to X-ray films (Kodak).
2.8. Combination index
The interaction between two compounds was quantified by determining the combination index (CI). The CI was calculated by the Chou–Talalay equation [23]. The general equation for the classic isobologram is given by: CI = (D)1/(Dx)1 + (D)2/(Dx)2. Where Dx indicates the dose of one compound alone required to produce an effect, and (D)1 and (D)2 are the doses of compounds 1 and 2, respectively, necessary to produce the same effect in combination. From this analysis, the combined effects of the two compounds can be summarized as follows: CI < 1 indicates synergism; CI = 1 indicates additive effect; and CI > 1 indicates antagonism.
2.9. Statistical methods
Mean + SD are presented where applicable. ANOVA was used where appropriate for determining statistical probabilities by using SPSS software. P values less than 0.05 indicate statistical significance.
3. Results
3.1. GA augments proteasome inhibitor-induced inhibition of cell viability in malignant cells
To verify our hypothesis and determine whether GA can sensitize cancer cells to treatment with proteasome inhibitors, we first tested the effects of various concentrations of gambogic acid alone on the viability of human leukemia K562 and murine hepatocarcinoma H22 cells. The results showed that even at the maximal dose tested, GA as a single agent inhibited cell viability by less than 20% in both cell lines (Fig. 1B and C). However, when the two cell lines were treated with the proteasome inhibitor MG262 at different doses with or without 0.4 µM of GA for 48 h, inhibition of cell viability was dramatically increased, for example, from 5% when treated with 6.25 nM of MG262 alone to 82% when combined with GA (K562 cells, Fig. 1D). When H22 cells were treated under the same treatment conditions, the addition of GA boosted the inhibitory effects of MG262 on cell viability/proliferation from 11% to 73% (Fig. 1E). GA was also tested in a combinatorial manner with another proteasome inhibitor MG132 in both K562 and H22 cell lines, and a similar synergistic effect was observed (Fig. 1F and G). An analysis of the combination index (CI) indicated that all values of CI were less than 1 (CI < 1), indicating that GA and proteasome inhibitors possess synergistic effects on inhibition of cancer cell viability (Fig. 1H and I).
3.2. GA is able to sensitize malignant cells to proteasome inhibitor-induced apoptotic cell death
We and others have reported that inhibition of proteasome activity, particularly inhibition of chymotrypsin-like activity, is associated with induction of apoptosis in malignant cells [26–28]. To determine whether GA has synergistic effects on proteasome inhibitor-induced apoptosis, H22 and K562 cells were treated with MG262 or MG132 in the presence or absence of GA, followed by the Annexin V Apoptosis assay for flow cytometric analysis. The results showed that co-treatment of these malignant cells, especially K562 cells, dramatically increased the population of both Annexin V and propidium iodide (PI) stained cells (Fig. 2A and B), indicating that the combination treatment induces more cell death than each treatment alone.
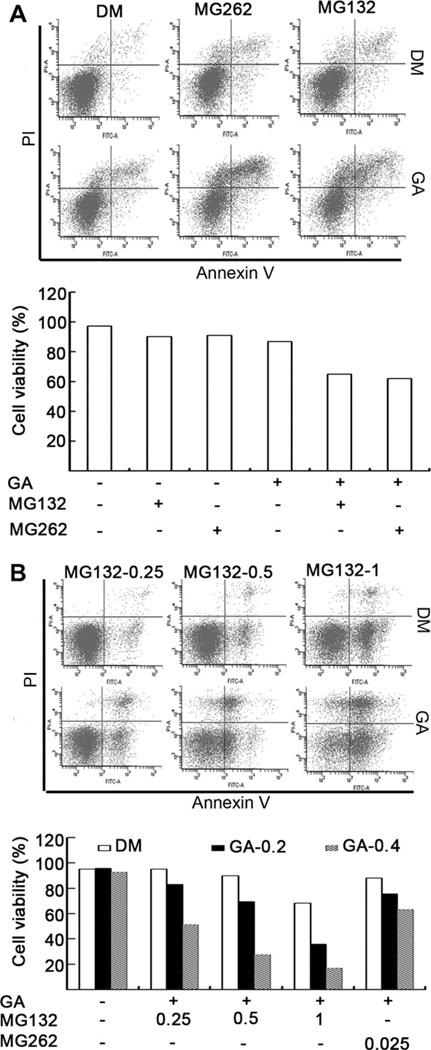
Fig. 2.
