Abstract
Reduced serotonergic neurotransmission is implicated in impulsive behavior. We studied the triallelic system of the serotonin transporter gene linked polymorphic region (5-HTTLPR) and acute manipulation of serotonin together to further delineate the mechanisms by which serotonergic neurotransmission affects impulsivity. Fifty-two healthy participants (38 men and 14 women) underwent acute tryptophan depletion (ATD) or placebo in a randomized, double-blind, parallel group experiment. Impulsive response style was measured on two versions of the Continuous Performance Task (CPT), and calculated using signal detection theory. We observed a dose-dependent effect for the short (S′) allele of the 5-HTTLPR on impulsive response style. Individuals who had the S′/S′ genotype were more impulsive than individuals with the L/S′ genotype. Participants with the L/S′ genotype were more impulsive than those with the L/L genotype. ATD increased impulsivity in men, and decreased impulsivity in women. These data demonstrate for the first time that reduced serotonergic tone as a result of either 5-HTTLPR genotype, or experimental ATD, are both independently and additively, associated with elevated impulsive response style in Caucasian men.
Keywords: Serotonin, 5-HTTLPR, Genetics, Impulsivity, Signal detection theory, Tryptophan depletion
A common polymorphism in the promoter region of the SLC6A4, the gene encoding the 5-HT transporter (5-HTT) protein, is a candidate gene region for studying the relationship between impulsivity and serotonin (5-HT) neurotransmission. This polymorphism (5-HTTLPR) results in a short (S) or long (L) allele. The S allele is associated with decreased transcriptional efficiency compared with the L allele [20,24] and is proposed to be a marker of less efficient 5-HTT functioning [31]. S allele carriers were found to have blunted central 5-HT function measured by fenfluramine-induced prolactin release [32] and have reduced levels of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) [47]. Recent data suggest that the 5-HTTLPR is triallelic with an A>G single nucleotide polymorphism (rs25531) in the first of two 22-bp imperfect repeats that define the 16-repeat L allele. LA is associated with higher expression of the transporter protein compared to the S and LG which are the low expression alleles [21,37].
Acute tryptophan depletion (ATD) is a widely used paradigm to study the effects of reduced 5-HT transmission on behavior [5] in several species including rodents, monkeys and humans. ATD reduces 5-HT function in the periphery and in the brain as indicated both by reductions in both plasma tryptophan and cerebrospinal fluid 5-HIAA concentrations [5]. Additionally, ATD decreases brain 5-HT synthesis and release [29]. Human studies carried out on high risk or predisposed individuals have found that ATD increases impulsive and aggressive behavior [2,10,22]. Recent rodent literature indicates that behavioral response to ATD is mediated by the SLC6A4 gene [30] and studying both together in humans is a potentially fruitful endeavor [19].
We studied the effects of 5-HTTLPR genotype and ATD on impulsive response style among healthy participants to further clarify the relationship between 5-HT neurotransmission and behavioral impulsivity. The participants were genotyped for the triallelic 5-HTTLPR [21,37], and were given placebo or ATD to experimentally reduce 5-HT neurotransmission. To measure impulsive response style, we used two versions of the Continuous Performance Task (CPT) and applied signal detection theory to calculate the participants’ preference to respond/not-respond. We expected participants with reduced 5-HT neurotransmission, either naturally through the 5-HTTLPR genotype or experimental during ATD, to express an increased impulsive response style. We discovered that reduced 5-HT increased impulsive response style in male participants.
Eighty-five students were recruited at the University of Oslo and screened for medical and mental health problems. Twenty-eight women were taking birth-control pills or other medication regulating hormones and were removed because ovarian steroids such as estrogen, the primary female sex hormone, affect 5-HT regulation [17,34,38].We could not extract the triallelic genotype for two women due to poor DNA quality. Three men did not complete the study because they could not tolerate the amino acid beverage. In total, 33 students were removed from the data set. The final sample consisted of 38 healthy men (mean age 24.2±2.9; range 20–33 years) and 14 healthy women (mean age 24.0±1.9; range 22–29 years) who were not taking any medication at the time. The women were tested during the luteal phase of their menstrual cycle. Alcohol use was not permitted 24 h before and during the course of the study, and all the participants were told to arrive well rested. We did not collect blood alcohol concentration or inquire about potential sleep debt, which would have better insured against these potential influences on the performance of the participants.
