Abstract
Understanding the underlying mechanisms that cause and exacerbate allergic asthmatic disease is of great clinical interest. Clinical studies have revealed that allergies and viral respiratory illnesses are strongly linked to the inception and exacerbation of asthma, and suggest the possibility that there are interactive inflammatory mechanisms. Recent work has revealed a number of mechanisms of virus and allergen cross-talk that may play a role in the pathophysiology of allergic asthma, including (1) deficiency in virus-induced interferon responses, (2) defective epithelial barrier function, (3) increased release of epithelium-derived cytokines (e.g., thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, IL-33), (4) dysregulation of lymphocytes (e.g., innate lymphoid cells (ILCs), regulatory T cells (Tregs)) and (5) altered activation of purinergic receptors. One or more of these processes may provide targets for new therapeutics to treat allergic asthma and prevent disease exacerbation.
Keywords: Asthma, Virus, Allergen, Atopy, Interactions, Rhinovirus, Exacerbations, eATP, TSLP, IL-33, IL-25, Treg, ILC, P2X7, FcεR1, Dendritic cells
Introduction
Viruses, allergens, and interactions between the two have been linked to the inception of asthma as well as exacerbations of established asthma. Asthma exacerbations and their associated costs have a significant clinical and societal impact including school/work absenteeism, interference with parental schedules, costs of medications, emergency department visits, and hospitalizations [1]. Given the increasing prevalence, cost and utilization of healthcare resources, and effect on long-term lung function, it is important to gain new insights into causes of asthma inception and exacerbation.
Viruses and Allergens in Disease Inception
Wheezing illnesses due to viral respiratory infections are frequent in childhood and are a common cause of morbidity, hospitalization, and utilization of healthcare resources. [2]. Up to 50% of children have had a wheezing illness by the age of six [3]. Since the first wheezing illnesses for most children with asthma are viral in origin, and the clinical features of viral wheeze and asthma are quite similar, this led to speculation that some viral respiratory infections could cause asthma. In fact, wheezing illnesses due to respiratory syncytial virus (RSV) infections were found to increase the risk of asthma in school aged children [4-6]. Whether severe RSV illnesses cause asthma is an area of significant controversy, with evidence supporting [6] and opposing [7] this theory. In an interventional study, treatment of mildly premature infants with palivizumab prevented recurrent wheeze, but only in non-atopic infants [8].
Further studies undertaken with improved molecular diagnostics suggest that the etiology of viral wheezing affects the risk of subsequent asthma. Infants who were hospitalized for human rhinovirus (HRV) wheezing illnesses in infancy were more likely (OR 4.14) to develop asthma compared to those infants hospitalized with wheezing illnesses due to other viruses [9]. A prospective birth cohort study found that among viral wheezing illnesses in infancy, human rhinovirus (HRV) infections were the most significant predictors of the subsequent development of asthma at age six [10]. While wheezing illnesses due to human rhinovirus (HRV) were associated with nearly a 10-fold increase in asthma at age six, the risk was noted to be even greater when infections occurred in the third year of life. Similarly, human rhinovirus (HRV) wheezing was a risk factor for subsequent asthma in a birth cohort based in Perth, Australia, especially if there were signs of atopy [11].
Atopy is a well-recognized risk factor for childhood asthma, and this is particularly true for children who are sensitized to allergens in the first two years [12]. While allergen sensitization has long been a recognized contributor to asthma inception, it was previously unclear whether allergen sensitization or early viral infections with wheezing occurred first. Recent studies have suggested that sensitization precedes viral wheezing. When examining allergic sensitization and viral wheezing in high-risk children enrolled in a prospective birth cohort, it was demonstrated that sensitization to aeroallergens in the first year of life predisposes children to HRV-induced wheezing illnesses [13]••.
Viruses and Allergens in Disease Exacerbation
Viral respiratory infections are important causes of exacerbations in both children and adults with asthma [14-16]. Heymann and colleagues evaluated viral infections in relation to age, atopic status, and season in which children had been admitted for wheezing [17]. In children younger than three years of age, RSV was the predominant virus isolated in winter admissions, but between the months of April and November, HRV was the most common virus isolated. Similarly, in patients older than three years of age, HRV was the most commonly identified virus and the only virus associated with wheezing [17, 18]•. In the outpatient setting, 80-85% of asthma exacerbations in children are also due to viral illnesses and up to two-thirds of these have been attributed to HRV infection [15, 19]. Interestingly, HRV burden in the lower airway was similar between asthmatics and nonatopic healthy adults, suggesting that mechanisms other than viral load account for the enhanced cold symptoms seen in asthmatics [19].
When examining the interplay between atopy and viral-induced wheeze, higher levels of immunoglobulin (Ig)E and more evidence of wheezing were found in children older than three years of age with wheezing [17]. Subsequent studies have demonstrated that concurrent viral illness and allergic sensitization increases the likelihood of asthma exacerbations and hospitalizations due to severe symptoms [20, 21]. Analysis of weekly samples of nasal secretions from asthmatic children during peak HRV seasons revealed that both virus-positive weeks and allergic sensitization were associated with greater cold and asthma symptom severity [21]. Although rates of infection were similar between sensitized and non-sensitized children, those sensitized to aeroallergens had 47% more virus-associated illnesses per season. In adults, a similar synergism between viruses and allergies was seen in asthmatics admitted to hospitals for exacerbations [20]. Interestingly, a recent study examining the serum of 287 school-age asthmatic children demonstrated that increasing serum titers of allergen-specific IgE antibody substantially increased the probability of HRV-induced wheeze, supporting a role for atopy in modulating host anti-viral responses [22]•. Finally, seasonal peaks for asthma exacerbations correlate with seasonal increases in viral illnesses, and significant peaks in wheezing illnesses and asthma have been noted in the fall [23, 24], with a smaller peak in the spring months. These peaks may be in part due to viral infections but may also correlate with fall and spring allergy seasons, which may be contributing to illness severity.