GA sensitizes malignant cells to the proteasome inhibitor-induced cell death. (A) H22 cells were treated with MG132 (0.5 µM) or MG262 (0.025 µM) in the presence or absence of GA (0.4 µM) for 24 h. The treated cells were collected and stained with FITC Annexin V and propidium iodide (PI), followed by flow cytometry analysis. The results of flow images (the upper panel) and summarized bar graphs (the lower panel) were presented. (B) K562 cells were treated with MG132 (0.25, 0.5, 1 µM) or MG262 (0.025 µM) in the presence or absence of GA (0.2, 0.4 µM) for 24 h, followed by staining with Annexin V and PI, and flow cytometry analysis.
To verify these findings, K562 and H22 cells were treated with MG262 or MG132 in the presence or absence of GA, followed by PI nucleic acid staining and analysis under inverted fluorescence microscope. The data showed that ∼30% and 35% of H22 cells co-treated with GA + MG262 or GA + MG132, respectively, were PI-positive (Table 1). In comparison, treatment of H22 cells with GA, MG262 or MG132 alone resulted in less than 5% PI-positive population (Table 1). Similarly, co-treatment of K562 cells with GA (0.4 µ,M)plus MG132 (1 µM)or MG262 (0.025 µM) resulted in 85% and 40% of PI-positive cells, respectively, compared with 8% and 3% of PI-positive populations after treatment with MG132 (1 µM) or MG262 (0.025 µM) alone (Table 1). It should be noted that after the cotreatment, many of the K562 cells were broken, while most of the H22 cells remained intact. Our data shows that GA appears to have a greater synergistic effect in K562 cells than in H22 cells under the same treatment conditions.
Table 1.
Percentage of PI-positive cells in living H22 or K562 cells treated with MG132 or MG262 in the absence or presence of gambogic acid.
| Cell type | Treatment | PI(+)% | Treatment | PI(+)% | ||
|---|---|---|---|---|---|---|
| K562 | MG132 | GA | MG262 | GA | ||
| 0.000 | 0.0 | – | 0.00000 | 0.0 | – | |
| 0.125 | 0.0 | – | 0.00625 | 0.0 | – | |
| 0.250 | 0.0 | – | 0.01250 | 0.0 | – | |
| 0.500 | 0.0 | 3 | 0.02500 | 0.0 | 3 | |
| 1.000 | 0.0 | 9 | 0.05000 | 0.0 | 10 | |
| 0.000 | 0.4 | 6 | 0.00000 | 0.4 | 5 | |
| 0.125 | 0.4 | 18 | 0.00625 | 0.4 | 4 | |
| 0.250 | 0.4 | 50 | 0.01250 | 0.4 | 15 | |
| 0.500 | 0.4 | 70 | 0.02500 | 0.4 | 40 | |
| 1.000 | 0.4 | 85 | 0.05000 | 0.4 | 65 | |
| H22 | 0.000 | 0.0 | – | 0.00000 | 0.0 | – |
| 0.000 | 0.4 | 3 | 0.00000 | 0.4 | 4 | |
| 1.000 | 0.0 | 5 | 0.02500 | 0.0 | 6 | |
| 1.000 | 0.4 | 35 | 0.02500 | 0.4 | 30 |
Percentage of PI-positive cells represents the average of three fields, “–” represents less than 3% PI-positive cells.