The study was carried out in accordance with the Helsinki Declaration. The protocol was reviewed and approved by the Norwegian Regional Committee for Medical Research Ethics. The study used a randomized double-blind placebo-controlled parallel group design. ATD was achieved following an overnight fast with a mixture of 15 amino acids devoid of tryptophan as described previously [1,5]. The composition of the active ATD and sham depletion mixture was based on the 100 g recipe previously used for men [1,45], but reduced to 86 g for women to adjust for differences in body mass [28]. During sham depletion, tryptophan was added to the amino acid mixture. Neuropsychological measures were collected approximately 6 h after the ingestion of the amino acid mixture, at the time of predicted peak effects of ATD [5].
Impulsivity was measured using two versions of the CPT test: the Immediate Memory Task (IMT) [12,13] and the Continuous Performance Test-Identical Pairs (CPT-IP) [9]. These are computer based neuropsychological tests that display numbers or nonsense shapes in rapid succession, record the response of the participants and calculate the output variables. The participants are instructed to quickly respond whenever two identical stimuli are presented in a row. A correct identification of a matching set is called “hit,” while a response to a stimulus similar to (but not identical to) the preceding stimuli is called “false alarm.” IMT and CPT-IP are both rapid-response tasks found to be valid [39] and reliable [26] measures of impulsivity.
Signal detection analysis combines the frequency of hits and false alarms into d′ and β to differentiate between the physiological capabilities to detect a signal (d′), and the willingness to report it (β). This also allows for controlling of false positive or negative data in cases where the participants are unengaged in the task and provide responses in a purely randomized manner. The d′ is a measure of the participant’s ability to discriminate between stimuli and reflect the observer’s attention and sensory capacity [25,40]. The β (also called impulsive response style, criterion and response bias) gives an individual’s preference to respond/not-respond in situations with competition between behavioral suppression and active responding. A tendency to over respond (low β) is considered risk taking and impulsive [7,35,36]. Low β is also associated with ADHD symptoms [14], adolescents with conduct and substance problems, Eysenck Impulsiveness Scale and the impulsivity factor in the Antisocial Process Screening Device [41]. β has also been related to changes in 5-HT neurotransmission [3,45]. β is a function of the ratio of target to nontarget stimuli and the participant’s tendency to respond too little or too much relative to the actual distribution of the signal. For a complete discussion on signal detection theory, see Swets et al. and Levine and Parkinson [25,40]. Easy to use “signal detection calculators” can be found on the internet (i.e. http://www.computerpsych.com/onlinedt.php).
For genotyping and assessment of tryptophan concentration, see previously published article [46]. The LG and S alleles are considered functionally equivalent and were grouped together as S′ (lower expressing allele); the LA allele was designated as L (the higher expressing allele).
The signal detection parameter β was collected from both CPT-IP and IMT and averaged to total scores. β was analyzed using a univariate general linear model with 5-HTTLPR genotype (S′/S′, L/S′, and L/L), intervention (ATD vs. sham depletion) and sex as between-subject factors. The significance for main effects and higher order interactions for β was interpreted at p < 0.05 two-tailed test.
As predicted, ATD significantly decreased plasma concentrations of total [t(27) = 27, p < 0.001] and free [t(26) = 17, p < 0.001] tryptophan by 84% and 79%, respectively. Sham depletion significantly increased the concentrations of total [t(23) = 6, p < 0.001] and free [t(23) = 6, p < 0.001] tryptophan. Sex and 5-HTTLPR genotype had no influence on tryptophan levels. These results show successful tryptophan depletion, comparable with other ATD studies [1,5,45]. The 5-HTTLPR genotypes [EA: χ2(2) = 0.66, p = 0.72] and rs25531 [EA: χ2(2) = 0.10, p = 0.95] were in Hardy Weinberg Equilibrium, consistent with prior studies of populations of Northern European Ancestry.
We analyzed the effects of 5-HTTLPR genotype, ATD intervention and sex on impulsive response style (β) to determine if 5-HT neurotransmission affects behavioral impulsivity.
