Abstract
Using a cell-based high-throughput screen, we identified isoxazolo[5,4-d]pyrimidines as novel small-molecule correctors of the cystic fibrosis mutant protein ΔF508-CFTR. 22 Isoxazolo[5,4-d]pyrimidine analogues were synthesized and tested. Synthesis of the key intermediate, 5-amino-3-arylisoxazole-4-carboxamide, was accomplished by nitrile oxide cycloaddition to (2-amino-1-cyano-2-oxoethyl)sodium. Formation of 3-arylisoxazolo-[5,4-d]pyrimidin-4(5H)-one and chlorination gave 4-chloro-3-arylisoxazolo[5,4-d]pyrimidine. Finally, functionalization at C-4 of the pyrimidine ring by nucleophilic substitution gave the targeted isoxazolo[5,4-d]pyrimidines. Six of the reported analogues had low micromolar potency for increasing halide transport in ΔF508-CFTR cells.
Keywords: cystic fibrosis, ΔF508-CFTR, corrector, isoxazolopyrimidine
Cystic fibrosis (CF) is a relatively common inherited disease caused by mutations in the CF transmembrane conductance regulator protein (CFTR). The most common CFTR mutation, deletion of phenylalanine at residue 508, ΔF508, results in a defective CFTR protein that fails to traffic to the plasma membrane and fails to activate as a chloride channel.1–3 As a consequence a viscous mucus accumulates in the lung, which promotes bacterial growth and progressive deterioration of lung function.4,5 Although CF patient care has improved considerably, there is no cure for CF. While available CF-relevant drugs target the various symptoms of CF, many current studies are focused on the discovery of compounds that restore normal ΔF508-CFTR processing, which are called correctors.6–14
Using screening methods we developed to discover bithiazoles as the first ΔF508-CFTR correctors,14 we screened a new collection of 50,000 diverse, drug-like small molecules (from Chemdiv Inc., San Diego CA). The goal was to identify new corrector scaffolds as potential drug candidates. Screening and secondary verification studies indicated isoxazolopyrimidines as ΔF508-CFTR correctors. Fused pyrimidines have broad-ranging biological activities, including antibacterial,15 CRF (corticotropin-releasing factor) antagonist,16 antimalarial,17 and antitumor activities.18 While isoxazolopyrimidines are not well studied, pyrimidine-based synthesis and application studies have been the focus of numerous research groups, including Laitonjam et al.,19 Badiger et al.,20 Wentrup et al.,21 Peinador et al.,22 and Sankyo Co., Ltd., Japan.23 Herein, we report the synthesis and ΔF508-CFTR corrector activity of twenty-two novel isoxazolopyrimidines. To the best of our knowledge, this is the first report of these heterocycles as ΔF508-CFTR correctors.
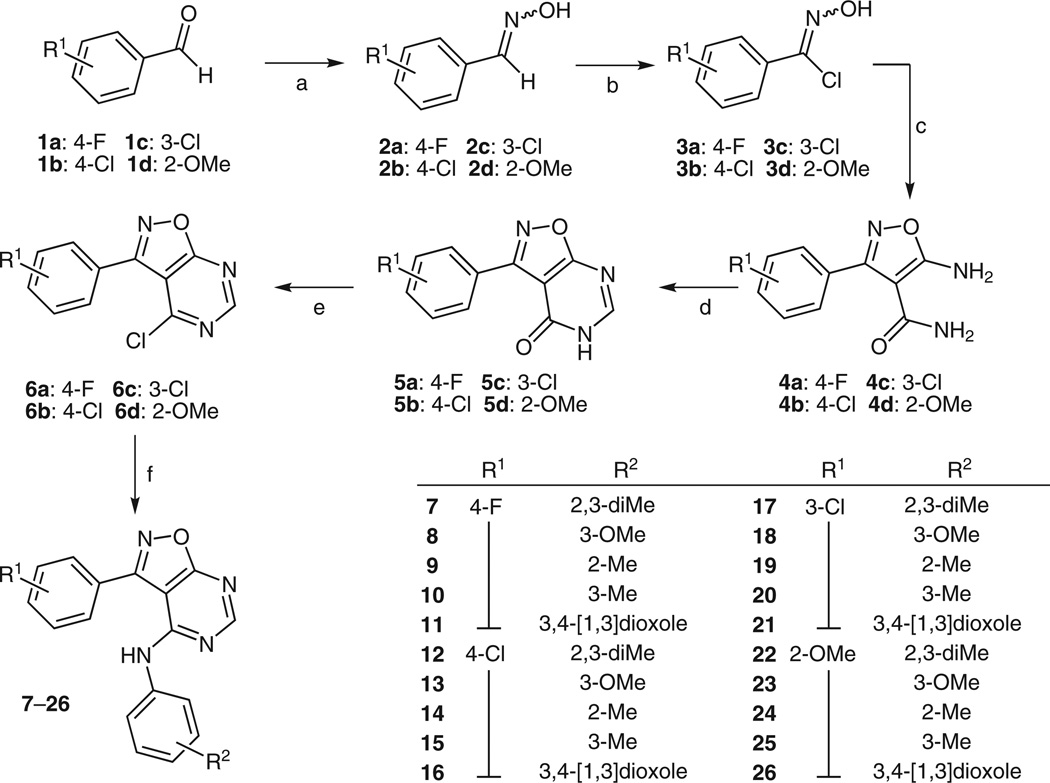
Our synthetic route, illustrated in Scheme 1, began with hydroximoyl chloride formation from aryl aldehyde 1 in two steps in quantitative yield.24 Hydroximoyl chlorides are well-established nitrile oxide precursors (via dehydrochlorination) that readily participate in 1,3-diploar cycloadditions. Adapting a protocol reported by Rajagopalan and Talaty,25 hydroximoyl chloride 3 was dissolved in ethanol and added dropwise to an ethanol suspension of (2-amino-1-cyano-2-oxoethyl)sodium to