Human rhinoviruses are classified into three species (A, B and C), and there is evidence that some HRVs may be more virulent with respect to respiratory illnesses and asthma symptoms. In children aged 2-16 years evaluated for acute asthma exacerbations, HRV C viral infections were associated with greater symptoms than the HRV A or B infections [25]. Other studies investigating relationships between HRV infections and illness have noted increased pathogenicity from both A and C strains [26-29]. A recent study described a correlation between winter seasonality and increased severity of HRV illnesses [29]•. In concordance with previous studies, HRV A and C strains were found to be more virulent than B strains in infants with risk for developing asthma and allergies, and HRV A and C strains were more associated with wheezing illnesses.
In experimental HRV inoculation studies of adult volunteers with vs. without asthma, cold symptoms severity is generally similar, while lower airway symptoms are increased with asthma [30, 31]. Notably, HRV infection enhanced eosinophilic responses to inhaled allergen in allergic individuals [32], but administration of inhaled allergen before HRV inoculation had little or no effect in the severity and duration of cold symptoms in adult rhinitis and asthmatic subjects [33, 34]. Thus, virus/allergen interactions that are observed in clinical studies have been difficult to reproduce in experimental infection models.
Mechanisms of virus-allergen interactions
It is widely accepted that allergic diseases are the result of an exaggerated immune response, leading to sensitivity to otherwise innocuous substances (i.e., allergens). Most allergic responses ultimately revolve around the activation of Th2-skewed CD4+ T cells, and recent work has revealed new insights into the mediators and immune cells that modulate the differentiation and activation of Th2 cells. There have been many proposed mechanisms to explain how allergy and viral infections interact, and some of the more recent hypotheses in the context of allergic asthma inception and exacerbation are discussed below, and summarized in Figure 1.
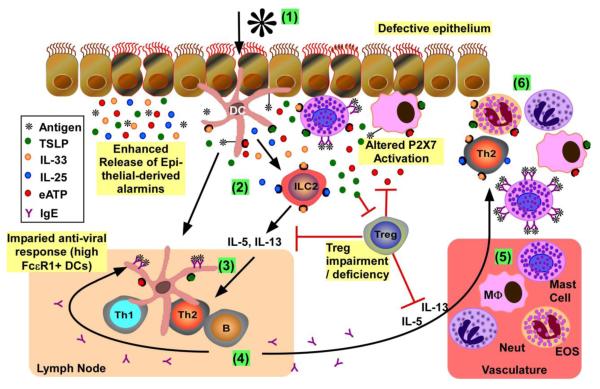
Figure 1. Overview of mucosal immune response to allergens and viruses in atopic asthma.
(1) Initial activation of epithelium and resident innate immune cells: Allergens and/or viruses activate epithelium to induce release of mediators, including alarmins (e.g., TSLP, IL-25, IL-33 and ATP). When epithelia are damaged, there is increased epithelial-mediated alarmin release together with exposure of resident immune cells (e.g., DCs, macrophages, mast cells) to pathogens to further promote pro-inflammatory mediator release. Resident antigen presenting cells (i.e., DCs, macrophages) process and present antigens to activate the adaptive immune response. Mast cells with bound IgE to FcεR1 can bind allergens, inducing FceR1 crosslinking and mediator release (e.g., histamine, TSLP, ATP). Released ATP from mast cells and/or damaged cells activates purinergic receptors on the surface of multiple immune cells (e.g., DCs, macrophages, mast cells) to further promote pro-inflammatory mediator release; the availability of active ATP for purinergic receptor binding is regulated by Tregs. (2) DC skewing and type 2 ILC (ILC2) expansion: Exposure of DCs to IL-33 and TSLP skews the DCs to support differentiation of naive T cells to Th2 in draining lymph nodes. IL-25 and IL-33 induce the maturation and activation of type 2 ILCs, which subsequently release IL-5 and IL-13. Tregs can inhibit the release of Th2 cytokines, but are suppressed by TSLP. (3) Th2 differentiation: The combination of primed DCs and released Th2-promoting cytokines (i.e., IL-4, IL-5, IL-13) lead to the maturation/activation of Th2 lymphocytes in the draining lymph nodes. (4) IgE production: Th2 cells induce B cell immunoglobulin isotype switching, leading to the release of antigen-specific IgE. Increased expression of FceR1 on DCs is inversely related to antiviral (i.e., IFN release) responses. (5) Recruitment of pro-inflammatory leukocytes from vasculature: Release of aforementioned mediators potentiates the release of chemokines from activated innate immune cells/epithelium to promote immune cell recruitment from the vasculature. (6) Chronic Inflammation: Recruited immune cells release more proinflammatory mediators, leading to exaggerated and chronic cellular inflammation.
Relationship of FcεR1+ Dendritic Cells to Release of Antivirals
The hallmark of atopy is the production of allergen-induced IgE, and elevated serum IgE levels are associated with increased asthma severity [35]. FcεRI is the high affinity surface receptor for IgE, and is expressed on dendritic cells as well as mast cells, basophils, and others [36]. Expression levels of FcεRI on peripheral blood cells correlate with serum IgE levels [36, 37]. Gill and colleagues found that there is a significant inverse correlation between FcεR1 on plasmacytoid dendritic cells (pDCs) and influenza-induced IFN-α responses [38]. Furthermore, influenza-induced IFN-α secretion was inversely correlated with serum IgE levels, and crosslinking IgE prior to influenza infection further attenuated IFN-α secretion from pDCs [38].