3.3. Antitumor activity of the proteasome inhibitor MG132 can be enhanced by co-treatment with GA in vivo
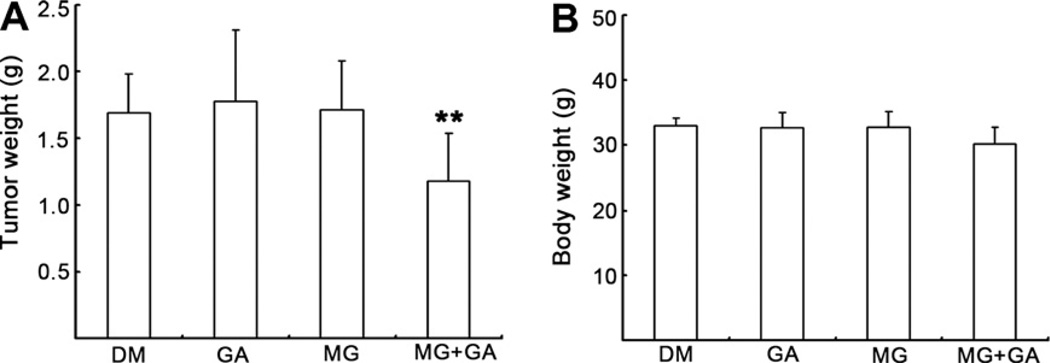
The data described above clearly demonstrate that GA exhibits sensitizing activity to proteasome inhibitors and could augment proteasome inhibitor-mediated inhibition of cell viability and induction of apoptosis in cultured cancer cells. To determine whether GA can exert the same synergistic effects on the inhibition of tumor growth in vivo, H22 cells were implanted s.c. in KMF mice. The mice were randomly divided into four groups (n = 10) and were treated daily with 1 mg/kg of GA alone, 2 mg/kg of MG132 alone, or a combination of both GA and MG132 for 7 days. A group treated with solvent was used as a control. The mice were euthanized 3 days after the last day of treatment and tumors were removed and measured. These in vivo data show that after a short period (7 days) of treatment, about 30% tumor growth inhibition was observed in the group co-treated with GA and MG132 (Fig. 3A). However, tumor weights of groups treated with GA or MG132 alone were not decreased compared with the solvent control group (Fig. 3A). At the endpoint of the in vivo study, the average body weight of the mice in each group were not significantly different, indicating little to no toxicity of GA and MG132 under the same treatment conditions (Fig. 3B).
Fig. 3.
Combinational treatment with GA and MG132 suppresses allograft tumor growth in animal models. H22 allograft was generated in male KMF mice as described in Section 2 and daily treated with either GA (1 mg/kg of body weight), MG132 (2 mg/kg of body weight) alone or combination of these two agents (10 mice per group) for 7 days. Two days after termination of the treatment, the mice were sacrificed and the tumor size (A) and body weigh (B) were analyzed. **P< 0.01, bars, SD, mean of 10 mice.
3.4. Potential mechanisms of synergistic effects of GA on proteasome inhibitor-induced apoptosis and antitumor activity
The results from our in vitro and in vivo studies have demonstrated that the combination treatment of GA and proteasome inhibitors results in synergistic inhibition of cell growth in malignant cultures and tumors grown in animal models. To explore potential mechanisms involved, we treated K562 cells with 0.4 µM GA, 25 nM MG262, various concentrations of MG132 as single agents, or in their combinations (GA + MG132 or GA + MG262) for 12 or 24 h, followed by Western analysis to measure caspase activation and PARP cleavage. After 12-h treatment, the GA + either proteasome inhibitor, but not GA or proteasome inhibitor alone, could induce cleavage of PARP as well as cleavage/activation of caspases 8 and 9 (Fig. 4A, left panel). Similar results were observed in the cells treated for 24 h (Fig. 4A, right panel). These findings indicate that GA is able to enhance the proteasome-inhibitor-induced apoptosis via caspase-dependent pathways.