produce 5-amino-3-arylisoxazole-4-carboxamide 4 (35–89% yield). Next, carboxamide 4 and triethyl orthoformate were dissolved in acetic anhydride, and the solution was refluxed for 2.5 hours to give 3-isoxazolo[5,4-d]pyrimidin-4(5H)-one 5 in 48–75% yield. Pyrimidinone 5 was treated with phosphorous oxychloride (0.83 equiv) in dry acetonitrile (alternatively, toluene can be used as solvent) at reflux in the presence of N, N-dimethylaniline (2 equiv) for 3 hours in a sealed tube under a N2 atmosphere to afford 4-chloropyrimidine 6.26 The chlorination of 5a to 6a was also attempted with excess phosphorous oxychloride as described by Rajagopalan and Talaty.25 However, after washing with water, only starting material 5a was recovered suggesting that, under acidic conditions, water displaces the chloride to reverse the transformation (e.g., 6a → 5a). We also found that treating chloropyrimidine 6a with 2,3-dimethylaniline under reflux in methanol for 24 hours in the presence of K2CO3 failed to deliver isoxazolopyrimidine 7 (4-chloro- → 4-hydroxypyrimidine occurred instead). Fortunately, target molecules 7–26 were obtained by refluxing an isopropanol solution of 6 with the appropriate aniline (2 equiv) in the presence of a catalytic amount of HCl gas.27
Scheme 1.
Reagents: (a) NH2OH·HCl, H2O–EtOH–THF, NaOAc, 66–100%; (b) NCS, DMF–MeCl, Et3N, pyridine (cat.), 60–95%; (c) i) 2-cyanoacetamide, NaOEt, EtOH, ii) 3, EtOH, 35–89%; (d) triethyl orthoformate, Ac2O, reflux, 2.5 h, 48–75%; (e) POCl3 (0.83 equiv), N, N-dimethylaniline (2 equiv), Et3N·HCl (2 equiv), MeCN, reflux under N2, sealed tube, 2.5 h, 83–92%; (f) 2,3-dimethylaniline, i-PrOH, HCl (gas), 30–76%.
Twenty isoxazolo[5,4-d]pyrimidine analogues (7–26) were synthesized using the method outlined in Scheme 1 by varying R1 (four inputs) and R2 (five inputs). ΔF508-CFTR corrector data for six active compounds are summarized in Table 1. Compounds were tested using a cell-based fluorimetry assay of iodide influx as described previously.30 Corrector activity of each compound was verified by the lack of compound effect on FRT-null cells (not expressing ΔF508-CFTR) and by inhibition of the increased iodide influx by the thiazolidinone CFTR inhibitor CFTRinh-172. Compound 7 exhibited a greater Vmax value than s-cis locked bithiazole 27 reported in our earlier studies.14 Compounds 10–14 also had comparable corrector activities (Table 1). An SAR trend is observed when the location of substituent R on the phenyl ring of the isoxazole portion of the molecule is varied. For instance, when R1 = 3-Cl or 2-OMe, corrector activity is diminished. Of the isoxazolopyrimidines tested, only those with a para substituent on the isoxazole phenyl moiety (4-F and 4-Cl) were active. This dramatic change in corrector activity in the 4-F or 4-Cl versus the 3-Cl or 2-OMe suggests that the aldehyde reagent employed in Scheme 1 is best fixed as 4-chloro- or 4-fluorobenzaldehyde. When R2 is fixed as 2,3-dimethyl (e.g., employing 3,4-dimethylaniline in Scheme 1), isoxazolopyrimidines 7 and 12 had comparable IC50 values, although the Vmax of 7 is much larger. The best IC50 value was obtained when 3-methoxyaniline (13) was employed.