A similar relationship was seen after HRV infection of peripheral blood mononuclear cells (PBMCs). Durrani and colleagues [39]• reported that allergic asthma was associated with significant reductions in HRV-induced IFN-α and IFN-λ1 secretion. Further, the percentages of FcεR1+ pDCs inversely correlated with HRV-induced IFN-α and IFN-λ1 responses. Interestingly, serum total IgE was positively correlated with the percentages of FcεR1+ pDCs, and inversely correlated with HRV-induced IFN-α production.
The mechanism by which FcεR1 expression on pDCs leads to decreased IFN production has yet to be determined, but may be through activation of intracellular signaling by the FcεR1γ subunit, which contains immune receptor tyrosine-based activation motifs (ITAMs) that can activate various kinases such as Src and Syc [40, 41]. These studies support a role for pDCs in predisposing atopic asthmatics to an impaired anti-viral response.
IFN Deficiency in Viral-Infected Atopics
Clearance of viral pathogens begins with interferon secretion, and the underproduction of these factors has been postulated to lead to viral-induced exacerbations [42-44]. There are three types of interferons (based on the receptors they bind): Type I (IFN-α/β), Type II (IFN-γ) and Type III (IFN-λ). Numerous in vivo and ex vivo experiments have monitored the virus-induced release of these IFNs from epithelial and mononuclear cells from atopic asthmatics (Table 1).
Table 1.
Summary of studies comparing IFN levels between atopic asthmatics and healthy controls.
| IFN | Virus | mRNA / Gene |
Protein Release |
Decrease in asthmatics? |
Reference |
|---|---|---|---|---|---|
| Epithelial Cells | |||||
| IFN-β | HRV-1 b, 16 | X | yes | [46] [*] | |
| IFN-β | HRV-1 b, 16 | X | ND | [46] [*] | |
| IFN-β | HRV-1a | X | no | [48] | |
| IFN-β | HRV-16 | X | no | [49] | |
| IFN-β | HRV-16 | X | X | yes | [44] |
| IFN-β | HRV-16 | X | yes | [52] [*] | |
| IFN-λ | HRV-1b, 16 | X | ND | [46] [*] | |
| IFN-λ | HRV-16 | X | yes | [42] | |
|
IFN-λ
IFN-λ IFN-λ1 |
HRV-1b | X | yes | [45] | |
| HRV-16 | X | yes | [52] [*] | ||
| HRV-1b, 16 | X | yes | [46] [*] | ||
| IFN-λ1 | HRV-16 | X | yes | [42] | |
| IFN-λ2 | HRV-1a | X | no | [48] | |
| IFN-λ2/3 | HRV-1b, 16 | X | yes | [46] [*] | |
| IFN-λ2/3 | HRV-16 | X | yes | [42] | |
| Blood Cells / Serum | |||||
| IFN-α | HRV-16 | X | yes [**] | [39] [*] | |
| IFN-α | influenza A | X | yes | [38] | |
| IFN-α (+α2) | HRV-16 | X | no | [47] | |
| IFN-β | HRV-16 | X | no | [47] | |
| IFN-γ | HRV-16 | X | yes | [43] | |
| IFN-λ1 | HRV-16 uninfected uninfected |
X | yes [**] | [39] [*] | |
| IFN-λ1 | X | no | [51] | ||
| IFN-λ2/3 | X | no | [51] | ||
| Nasal and Bronchial Lavage / Sputum | |||||
|
IFN-α2
IFN-α (+α2) |
HRV-16 | X | no | [30] | |
| HRV-16 | X | yes | [47] | ||
| IFN-β | HRV-16 | X | yes | [47] | |
| IFN-γ | HRV-16 | X | no | [30] | |
|
IFN-λ IFN-λ1 IFN-λ2/3 |
HRV-16 uninfected uninfected |
X X |
X | yes no no |
[42] [50] [50] |
ND= not detected,
Pediatric Study;
need FcepsilonR1 cross-link to see differences
Results of a number of studies suggest that virus-induced IFN responses may be impaired in asthma [38, 39, 42-47], however, others have found no difference, or even enhanced IFN responses, in atopic asthmatics [18, 30, 48-51]. Of note, a recent prospective study of respiratory secretions in 409 asthmatic children found that in vivo IFN-λ1 levels during HRV infections were higher in wheezing versus non-wheezing children and IFN-λ1 levels were positively related to symptom severity [18]. In the absence of infection, adults with allergic asthma were found to have increased IFN-λ levels in allergic airway mucosa during peak allergy season, and allergen stimulation of PBMCs led to increased expression of IFN-λ1 by CD14+ cells [51]. These contradictory findings in different studies may be due to a number of factors, including timing, magnitude and location of the IFN response. These differences also suggest that exuberant IFN responses could lead to more severe clinical symptoms by enhancing airway inflammation,
Epithelial Cell Barrier Abnormalities
The airway epithelium provides the first line of defense against inhaled pathogens, but can lose barrier function after mechanical damage or exposure to numerous agents, including viruses [52-54]. Moreover, there is evidence that both atopy and asthma in children are associated with damaged airway epithelium [52]•. The apical layer of epithelium is more resistant to viral infection than the basal layer [55, 56], therefore agents that decrease the integrity of the epithelium could increase the susceptibility of the host to pathogen-induced damage. Furthermore, compromise of the epithelial barrier in asthma is associated with disrupted tight junctions, impaired innate immunity and attenuated antioxidant properties [57], which may contribute to increased sensitivity to infections and allergens.
Common Epithelial-derived Mediators in Allergy and Viral Illnesses
There is emerging evidence that viruses and allergens can act on the epithelium to initiate innate immune responses in the respiratory microenvironment that promote Th2 inflammation. In particular, both viruses and allergens induce epithelial cell-derived “alarmins”, TSLP, IL-33 and IL-25, which promote the differentiation and activation of innate lymphoid cells (ILCs) and suppress the activation of regulatory T (Treg) cells. Furthermore, there is increasing support for a role for extracellular nucleotides and the activation of nucleotide receptors in potentiating allergen- and viral-effects in human cells.