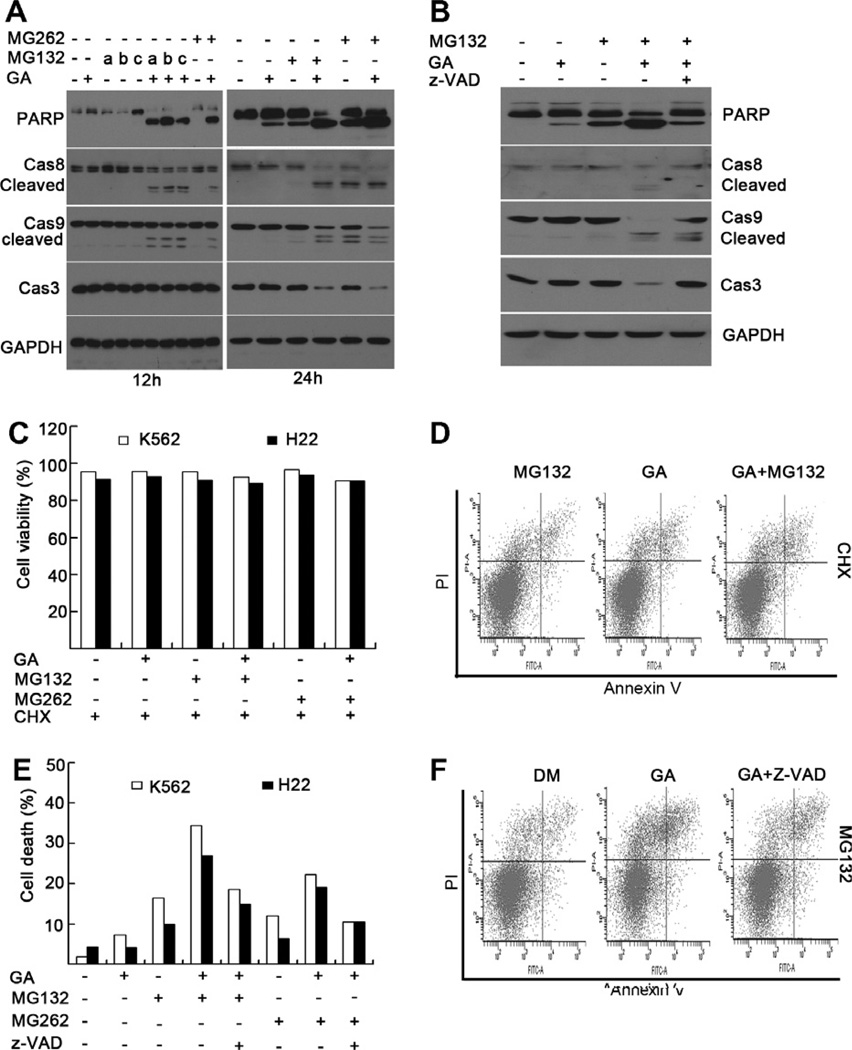
Fig. 4.
Combinational treatment with GA and proteasome inhibitors can activate caspase pathway and induce cell death, which can be blocked by caspase inhibitor (z-VAD) and protein synthesis inhibitor cycloheximide (CHX). (A) K562 cells were treated with GA (0.4 µM), MG132 (at a-0.25, b-0.5, c-1 µM for 12 h; or 0.5 µM for 24 h), MG262 (0.025 µM) or combined GA and MG132/MG262 for 12 or 24 h. Proteins extracts from the treated cells were subjected to Western blot analysis by using anti-caspase-9, anti-caspase-8, anti-caspase-3, or anti-PARP antibodies. GAPDH blot was used as a loading control. (B) K562 cells were treated with GA (0.4 µM), MG132 (1 µM), or combination of GA and MG132 for 12 h in the presence of a pan-caspase inhibitor z-VAD-fmk (20 µM), followed by Western blot analysis as in (A). (C and D) K562 or H22 cells were treated with GA (0.4 µM), MG132 (1 µM), MG262 (0.025 µM) or combination of GA and MG132/MG262 for 12 h in the presence or absence of cycloheximide (25 µg/ml), followed by flow cytometry. A summary of cell viability was shown in (C) and representative flow images from at least three independent experiments were shown in (D). (E and F) K562 or H22 cells were treated with GA (0.4 µM), MG132 (1 µM), MG262 (0.025 µM) or combination of GA and MG132/MG262 for 12 h in the presence or absence of caspase inhibitor z-VAD-fmk 20 µM), followed by flow cytometry. A summary of cell death was shown in (E) and representative images from at least three independent experiments were shown in (F).