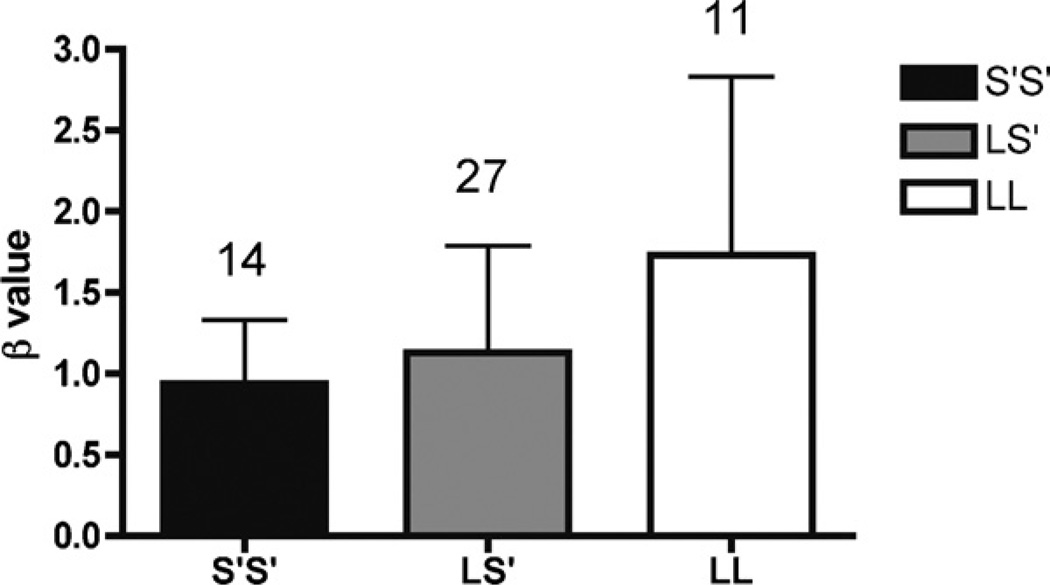
The overall model was statistically significant [adjusted R2 = 0.49(11,51), p < 0.001]. β was found to be significantly influenced by genotype [F(2,51) = 4.0, p = 0.026] (Fig. 1), intervention [F(1,51) = 6.8, p = 0.013] and sex of the participants [F(1,51) = 8.6, p = 0.006]. There was a significant ATD × sex interaction [F(1,51) = 22, p < 0.001], but the ATD × genotype interaction did not reach statistical significance [F(2,51) = 2.6, p = 0.089]. The ATD × sex interaction was produced by opposite reactions to the intervention in men and women. Tryptophan depleted men became more impulsive and tryptophan depleted women became less impulsive. The three-way interaction for genotype × intervention × sex [F(2,51) = 5.0, p = 0.011] relates to the main effect of genotype and the intervention × sex interaction. The S′/S′ genotype was the most impulsive and the L/L allele was the most cautious on average for both sexes, in addition to a sex-dependent effect from the ATD intervention on impulsivity.
Fig. 1.
Male and female participants. Low β indicates higher impulsivity. The effects of 5-HTTLPR genotype on impulsive response style (β) in male (N= 38) and female (N= 14). Individuals who had the S′/S′ genotype were more impulsive than individuals with the L/S′ genotype, who in turn were more impulsive than those with the L/L genotype (p = 0.026). The number of participants in each group is displayed on top of the bars, and the Whiskers indicate standard deviations.