TSLP
TSLP (an IL-7-like cytokine) is primarily released from epithelial cells and keratinocytes at barrier surfaces, but is also expressed in lung fibroblasts, airway smooth muscles, and various immune cells including mast cells, macrophages and dendritic cells [58]. For instance, mast cells release TSLP following FcεR1 aggregation [59]. TSLP expression is induced by both allergens and viruses. [58, 59]. Of note, HRV infection of bronchial epithelial cells can induce TSLP expression and release [60]. In addition to numerous murine allergic inflammation models supporting a role for TSLP in driving Th2 inflammation [61], elevated levels of TSLP in the epithelium of atopic asthmatics and allergic rhinitis patients positively correlate with airway obstruction (FEV1) [62-64].
Human TSLP potently upregulates antigen presenting receptors on myeloid dendritic cells (mDCs) and subsequently prime mDCs to release Th2-attracting chemokines and induce the differentiation of naïve Th0 cells to Th2 cells [65, 66]. Interestingly, TSLP-activated thymus-derived human mDCs and pDCs in young children (0–2 years old) have been linked to the generation of Fox3P+ Tregs, which are general suppressors of inflammation [67]. The link between TSLP and the development of immunologic tolerance during infancy suggests that TSLP induces age-dependent differential effects on T cell maturation/activation.
IL-33
IL-33 expression in humans appears to be primarily confined to epithelial cells, endothelial cells, fibroblasts and smooth muscle cells [68, 69]. IL-33 is released from necrotic cells in response to inflammation/infection, and has been recently proposed to be a potential asthma therapeutic target [70], given that asthmatic lung epithelia basally express more IL-33 than healthy controls [71], and IL-33 levels correlate with increased asthma severity [71]. Exposure of primary human epithelial cells to airborne allergens also leads to IL-33 release, possibly be releasing ATP which stimulates purinergic receptors (P2X7 or P2Y2) [72]. Infection of human epithelial cells with influenza also increases IL-33 mRNA expression [73].
IL-33 can activate numerous immune cells (e.g., mast cells, DCs, Th2 cells, eosinophils) to amplify Th2-type responses [69, 70, 74]. Current work exploring the therapeutic use of anti-IL-33 for allergic rhinitis is promising; treatment of OVA-sensitized mice with anti-IL-33 decreases both IgE levels and eosinophil infiltration to the nasal cavity [75].
IL-25
IL-25, unlike other IL-17 family members that promote neutrophilic inflammation, induces Th2 cytokines from memory Th2 cells, basophils, and other immune cells [61, 76]. IL-25 is primarily made by epithelial cells, but eosinophils and basophils may also contribute [76]. Elevated expression of IL-25 and IL-25 receptor transcripts has been detected in asthmatic lung tissues, and the presence of eosinophil-derived IL-25 promotes IL-5 and IL-13 synthesis and Th2 cell expansion [76]. Interestingly, when dsRNA-treated nasal epithelium is cotreated with IL-25, TSLP release from nasal epithelium is significantly elevated, suggesting a positive feedback loop for Th2-mediated inflammation [63].
ILCs
Many of the Th2-promoting events induced by IL-33 and IL-25 are attributed to the activation of ILCs, which produce IL-13 and IL-5 after stimulation with these alarmins. ILCs are lineage-negative cells that are potent sources of cytokines at epithelial surfaces, including the lung. Of the four types described to date, CRTH2+ type 2 ILCs (found in the gut, lung and peripheral blood) [77, 78] are of particular interest. Stimulation of human type 2 ILCs with IL-25 or IL-33 leads to the secretion of IL-5 and IL-13, further promoting a Th2 microenvironment [77, 78]. Considering both allergens and viruses are able to induce the release of IL-25 and IL-33, ILCs may provide an important aspect of virus/allergen interactions in asthma.
Role of Tregs in Allergen/Viral Immunity
Tregs play in important role in fine-tuning the balance between effector and tolerogenic immune responses. There are two major Treg types: natural Tregs (nTregs), and induced/adaptive Tregs (iTregs) [79]. nTregs are a subset of CD4+ T cells that differentiate in the thymus express constitutively high amounts of the IL-2 receptor α-chain (CD25) and the transcription factor forkhead box P3 (Foxp3), and play a pivotal role in maintaining peripheral tolerance and limiting chronic inflammation [80]. In addition to nTregs, iTreg cells can also be generated in the presence of TGF-β, IL-10 and IL-35, and these cytokines are subsequently produced by iTregs to limit inflammation [80].
There is evidence that Treg cells might be functionally deficient in atopy and asthma. TSLP levels in bronchial alveolar lavage fluid (BALF) of allergic asthmatics have been correlated with impaired Treg suppressive function [79, 81, 82]. Moreover, CD4+CD25hi Treg cells expressed in atopic subjects were significantly less effective at suppressing proliferation and IL-5 release from CD4+CD25− T cells, as compared to non-atopic subjects [83]. Furthermore, BALF isolated from non-ICS treated asthmatic children had fewer CD4+CD25hi Treg cells than non-asthmatic controls [84]. Interestingly, asthmatic children who were on ICS treatment had similar CD4+CD25hi Treg levels as non-asthmatic controls [84], supporting a role for corticosteroids in activating Treg differentiation and subsequent IL-10 release [79].
During viral infection, Tregs may protect against viral-induced inflammation by suppressing the proliferation, cytokine production and cytotoxicity of effector CD8+ T cells, as well as suppressing CD4+ T cell function [85]. Accordingly, during HRV infections, Tregs suppress DC activation of T cells [86].