To study if newly synthesized proteins are required for GA-mediated synergetic effect to proteasome inhibitors, we used cycloheximide (CHX), an inhibitor of protein synthesis. We found that in the presence of CHX, GA + a proteasome inhibitor was unable to give any synergetic/additive effect anymore, comparing to each treatment alone, regarding either cell viability or apoptosis, tested in both K562 and H22 cell lines (Fig. 4C and D). These results indicate that accumulation of newly synthesized pro-apoptotic proteins, such as caspases, is required for the synergetic effect of GA plus proteasome inhibitor. Co-treatment with the caspase inhibitor z-VAD-FMK partially abolished the GA-proteasome inhibitor combination-induced PARP and caspase cleavage (Fig. 4B) as well as partially rescued the induced cell death in K562 cells (Fig. 4E and F). These findings indicate that activation of caspases involved the apoptotic pathway is one of the major mechanisms by which GA exerts its synergistic effects on proteasome inhibitor-treated cancer cells.
4. Discussion
Discovery of novel agents with anticancer activity from natural resources has gained significant importance in cancer prevention and therapy. The number of natural compounds with anticancer properties discovered and tested in vitro and in vivo is increasing exponentially. However, due to the lack of potency, many of these natural compounds have failed to gain favor as single-agent anticancer drugs. Conventional chemotherapy plays an important role in the treatment of cancers, but clinical limitations exist because of dose limiting side effects and drug resistance.
Accordingly, the combinatorial use of chemotherapeutic agents and natural compounds with distinct molecular mechanisms are considered to be a promising therapeutic strategy with higher clinical efficacy and better patient survival. In recent years, more natural compounds, including genistein, curcumin, (–)-epigallocatechin–3-gallate (EGCG), and resveratrol, have been recognized as cancer chemopreventive agents because of their anticarcinogenic activity [29]. Moreover, these compounds exert their antitumor effects through modulation of different cell signaling pathways. Therefore, conventional therapies combined with these natural compounds may exert enhanced antitumor activity through synergistic or additive properties. Combination treatment may also decrease the systemic toxicity caused by chemotherapies or radiotherapies since efficacy can be achieved with lower doses. Thus far, scientists have been exploring a series of sensitizers to conventional chemotherapy and radiotherapy from natural compounds, including curcumin [30–33], genistein [34– 36], EGCG [37–40] and resveratrol [41], and many positive findings in this field have been published.
Inhibition of cell viability/proliferation and induction of apoptotic cell death are two major mechanisms by which chemotherapeutic agents kill cancer cells. To determine whether GA sensitizes cancer cells to proteasome inhibitors, we conducted cell growth inhibition and apoptosis assays. We found that MG262 or MG132 as single agents could inhibit the viability/proliferation of cancer cells and induce apoptosis. However, co-treatment with GA resulted in significantly greater inhibition of cell viability/proliferation and induction of apoptosis, suggesting that GA plays a synergistic role in proteasome inhibitor-induced apoptosis and growth inhibition in cancer cells.
To investigate whether the enhanced cell growth inhibition and apoptosis by GA also occurs in vivo, we treated allografts in KMF mice with GA, MG132 or the combination of GA plus MG132. We found that the combination treatment resulted in significant tumor growth inhibition compared to treatment with either agent alone. To achieve greater inhibitory effects in cancer cells, combination of two or more chemotherapeutic agents for treatment is commonly considered for designing better therapeutic strategies. However, combination treatment may result in varying levels of systemic toxicities [42,43]. Thus, optimization of combination chemotherapy based on molecular mechanisms may improve the therapeutic index, and such strategies are urgently needed for the treatment of cancer. In the present study, our results convincingly demonstrate that the combination of proteasome inhibitors with GA achieves greater therapeutic effect without systemic toxicities, as indicated by the results in Fig. 3, which showed no significant change in body weight of mice after treatment.
In conclusion, our results clearly show that GA is able to activate the caspase pathway and, in turn, sensitize cancer cells to growth inhibition and apoptosis induction by pro-teasome inhibitors in vitro and in vivo. These findings suggest that GA may serve as a potent chemosensitizer in the treatment of human cancers. However, further in-depth studies including clinical trials are needed to fully validate the intrinsic value of GA in combination with proteasome inhibitors, such as bortezomib (Velcade), for the treatment of human cancers.