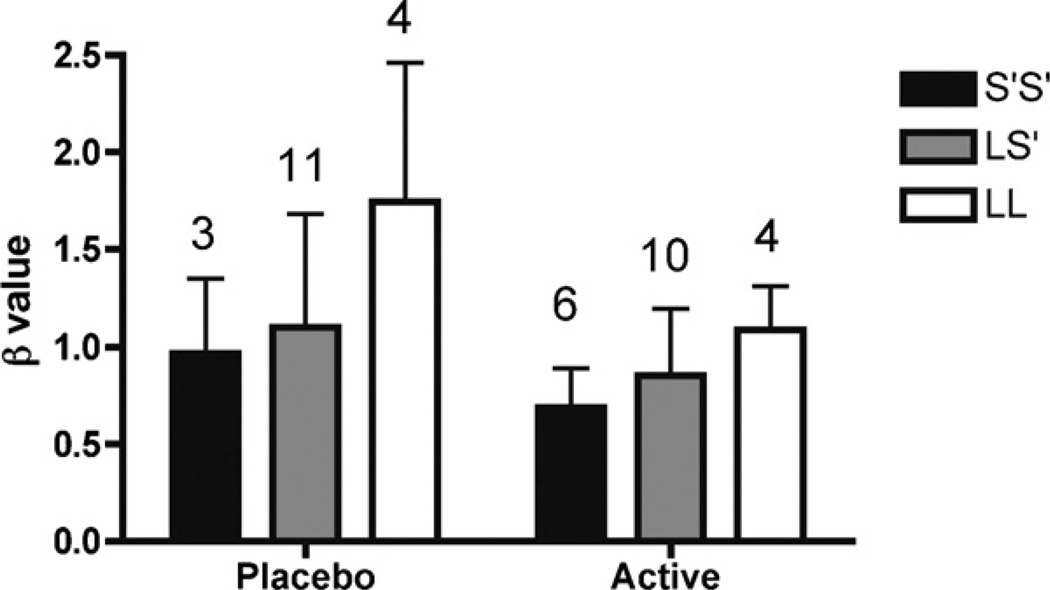
Given that the effect from 5-HT manipulation on β was sex-dependent, further analyses on impulsivity was carried out separately for men and women. Men had significant main effects for genotype [F(2,37) = 3.8, p < 0.033] and intervention [F(1,37) = 5.7, p < 0.023], but no genotype × intervention interaction (Fig. 2). Women had a significant main effect for intervention [F(1,13) = 8.4, p < 0.020], but not for genotype and no interaction. The tryptophan depleted women were more cautious compared to the sham depleted women.
Fig. 2.
Male participants only. Low β indicates higher impulsivity. The effects of 5-HTTLPR genotype and acute tryptophan depletion (ATD) on impulsive response style (β) in healthy men. Individuals who had the S′/S′ genotype were more impulsive than individuals with the L/S′ genotype, who in turn were more impulsive than those with the L/L genotype (p = 0.033). Furthermore, the tryptophan depleted men (active) were more impulsive compared to sham depleted men(p = 0.023). The number of participants in each group is displayed on top of the bars, and the Whiskers indicate standard deviations.
The results demonstrate a dose-dependent effect for the S′ allele of the 5-HTTLPR on a neuropsychological measure of impulsivity. Individuals with the S′/S′ genotype were more impulsive than individuals with the L/S′ genotype. Those with the L/S′ genotype were more impulsive than those with the L/L genotype. The effect of ATD on impulsivity was sex-dependent. Reduced 5-HT neurotrans-mission increased impulsivity in men, but decreased impulsivity in women. In the male participants, reduced 5-HT as a result of either 5-HTTLPR genotype or ATD was associated with elevated impulsive response style, confirming the research hypothesis.
Current research supports investigating sex effects and 5-HTTLPR together when studying psychiatric disorders related to impulsivity [42]. A study by Cadoret et al. [4] investigating an adoptee population found that males with the S allele were more likely to have externalizing behavior like conduct disorder, aggressivity and ADHD. In contrast, the females with the S allele were less likely to display such behavior [4]. The results from the male part of our sample are consistent with the findings of Cadoret et al. [4], further demonstrating an association between 5-HTTLPR S allele and impulsivity in men. Conversely, a recent research study in individuals with alcohol use disorder reported that male L homozygotes and females S homozygotes self-reported the highest levels of antisocial behavior [18].
A review of ATD experiments using neuropsychological measures of impulsivity indicates that some studies report a null effect between ATD and impulsivity [6,8,11,16,23,33]. Other studies found increased impulsivity in response to ATD in normal controls [13], and in nonalcoholic men with a family history of alcoholism [10,22]. Finally, three studies found increased impulsive response style (β) in response to ATD [3,45,46]. All studies using the signal detection theory to calculate β from the frequency of false alarms and correct responses observed an effect of ATD on impulsivity. ATD studies exclusively analyzing false alarms (response inhibition) or reaction times frequently report negative findings [6,8,16,23,33].