Although Tregs have been studied extensively in the context of allergies and viral infections, very little work has examined the effect of both allergens and virus together on Treg function. Recently a study in mice has implicated a potential role for early RSV exposure in suppressing Treg development and increasing subsequent susceptibility to allergic disease [87]. More work in human in vivo models need to be performed to determine whether Tregs are key players in allergen/virus signaling crosstalk.
Extracellular ATP and Purinergic Receptors
Viral infection and allergen exposure can both cause cells to release nucleotides into the extracellular space [88, 89], leading to millimolar concentrations of extracellular ATP (eATP) in an inflammatory microenvironment [90]. Of note, allergen activation of mast cells leads to the release high concentrations of eATP stored in their granules [90]. One mechanism by which Tregs are believed to suppress inflammation is through expression of ectoATPases (e.g., CD39, CD73) that metabolize eATP into adenosine, leading to attenuation of effector T cell proliferation and decreased DC function [80].
Cellular nucleotide receptors have been linked to inflammatory responses and asthma. Approximately 35% of asthmatics have attenuated P2X7 function [91], and recent clinical studies also support a potential role for the P2X7 in allergen sensitization and viral-induced asthma exacerbations. P2X7 is expressed on the surface of epithelial cells and numerous innate immune cells, including macrophages, DCs, eosinophils and mast cells [89, 90, 92]. Interestingly, there appears to be a difference between P2X7 function on the cells of children versus adults. In children, attenuated P2X7 function was associated with lower rate of asthma and less sensitization to aeroallergens [93]. Conversely, adults with attenuated P2X7 function present with decreased viral-induced nasal inflammation and are at a higher risk for viral-induced loss of asthma control despite maintenance therapy [91, 94].
Recent Treatments to Control Asthma Exacerbations
Current therapeutic research is primarily focused on preventing exacerbations of asthma and maintaining symptom control. Eosinophilic inflammation is a risk factor for more severe viral illnesses and asthma exacerbations [17, 30], suggesting that treatments that inhibit eosinophilic airway inflammation might be beneficial. In that respect, mepolizumab, a humanized monoclonal antibody against IL-5, significantly reduces eosinophil levels in the lungs and circulation [95], and reduces asthma exacerbations [96]. However, it must be noted that mepoluzimab treatment did not significantly improve persistent asthma symptoms for most individuals [95]. Another medication known to reduce eosinophilic inflammation and asthma exacerbations is montelukast (a leukotriene receptor antagonist). Addition of montelukast to asthma maintenance therapy has been shown to reduce September exacerbations and recurrent wheeze post-RSV bronchiolitis in some, but not all, studies [97, 98].
Another promising drug in preventing exacerbations and maintaining symptom control is omalizumab, a humanized monoclonal anti-IgE antibody. Omalizumab lowers the frequency of exacerbations and reduces asthma symptoms and requirements for other controller medications [99, 100]. Interestingly, omalizumab therapy substantially inhibited seasonal peaks in asthma exacerbation in children with moderate to severe asthma, supporting its potential efficacy in preventing viral-induced exacerbations [99]••.
Conclusions
Both allergens and viruses are important risk factors for the inception and exacerbation of asthma. Improving our understanding of the mechanisms for these effects is important to identify opportunities for treatment and to identify strategies for primary prevention of childhood asthma.
Acknowledgments
James E. Gern has served as a consultant for GlaxoSmithKline, Biota Pharmaceuticals, Centocor, Boehringer Ingelheim GmbH, MedImmune, Theraclone Sciences, Merck & Co., and Gilead Sciences, and has received grant support from AstraZeneca, GlaxoSmithKline, and Merck & Co.
Footnotes
Disclosure Monica L. Gavala declares that she has no conflict of interest.
Hiba Bashir declares that she has no conflict of interest.
References
Papers of interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Platts-Mills TA, Erwin EA, Woodfolk JA, Heymann PW. Environmental factors influencing allergy and asthma. Chemical immunology and allergy. 2006;91:3–15. doi: 10.1159/000090225. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(Suppl 3):S131–45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. The New England journal of medicine. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 4.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. American journal of respiratory and critical care medicine. 2005;171:137–41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 5.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 6.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, Hartert TV. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. American journal of respiratory and critical care medicine. 2008;178:1123–9. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomsen SF, van der Sluis S, Stensballe LG, Posthuma D, Skytthe A, Kyvik KO, Duffy DL, Backer V, Bisgaard H. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. American journal of respiratory and critical care medicine. 2009;179:1091–7. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 8.Simoes EA, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick L, Groothuis JR. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. The Journal of allergy and clinical immunology. 2010;126:256–62. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? The Journal of allergy and clinical immunology. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF., Jr. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. American journal of respiratory and critical care medicine. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. The Journal of allergy and clinical immunology. 2007;119:1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sly PD, Boner AL, Bjorksten B, Bush A, Custovic A, Eigenmann PA, Gern JE, Gerritsen J, Hamelmann E, Helms PJ, Lemanske RF, Martinez F, Pedersen S, Renz H, Sampson H, von Mutius E, Wahn U, Holt PG. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–6. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, Gern JE, Lemanske RF., Jr. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. American journal of respiratory and critical care medicine. 2012;185:281–5. doi: 10.1164/rccm.201104-0660OC. •• Provide evidence that exposure to allergens proceeds HRV-induced wheeze in a high risk prospective birth cohort