Acknowledgements
This work was supported by The National High Technology Research and Development Program of China (Project 2006AA02Z4B5, to J.L.) and Grants from the National Cancer Institute (1R01CA120009 and 3R01CA120009-04S1, to Q.P.D.). We thank Sara Schmitt and Michael Frezza for critical reading of the manuscript.
Footnotes
Conflicts of interest
None declared.
References
- 1.Adams J. The proteasome: a suitable antineoplastic target. Nat. Rev. Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A. The ubiquitin–proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 4.Hochstrasser M, Ubiquitin proteasomes. and the regulation of intracellular protein degradation. Curr. Opin. Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 5.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 6.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 7.Orlowski RZ, Dees EC. The role of the ubiquitination–proteasome pathway in breast cancer: applying drugs that affect the ubiquitin–proteasome pathway to the therapy of breast cancer. Breast Cancer Res. 2003;5:1–7. doi: 10.1186/bcr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals) IDrugs. 2002;5:828–834. [PubMed] [Google Scholar]
- 9.Kane RC, Farrell AT, Sridhara R, Pazdur R. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin. Cancer Res. 2006;12:2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 10.Oakervee HE, Popat R, Curry N, Smith P, Morris C, Drake M, Agrawal S, Stec J, Schenkein D, Esseltine DL, Cavenagh JD. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br. J. Haematol. 2005;129:755–762. doi: 10.1111/j.1365-2141.2005.05519.x. [DOI] [PubMed] [Google Scholar]
- 11.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 12.Gruenwald TBJ, Jaenicke C. PDR for Herbal Medicines. second ed. Montvale, NJ: Medical Economics; 2000. pp. 325–326. [Google Scholar]
- 13.Yang Y, Yang L, You QD, Nie FF, Gu HY, Zhao L, Wang XT, Guo QL. Differential apoptotic induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Lett. 2007;256:259–266. doi: 10.1016/j.canlet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Zhang HZ, Kasibhatla S, Wang Y, Herich J, Guastella J, Tseng B, Drewe J, Cai SX. Discovery, characterization and SAR of gambogic acid as a potent apoptosis inducer by a HTS assay. Bioorg. Med. Chem. 2004;12:309–317. doi: 10.1016/j.bmc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Guo QL, You QD, Zhao L, Gu HY, Yuan ST. Anticancer effect and apoptosis induction of gambogic acid in human gastric cancer line BGC-823. World J. Gastroenterol. 2005;11:3655–3659. doi: 10.3748/wjg.v11.i24.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu H, Wang X, Rao S, Wang J, Zhao J, Ren FL, Mu R, Yang Y, Qi Q, Liu W, Lu N, Ling H, You Q, Guo Q. Gambogic acid mediates apoptosis as a p53 inducer through down-regulation of mdm2 in wild-type p53-expressing cancer cells. Mol. Cancer Ther. 2008;7:3298–3305. doi: 10.1158/1535-7163.MCT-08-0212. [DOI] [PubMed] [Google Scholar]
- 17.Guo QL, You QD, Wu ZQ, Yuan ST, Zhao L. General gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitro and in nude mice. Acta Pharmacol. Sin. 2004;25:769–774. [PubMed] [Google Scholar]
- 18.Pandey MK, Sung B, Ahn KS, Kunnumakkara AB, Chaturvedi MM, Aggarwal BB. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood. 2007;110:3517–3525. doi: 10.1182/blood-2007-03-079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi T, Yi Z, Cho SG, Luo J, Pandey MK, Aggarwal BB, Liu M. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008;68:1843–1850. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Guo QL, You QD, Zhao L, Gu HY, Yang Y, Zhang HW, Tan Z, Wang X. Gambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis. 2007;28:632–638. doi: 10.1093/carcin/bgl168. [DOI] [PubMed] [Google Scholar]
- 21.Zhai D, Jin C, Shiau CW, Kitada S, Satterthwait AC, Reed JC. Gambogic acid is an antagonist of antiapoptotic Bcl-2 family proteins. Mol. Cancer Ther. 2008;7:1639–1646. doi: 10.1158/1535-7163.MCT-07-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou JWWZT. Phase I human tolerability trial of gambogic acid. Chin. J. New drugs. 2007;16:79–82. [Google Scholar]