Several methodological issues are relevant when discussing why some report effects of ATD whereas others do not. First is the question of how impulsivity is measured [15]. Self-report scales do not lend themselves easily to pharmacological studies of impulsivity because they are subjective and measure relatively stable characteristics. The use of neuropsychological computer measures such as the Continuous Performance Test (CPT) has been recommended to determine impulsivity [39,41].A second methodological issue is the carry-over effect identified in ATD studies [44]. ATD studies using a cross-over design [6,11,16,23,33] must insure the intervention has the same effect for those given ATD on the first test day when the environment is novel, compared with those given ATD on the second test day when the environment is familiar. To avoid a type II error caused by carry-over in ATD studies, one should use a between-subject parallel design similar to this study, or analyzing the first test day only. A third methodological issue stems from reports that free plasma tryptophan must be reduced by at least 60% for the ATD to have an effect [27,43]. ATD studies using amino acid mixtures smaller than the original 100 g [6,8,11,16], may not reach the threshold required to obtain behavioral effects following ATD. A fourth methodological issue is the sex-dependent effect of ATD on impulsivity [46]. ATD studies including female participants [6,11,28,33] must have a large cohort and use sex as a between-subjects factor to observe an effect of ATD on impulsivity.
The effect of 5-HTTLPR genotype on central nervous system (CNS) 5-HT levels may depend on sex and race [48]. Using cerebrospinal fluid levels of 5-HIAA as an index for CNS 5-HT turnover, Williams et al. found that the S/S genotype was associated with lower 5-HIAA levels in men, but with higher levels in women. Furthermore, the S/S genotype was associated with lower 5-HIAA levels in Caucasian, but with higher levels in African-Americans [48]. A main limitation to our study is that our results are only valid in a Caucasian population. Future studies would benefit from ethnically diverse samples including African-American and Asian individuals. Additionally, studying other gene polymorphisms in the 5-HT system, using the same impulsivity measurement and ATD intervention, may increasingly clarify the mechanisms by which 5-HT neurotransmission affects impulsivity.
In conclusion, we observed that the S′ allele of the 5-HTTLPR and ATD independently increased impulsivity in men. The S′ allele is a marker of less efficient 5-HT functioning in Caucasian men, and ATD is an experimental intervention commonly used to reduce the CNS levels of 5-HT. The S′ allele of the 5-HTTLPR was associated with increased impulsivity in a dose-dependent manner for the whole sample, but it should be noted that the sample comprised more than twice as many males than females and thus may not apply to the female participants as a subset. Our results support the hypothesis that reduced 5-HT transmission increases impulsive response style, at least for Caucasian men.
Acknowledgements
This study was supported by grants from the Research Council of Norway, Division for Science and the Department of Psychology, University of Oslo. Additionally this study was funded by K12 DA000167-18 award. The authors would also like to thank Natalie Johnston for acquiring and managing the behavioral data.
References
- 1.Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young SN. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch. Gen. Psychiatry. 1994;51:687–697. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- 2.Bjork JM, Dougherty DM, Moeller FG, Swann AC. Differential behavioral effects of plasma tryptophan depletion and loading in aggressive and nonaggressive men. Neuropsychopharmacology. 2000;22:357–369. doi: 10.1016/S0893-133X(99)00136-0. [DOI] [PubMed] [Google Scholar]
- 3.Booij L, Swenne CA, Brosschot JF, Haffmans PMJ, Thayer JF, Van der Does AJW. Tryptophan depletion affects heart rate variability and impulsivity in remitted depressed patients with a history of suicidal ideation. Biol. Psychiatry. 2006;60:507–514. doi: 10.1016/j.biopsych.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Cadoret RJ, Langbehn D, Caspers K, Troughton EP, Yucuis R, Sandhu HK, Philibert R. Associations of the serotonin transporter promoter polymorphism with aggressivity, attention deficit, and conduct disorder in an adoptee population. Compr. Psychiatry. 2003;44:88–101. doi: 10.1053/comp.2003.50018. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter LL, Anderson GM, Pelton GH, Gudin JA, Kirwin PDS, Price LH, Heninger GR, McDougle CJ. Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology. 1998;19:26–35. doi: 10.1016/S0893-133X(97)00198-X. [DOI] [PubMed] [Google Scholar]