- 14.Murray CS, Poletti G, Kebadze T, Morris J, Woodcock A, Johnston SL, Custovic A. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376–82. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint SH, Tyrrell DA, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, Erwin EA, Shaker MS, Hellems M, Peerzada J, Hayden FG, Hatley TK, Chamberlain R. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. The Journal of allergy and clinical immunology. 2004;114:239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller EK, Hernandez JZ, Wimmenauer V, Shepherd BE, Hijano D, Libster R, Serra ME, Bhat N, Batalle JP, Mohamed Y, Reynaldi A, Rodriguez A, Otello M, Pisapia N, Bugna J, Bellabarba M, Kraft D, Coviello S, Ferolla FM, Chen A, London SJ, Siberry GK, Williams JV, Polack FP. A mechanistic role for type III IFN-lambda1 in asthma exacerbations mediated by human rhinoviruses. American journal of respiratory and critical care medicine. 2012;185:508–16. doi: 10.1164/rccm.201108-1462OC. • A prospective study showing a correlation between increased HRV-induced IFN-λ release and asthma exacerbations in children
- 19.Denlinger LC, Sorkness RL, Lee WM, Evans MD, Wolff MJ, Mathur SK, Crisafi GM, Gaworski KL, Pappas TE, Vrtis RF, Kelly EA, Gern JE, Jarjour NN. Lower airway rhinovirus burden and the seasonal risk of asthma exacerbation. American journal of respiratory and critical care medicine. 2011;184:1007–14. doi: 10.1164/rccm.201103-0585OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, Evans MD, Bork J, Roberg K, Lemanske RF, Jr., Gern JE. Weekly monitoring of children with asthma for infections and illness during common cold seasons. The Journal of allergy and clinical immunology. 2010;125:1001–06. e1. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, Murphy DD, Odio S, James HR, Patrie JT, Hunt W, O’Rourke AK, Davis MD, Steinke JW, Lu X, Kennedy J, Heymann PW. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. The Journal of allergy and clinical immunology. 2012;129:1499–505. e5. doi: 10.1016/j.jaci.2012.03.040. • Found HRV-induced wheeze correlated with high serum IgE
- 23.Johnston NW, Johnston SL, Duncan JM, Greene JM, Kebadze T, Keith PK, Roy M, Waserman S, Sears MR. The September epidemic of asthma exacerbations in children: a search for etiology. The Journal of allergy and clinical immunology. 2005;115:132–8. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston NW, Johnston SL, Norman GR, Dai J, Sears MR. The September epidemic of asthma hospitalization: school children as disease vectors. The Journal of allergy and clinical immunology. 2006;117:557–62. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 25.Bizzintino J, Lee WM, Laing IA, Vang F, Pappas T, Zhang G, Martin AC, Khoo SK, Cox DW, Geelhoed GC, McMinn PC, Goldblatt J, Gern JE, Le Souef PN. Association between human rhinovirus C and severity of acute asthma in children. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2011;37:1037–42. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khetsuriani N, Lu X, Teague WG, Kazerouni N, Anderson LJ, Erdman DD. Novel human rhinoviruses and exacerbation of asthma in children. Emerging infectious diseases. 2008;14:1793–6. doi: 10.3201/eid1411.080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, Morin LL, Heil LH, Lu X, Williams JV. A novel group of rhinoviruses is associated with asthma hospitalizations. The Journal of allergy and clinical immunology. 2009;123:98–104. e1. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwane MK, Prill MM, Lu X, Miller EK, Edwards KM, Hall CB, Griffin MR, Staat MA, Anderson LJ, Williams JV, Weinberg GA, Ali A, Szilagyi PG, Zhu Y, Erdman DD. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. The Journal of infectious diseases. 2011;204:1702–10. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 29.Lee WM, Lemanske RF, Jr., Evans MD, Vang F, Pappas T, Gangnon R, Jackson DJ, Gern JE. Human rhinovirus species and season of infection determine illness severity. American journal of respiratory and critical care medicine. 2012;186:886–91. doi: 10.1164/rccm.201202-0330OC. • Provide data that HRV-A and HRV-C groups cause more respiratory illnesses in infants, with season effecting HRV prevalence and virulence
- 30.DeMore JP, Weisshaar EH, Vrtis RF, Swenson CA, Evans MD, Morin A, Hazel E, Bork JA, Kakumanu S, Sorkness R, Busse WW, Gern JE. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. The Journal of allergy and clinical immunology. 2009;124:245–52. 52, e1–3. doi: 10.1016/j.jaci.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, Jeffery PK, Stanciu LA, Johnston SL. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. The Journal of clinical investigation. 1994;94:2200–8. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avila PC, Abisheganaden JA, Wong H, Liu J, Yagi S, Schnurr D, Kishiyama JL, Boushey HA. Effects of allergic inflammation of the nasal mucosa on the severity of rhinovirus 16 cold. The Journal of allergy and clinical immunology. 2000;105:923–32. doi: 10.1067/mai.2000.106214. [DOI] [PubMed] [Google Scholar]
- 34.de Kluijver J, Evertse CE, Sont JK, Schrumpf JA, van Zeijl-van der Ham CJ, Dick CR, Rabe KF, Hiemstra PS, Sterk PJ. Are rhinovirus-induced airway responses in asthma aggravated by chronic allergen exposure? American journal of respiratory and critical care medicine. 2003;168:1174–80. doi: 10.1164/rccm.200212-1520OC. [DOI] [PubMed] [Google Scholar]
- 35.Kovac K, Dodig S, Tjesic-Drinkovic D, Raos M. Correlation between asthma severity and serum IgE in asthmatic children sensitized to Dermatophagoides pteronyssinus. Archives of medical research. 2007;38:99–105. doi: 10.1016/j.arcmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nature reviews Immunology. 2007;7:365–78. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 37.Dehlink E, Baker AH, Yen E, Nurko S, Fiebiger E. Relationships between levels of serum IgE, cell-bound IgE, and IgE-receptors on peripheral blood cells in a pediatric population. PloS one. 2010;5:e12204. doi: 10.1371/journal.pone.0012204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, Gangnon RE, Gill MA, Gern JE, Lemanske RF, Jr., Jackson DJ. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. The Journal of allergy and clinical immunology. 2012;130:489–95. doi: 10.1016/j.jaci.2012.05.023. • Data reveal a role for IgE receptor activation on pDCs in attenuating anti-viral responses in asthmatic children