- 23.Huang H, Zhang X, Li S, Liu N, Lian W, McDowell EM, Zhou P, Zhao C, Guo H, Zhang C, Yang C, Wen G, Dong X, Lu L, Ma N, Dong W, Dou QP, Wang X, Liu J. Physiological levels of ATP negatively regulate proteasome function. Cell Res. 2010;20:1372–1385. doi: 10.1038/cr.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H, Zhou P, Huang H, Chen D, Ma N, Cui QC, Shen S, Dong W, Zhang X, Lian W, Wang X, Dou QP, Liu J. Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. Int. J. Cancer. 2009;124:2450–2459. doi: 10.1002/ijc.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 26.An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts, human fibroblasts. Cell Death Differ. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 27.Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem. Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Lopes UG, Erhardt P, Yao R, Cooper GM. p53-Dependent induction of apoptosis by proteasome inhibitors. J. Biol. Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 29.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 30.Lev-Ari S, Strier L, Kazanov D, Madar-Shapiro L, Dvory-Sobol H, Pinchuk I, Marian B, Lichtenberg D, Arber N. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin. Cancer Res. 2005;11:6738–6744. doi: 10.1158/1078-0432.CCR-05-0171. [DOI] [PubMed] [Google Scholar]
- 31.Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D’Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, Price JE. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 33.Bava SV, Puliappadamba VT, Deepti A, Nair A, Karunagaran D, Anto RJ. Sensitization of taxol-induced apoptosis by curcumin involves down-regulation of nuclear factor-kappaB and the serine/threonine kinase Akt and is independent of tubulin polymerization. J. Biol. Chem. 2005;280:6301–6308. doi: 10.1074/jbc.M410647200. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, Philip PA, Abbruzzese J, Sarkar FH. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 36.Hillman GG, Forman JD, Kucuk O, Yudelev M, Maughan RL, Rubio J, Layer A, Tekyi-Mensah S, Abrams J, Sarkar FH. Genistein potentiates the radiation effect on prostate carcinoma cells. Clin. Cancer Res. 2001;7:382–390. [PubMed] [Google Scholar]
- 37.Chisholm K, Bray BJ, Rosengren RJ. Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to MDA-MB-231 human breast cancer cells. Anticancer Drugs. 2004;15:889–897. doi: 10.1097/00001813-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Wei D, Liu J. In vivo reversal of doxorubicin resistance by (—)-epigallocatechin gallate in a solid human carcinoma xenograft. Cancer Lett. 2004;208:179–186. doi: 10.1016/j.canlet.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 39.McLaughlin N, Annabi B, Lachambre MP, Kim KS, Bahary JP, Moumdjian R, Beliveau R. Combined low dose ionizing radiation and green tea-derived epigallocatechin-3-gallate treatment induces human brain endothelial cells death. J. Neurooncol. 2006;80:111–121. doi: 10.1007/s11060-006-9171-8. [DOI] [PubMed] [Google Scholar]
- 40.Lesnick C, Kay NE, LaPlant B, Shanafelt TD. The green tea extract EGCG demonstrates synergist activity against CLL B-Cells when combined with fludarabine and chlorambucil. New Orleans: 51th American Society of Hematology Annual Meeting; 2009. [Google Scholar]
- 41.Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin’s lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol. Cancer Ther. 2004;3:71–84. [PubMed] [Google Scholar]
- 42.Ravdin PM, Burris HA, 3rd, Cook G, Eisenberg P, Kane M, Bierman WA, Mortimer J, Genevois E, Bellet RE. Phase II trial of docetaxel in advanced anthracycline-resistant or anthracenedioneresistant breast cancer. J. Clin. Oncol. 1995;13:2879–2885. doi: 10.1200/JCO.1995.13.12.2879. [DOI] [PubMed] [Google Scholar]
- 43.To H, Ohdo S, Shin M, Uchimaru H, Yukawa E, Higuchi S, Fujimura A, Kobayashi E. Dosing time dependency of doxorubicininduced cardiotoxicity and bone marrow toxicity in rats. J. Pharm. Pharmacol. 2003;55:803–810. doi: 10.1211/002235703765951410. [DOI] [PubMed] [Google Scholar]