- 6.Clark L, Roiser JP, Cools R, Rubinsztein DC, Sahakian BJ, Robbins TW. Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology. 2005;182:570–578. doi: 10.1007/s00213-005-0104-6. [DOI] [PubMed] [Google Scholar]
- 7.Conners CK, Epstein JN, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. J. Abnorm. Child Psychol. 2003;31:555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- 8.Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW. Tryptophan depletion disrupts the motivational guidance of goal-directed behavior as a function of trait impulsivity. Neuropsychopharmacology. 2005;30:1362–1373. doi: 10.1038/sj.npp.1300704. [DOI] [PubMed] [Google Scholar]
- 9.Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res. 1989;29:65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- 10.Crean J, Richards JB, de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behav. Brain Res. 2002;136:349–357. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 11.Crockett MJ, Clark L, Robbins TW. Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J. Neurosci. 2009;29:11993–11999. doi: 10.1523/JNEUROSCI.2513-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougherty DM, Marsh DM. Immediate & Delayed Memory Tasks (IMT/DMT 2.0): A Research Tool for Studying Attention, Memory, and Impulsive Behavior, Neurobehavioral Research Laboratory and Clinic. Houston: University of Texas Health Science Center at Houston; 2003. [Google Scholar]
- 13.Dougherty DM, Marsh DM, Mathias CW, Dawes MA, Bradley DM, Morgan CJ, Badawy AAB. The effects of alcohol on laboratory-measured impulsivity after l-tryptophan depletion or loading. Psychopharmacology. 2007;193:137–150. doi: 10.1007/s00213-007-0763-6. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between continuous performance test performance measures and ADHD behaviors. J. Abnorm. Child Psychol. 2003;31:543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- 15.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl.) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 16.Evers EAT, van der Veen FM, Jolles J, Deutz NEP, Schmitt JAJ. Acute tryptophan depletion improves performance and modulates the BOLD response during a Stroop task in healthy females. Neuroimage. 2006;32:248–255. doi: 10.1016/j.neuroimage.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Fink G, Sumner BEH, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin. Exp. Pharmacol. Physiol. 1998;25:764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 18.Herman AI, Connor TS, Kranzler HR, Anton RF, Gelernter J, Covault J. Variation in the gene encoding the serotonin transporter is associated with ameasure of sociopathy in alcoholics. Addict. Biol. 2010 doi: 10.1111/j.1369-1600.2009.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homberg JR, Pattij T, Janssen MCW, Ronken E, Boer SFD, Schoffelmeer ANM, Cuppen E. Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility. Eur. J. Neurosci. 2007;26:2066–2073. doi: 10.1111/j.1460-9568.2007.05839.x. [DOI] [PubMed] [Google Scholar]
- 20.Hranilovic D, Stefulj J, Schwab S, Borrmann-Hassenbach M, Albus M, Jernej B, Wildenauer D. Serotonin transporter promoter and intron 2 polymorphisms: relationship between allelic variants and gene expression. Biol. Psychiatry. 2004;55:1090–1094. doi: 10.1016/j.biopsych.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin. Exp. Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 22.LeMarquand DG, Benkelfat C, Pihl RO, Palmour RM, Young SN. Behavioral disinhibition induced by tryptophan depletion in nonalcoholic youngmenwith multigenerational family histories of paternal alcoholism. Am. J. Psychiatry. 1999;156:1771–1779. doi: 10.1176/ajp.156.11.1771. [DOI] [PubMed] [Google Scholar]
- 23.LeMarquand DG, Pihl RO, Young SN, Tremblay RE, Seguin JR, Palmour RM, Benkelfat C. Tryptophan depletion, executive functions, and disinhibition in aggressive, adolescent males. Neuropsychopharmacology. 1998;19:333–341. doi: 10.1016/S0893-133X(98)00029-3. [DOI] [PubMed] [Google Scholar]
- 24.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 25.Levine G, Parkinson S. In: Experimental Methods in Psychology. Erlbaum L, editor. Hillsdale, NJ: 1994. [Google Scholar]