- 40.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. The Journal of experimental medicine. 2006;203:1399–405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao W, Zhang L, Rosen DB, Bover L, Watanabe G, Bao M, Lanier LL, Liu YJ. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS biology. 2007;5:e248. doi: 10.1371/journal.pbio.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. Role of deficient type III interferon-lambda production in asthma exacerbations. Nature medicine. 2006;12:1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 43.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57:328–32. doi: 10.1136/thorax.57.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. The Journal of experimental medicine. 2005;201:937–47. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collison A, Hatchwell L, Verrills N, Wark PA, de Siqueira AP, Tooze M, Carpenter H, Don AS, Morris JC, Zimmermann N, Bartlett NW, Rothenberg ME, Johnston SL, Foster PS, Mattes J. The E3 ubiquitin ligase midline 1 promotes allergen and rhinovirus-induced asthma by inhibiting protein phosphatase 2A activity. Nature medicine. 2013 doi: 10.1038/nm.3049. [DOI] [PubMed] [Google Scholar]
- 46.Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, Saglani S, Sykes A, Macintyre J, Davies J, Bossley C, Bush A, Johnston SL. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal immunology. 2012 doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, Kon OM, Mallia P, McHale M, Johnston SL. Rhinovirus 16-induced IFN-alpha and IFN-beta are deficient in bronchoalveolar lavage cells in asthmatic patients. The Journal of allergy and clinical immunology. 2012;129:1506–14. e6. doi: 10.1016/j.jaci.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 48.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal immunology. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, Finkbeiner WE, Dolganov GM, Widdicombe JH, Boushey HA, Avila PC. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. The Journal of allergy and clinical immunology. 2009;123:1384–90. e2. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bullens DM, Decraene A, Dilissen E, Meyts I, De Boeck K, Dupont LJ, Ceuppens JL. Type III IFN-lambda mRNA expression in sputum of adult and school-aged asthmatics. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2008;38:1459–67. doi: 10.1111/j.1365-2222.2008.03045.x. [DOI] [PubMed] [Google Scholar]
- 51.He S, Li T, Chen H, Ma W, Yao Q, Yang H, Wang H, Wang F, Zhao C, Yang P. CD14+ cell-derived IL-29 modulates proinflammatory cytokine production in patients with allergic airway inflammation. Allergy. 2011;66:238–46. doi: 10.1111/j.1398-9995.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 52.Baraldo S, Contoli M, Bazzan E, Turato G, Padovani A, Marku B, Calabrese F, Caramori G, Ballarin A, Snijders D, Barbato A, Saetta M, Papi A. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. The Journal of allergy and clinical immunology. 2012;130:1307–14. doi: 10.1016/j.jaci.2012.08.005. • Show asthmatic children have greater epithelia damage and impaired HRV-induced cytokine production that correlates inversely with HRV replication
- 53.Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. American journal of respiratory and critical care medicine. 2008;178:1271–81. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clinical microbiology reviews. 2011;24:210–29. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. American journal of respiratory cell and molecular biology. 2008;38:517–23. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez-Souza N, Dolganov G, Dubin R, Sachs LA, Sassina L, Sporer H, Yagi S, Schnurr D, Boushey HA, Widdicombe JH. Resistance of differentiated human airway epithelium to infection by rhinovirus. American journal of physiology Lung cellular and molecular physiology. 2004;286:L373–81. doi: 10.1152/ajplung.00300.2003. [DOI] [PubMed] [Google Scholar]
- 57.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proceedings of the American Thoracic Society. 2009;6:655–9. doi: 10.1513/pats.200907-072DP. [DOI] [PubMed] [Google Scholar]
- 58.Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergology international : official journal of the Japanese Society of Allergology. 2012;61:3–17. doi: 10.2332/allergolint.11-RAI-0395. [DOI] [PubMed] [Google Scholar]
- 59.Okayama Y, Okumura S, Sagara H, Yuki K, Sasaki T, Watanabe N, Fueki M, Sugiyama K, Takeda K, Fukuda T, Saito H, Ra C. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2009;34:425–35. doi: 10.1183/09031936.00121008. [DOI] [PubMed] [Google Scholar]
- 60.Calven J, Yudina Y, Hallgren O, Westergren-Thorsson G, Davies DE, Brandelius A, Uller L. Viral stimuli trigger exaggerated thymic stromal lymphopoietin expression by chronic obstructive pulmonary disease epithelium: role of endosomal TLR3 and cytosolic RIG-I-like helicases. Journal of innate immunity. 2012;4:86–99. doi: 10.1159/000329131. [DOI] [PubMed] [Google Scholar]
- 61.Holtzman MJ. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. The Journal of clinical investigation. 2012;122:2741–8. doi: 10.1172/JCI60325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, Shelley M, Abbas AR, Austin CD, Jackman J, Wu LC, Heaney LG, Arron JR, Bradding P. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. The Journal of allergy and clinical immunology. 2012;129:104–11. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 63.Xu G, Zhang L, Wang DY, Xu R, Liu Z, Han DM, Wang XD, Zuo KJ, Li HB. Opposing roles of IL-17A and IL-25 in the regulation of TSLP production in human nasal epithelial cells. Allergy. 2010;65:581–9. doi: 10.1111/j.1398-9995.2009.02252.x. [DOI] [PubMed] [Google Scholar]
- 64.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 65.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. The Journal of experimental medicine. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature immunology. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 67.Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, Arima K, Wang YH, Voo KS, Cao W, Liu YJ. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol. 2010;184:2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PloS one. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Borish L, Steinke JW. Interleukin-33 in asthma: how big of a role does it play? Current allergy and asthma reports. 2011;11:7–11. doi: 10.1007/s11882-010-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemiere C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 72.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–87. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Goffic R, Arshad MI, Rauch M, L’Helgoualc’h A, Delmas B, Piquet-Pellorce C, Samson M. Infection with influenza virus induces IL-33 in murine lungs. American journal of respiratory cell and molecular biology. 2011;45:1125–32. doi: 10.1165/rcmb.2010-0516OC. [DOI] [PubMed] [Google Scholar]
- 74.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. The Journal of allergy and clinical immunology. 2008;121:1484–90. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim YH, Yang TY, Park CS, Ahn SH, Son BK, Kim JH, Lim DH, Jang TY. Anti-IL-33 antibody has a therapeutic effect in a murine model of allergic rhinitis. Allergy. 2012;67:183–90. doi: 10.1111/j.1398-9995.2011.02735.x. [DOI] [PubMed] [Google Scholar]
- 76.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. The Journal of experimental medicine. 2007;204:1837–47. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hams E, Fallon PG. Innate type 2 cells and asthma. Current opinion in pharmacology. 2012;12:503–9. doi: 10.1016/j.coph.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature immunology. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 79.Nandakumar S, Miller CW, Kumaraguru U. T regulatory cells: an overview and intervention techniques to modulate allergy outcome. Clinical and molecular allergy : CMA. 2009;7:5. doi: 10.1186/1476-7961-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. The Journal of experimental medicine. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nguyen KD, Vanichsarn C, Nadeau KC. TSLP directly impairs pulmonary Treg function: association with aberrant tolerogenic immunity in asthmatic airway. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2010;6:4. doi: 10.1186/1710-1492-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 84.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, Griese M, Krauss-Etschmann S. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. The Journal of allergy and clinical immunology. 2007;119:1258–66. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 85.Keynan Y, Card CM, McLaren PJ, Dawood MR, Kasper K, Fowke KR. The role of regulatory T cells in chronic and acute viral infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46:1046–52. doi: 10.1086/529379. [DOI] [PubMed] [Google Scholar]
- 86.Seyerl M, Kirchberger S, Majdic O, Seipelt J, Jindra C, Schrauf C, Stockl J. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. European journal of immunology. 2010;40:321–9. doi: 10.1002/eji.200939527. [DOI] [PubMed] [Google Scholar]
- 87.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, Wenzel SE, Moore ML, Peebles RS, Jr., Ray A, Ray P. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nature medicine. 2012;18:1525–30. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Esther CR, Jr., Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Ribeiro CM, Moore CG, Davis SD, Boucher RC. Extracellular purines are biomarkers of neutrophilic airway inflammation. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2008;31:949–56. doi: 10.1183/09031936.00089807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Jr., Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nature medicine. 2007;13:913–9. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 90.Bulanova E, Bulfone-Paus S. P2 receptor-mediated signaling in mast cell biology. Purinergic signalling. 2010;6:3–17. doi: 10.1007/s11302-009-9173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Denlinger LC, Manthei DM, Seibold MA, Ahn K, Bleecker E, Boushey HA, Calhoun WJ, Castro M, Chinchili VM, Fahy JV, Hawkins GA, Icitovic N, Israel E, Jarjour NN, King T, Kraft M, Lazarus SC, Lehman E, Martin RJ, Meyers DA, Peters SP, Sheerar D, Shi L, Sutherland ER, Szefler SJ, Wechsler ME, Sorkness CA, Lemanske RF., Jr. P2X7-Regulated Protection from Exacerbations and Loss of Control Is Independent of Asthma Maintenance Therapy. American journal of respiratory and critical care medicine. 2013;187:28–33. doi: 10.1164/rccm.201204-0750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi L, Manthei DM, Guadarrama AG, Lenertz LY, Denlinger LC. Rhinovirus-induced IL-1beta release from bronchial epithelial cells is independent of functional P2X7. American journal of respiratory cell and molecular biology. 2012;47:363–71. doi: 10.1165/rcmb.2011-0267OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manthei DM, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Gern JE, Lemanske RF, Jr., Denlinger LC. Protection from asthma in a high-risk birth cohort by attenuated P2X(7) function. The Journal of allergy and clinical immunology. 2012;130:496–502. doi: 10.1016/j.jaci.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Denlinger LC, Shi L, Guadarrama A, Schell K, Green D, Morrin A, Hogan K, Sorkness RL, Busse WW, Gern JE. Attenuated P2X7 pore function as a risk factor for virus-induced loss of asthma control. American journal of respiratory and critical care medicine. 2009;179:265–70. doi: 10.1164/rccm.200802-293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, Robinson D, Wenzel S, Busse W, Hansel TT, Barnes NC. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. American journal of respiratory and critical care medicine. 2007;176:1062–71. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 96.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. The New England journal of medicine. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnston NW, Mandhane PJ, Dai J, Duncan JM, Greene JM, Lambert K, Sears MR. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics. 2007;120:e702–12. doi: 10.1542/peds.2006-3317. [DOI] [PubMed] [Google Scholar]
- 98.Kim CK, Choi J, Kim HB, Callaway Z, Shin BM, Kim JT, Fujisawa T, Koh YY. A randomized intervention of montelukast for post-bronchiolitis: effect on eosinophil degranulation. The Journal of pediatrics. 2010;156:749–54. doi: 10.1016/j.jpeds.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 99.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, Chmiel JF, Steinbach SF, Calatroni A, Togias A, Thompson KM, Szefler SJ, Sorkness CA. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. The New England journal of medicine. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. •• Describe the benefits of anti-IgE (omalizumab) therapy for improved asthma control and reduce exacerbations in an inner-city children cohort
- 100.Chen H, Eisner MD, Haselkorn T, Trzaskoma B. Concomitant asthma medications in moderate-to-severe allergic asthma treated with omalizumab. Respiratory medicine. 2013;107:60–7. doi: 10.1016/j.rmed.2012.09.008. [DOI] [PubMed] [Google Scholar]