- 26.Mathias CW, Marsh DM, Dougherty DM. Reliability estimates for the immediate and delayed memory tasks. Percept. Mot. Skills. 2002;95:559–569. doi: 10.2466/pms.2002.95.2.559. [DOI] [PubMed] [Google Scholar]
- 27.Merens W, van der Does W. Low-dose tryptophan depletion. Biol. Psychiatry. 2007;62:542–543. doi: 10.1016/j.biopsych.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl.) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- 29.Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olivier JD, Jans LA, Korte-Bouws GA, Korte SM, Deen PM, Cools AR, Ellenbroek BA, Blokland A. Acute tryptophan depletion dose dependently impairs object memory in serotonin transporter knockout rats. Psychopharmacology (Berl.) 2008;200:243–254. doi: 10.1007/s00213-008-1201-0. [DOI] [PubMed] [Google Scholar]
- 31.Paaver M, Nordquist N, Parik J, Harro M, Oreland L, Harro J. Platelet MAO activity and the 5-HTT gene promoter polymorphism are associated with impulsivity and cognitive style in visual information processing. Psychopharmacology. 2007;194:545–554. doi: 10.1007/s00213-007-0867-z. [DOI] [PubMed] [Google Scholar]
- 32.Reist C, Mazzanti C, Vu R, Tran D, Goldman D. Rapid publication – serotonin transporter promoter polymorphism is associated with attenuated prolactin response to fenfluramine. Am. J. Med. Genet. 2001;105:363–368. doi: 10.1002/ajmg.1360. [DOI] [PubMed] [Google Scholar]
- 33.Rubia K, Lee F, Cleare AJ, Tunstall N, Fu CH, Brammer M, McGuire P. Tryptophan depletion reduces right inferior prefrontal activation during response inhibition in fast, event-related fMRI. Psychopharmacology (Berl.) 2005;179:791–803. doi: 10.1007/s00213-004-2116-z. [DOI] [PubMed] [Google Scholar]
- 34.Rubinow DR, Schmidt PJ, Roca CA. Estrogen–serotonin interactions: implications for affective regulation. Biol. Psychiatry. 1998;44:839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 35.Rutschmann J, Cornblatt B, Erlenmeyer-Kimling L. Sustained attention in children at risk for schizophrenia. Report on a continuous performance test. Arch. Gen. Psychiatry. 1977;34:571–575. doi: 10.1001/archpsyc.1977.01770170081007. [DOI] [PubMed] [Google Scholar]
- 36.Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1998. [Google Scholar]
- 37.Stein MB, Seedat S, Gelernter J. Serotonin transporter gene promoter polymorphism predicts SSRI response in generalized social anxiety disorder. Psychopharmacology (Berl.) 2006;187:68–72. doi: 10.1007/s00213-006-0349-8. [DOI] [PubMed] [Google Scholar]
- 38.Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier KH, Fink G. Raloxifene blocks estradiol induction of the serotonin transporter and 5- hydroxytryptamine 2A receptor in female rat brain. Neurosci. Lett. 2007;417:95–99. doi: 10.1016/j.neulet.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 39.Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biol. Psychiatry. 2002;51:988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- 40.Swets J, Tanner WP, Jr, Birdsall TG. Decision processes in perception. Psychol. Rev. 1961;68:301–340. [PubMed] [Google Scholar]
- 41.Thompson LL, Whitmore EA, Raymond KM, Crowley TJ. Measuring impulsivity in adolescents with serious substance and conduct problems. Assessment. 2006;13:3–15. doi: 10.1177/1073191105282247. [DOI] [PubMed] [Google Scholar]
- 42.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol. Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- 43.Van der does AJW. The mood-lowering effect of tryptophan depletion: possible explanation for discrepant findings. Arch. Gen. Psychiatry. 2001;58:200–201. doi: 10.1001/archpsyc.58.2.200. [DOI] [PubMed] [Google Scholar]
- 44.Walderhaug E, Landro NI, Magnusson A. A synergic effect between lowered serotonin and novel situations on impulsivity measured by CPT. J. Clin. Exp. Neuropsychol. 2008;30:204–211. doi: 10.1080/13803390701346311. [DOI] [PubMed] [Google Scholar]
- 45.Walderhaug E, Lunde H, Nordvik JE, Landro NI, Refsum H, Magnusson A. Lowering of serotonin by rapid tryptophan depletion increases impulsiveness in normal individuals. Psychopharmacology (Berl.) 2002;164:385–391. doi: 10.1007/s00213-002-1238-4. [DOI] [PubMed] [Google Scholar]
- 46.Walderhaug E, Magnusson A, Neumeister A, Lappalainen J, Lunde H, Refsum H, Landro NI. Interactive effects of sex and 5-HTTLPR on mood and impulsivity during tryptophan depletion in healthy people. Biol. Psychiatry. 2007;62:593–599. doi: 10.1016/j.biopsych.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Central nervous system serotonin function and cardiovascular responses to stress. Psychosom. Med. 2001;63:300–305. doi: 10.1097/00006842-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]