Table 1.
IC50 and Vmax Data for Active Isoxazolopyrimidinesa
 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | IC50 µM | Vmax µM/s |
| 7 | 4-F | 2,3-diMe | 3.9 | 161 |
| 10 | 4-F | 3-Me | 5.8 | 113 |
| 11 | 4-F | 3,4-[1,3]dioxole | 5.7 | 90 |
| 12 | 4-Cl | 2,3-diMe | 3.9 | 109 |
| 13 | 4-Cl | 3-OMe | 3.2 | 100 |
| 14 | 4-Cl | 2-Me | 6.9 | 103 |
For comparison, IC50 and Vmax of reference compound corr-4a11 were 0.92 µM and 152 µM/s.
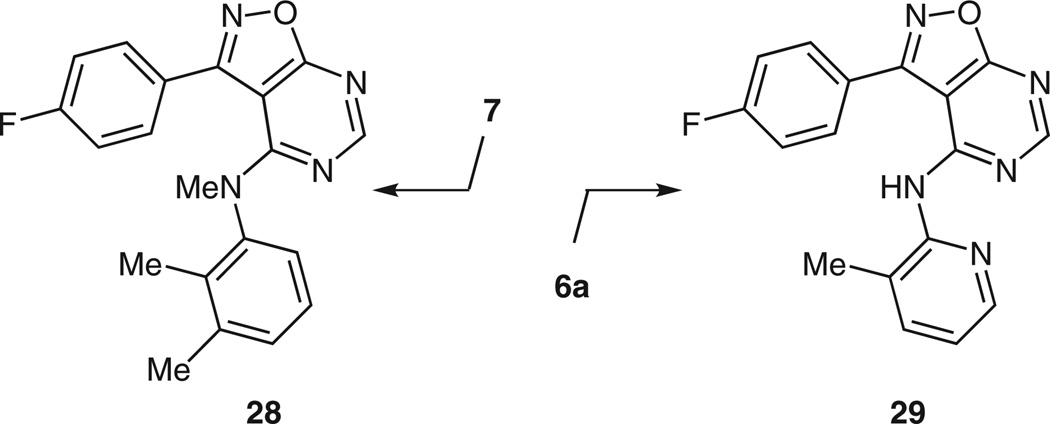
The hydrogen bond donor/acceptor profile of these isoxazolopyrimidines was then considered. For example, we investigated changing the NH of isoxazolopyrimidine 7 to an NMe (Figure 1; 28 was prepared by N-methylating 7 with iodomethane in methanol + NaH). We also modified 7 (starting from 6a) by replacing the 2,3-dimethylaniline moiety with a 3-methylpyridin-2-amine moiety to give analogue 29. When comparing the LogP values of compound 7, 28, and 29 (Figure 1), there is no significant difference between 7 and 28, while 29 has a lower LogP value (7: LogP 5.4813; 28: LogP 5.7171; 29: LogP 4.3731; data generated with ChemPropPro 8.03). Unfortunately, isoxazolopyrimidines 28 and 29 showed little corrector activity compared to 7.
Figure 1.
N-Methyl and pyridyl analogues of 7
In summary, a collection of 4-aniline substituted derivatives of isoxazole[5,4-d]pyrimidines was synthesized. The synthetic protocol outlined in Scheme 1 is suitable to high-volume combinatorial analogue generation. The six isoxazolopyrimidine-fused heterocycles listed in Table 1 demonstrate corrector activity with low micromolar potency. Isoxazolopyrimidines might thus serve as novel correctors to probe defective ΔF508-CFTR cellular processing and for further preclinical development.
4-Fluorobenzaldehyde Oxime (2a)
To a stirred solution of hydroxylamine·HCl (1.85 g, 26.6 mmol) in THF–EtOH–H2O (30 mL:75 mL:15 mL) was added 4-fluorobenzaldehyde (1a, 3.0 g, 24.2 mmol), and the mixture was stirred at r.t. for 25 min at which time EtOH and THF were removed in vacuo. The residue was extracted with Et2O (3 × 30), washed with brine, dried over anhyd Na2SO4, and filtered. Evaporation of the solvent afforded 2a (3.36 g, 100%), which was used in the next step without further purification. 1H NMR matches the literature data;28 ESI-MS: m/z = 139.99 [M + H]+. Oximes 2b–d were prepared following analogous procedures with or without NaOAc.
4-Fluoro-N-hydroxybenzimidoyl Chloride (3a)
To a stirred solution of NCS (3.55 g, 26.6 mmol) in DMF–CH2Cl2 (20 mL:120 mL) was added dropwise a CH2Cl2 (120 mL) solution of pyridine (200 µL, 2.42 mmol), Et3N (3.37 mL, 24.2 mmol), and 4-fluorobenzaldehyde oxime (2a: 3.36 g, 24.2 mmol). The solution was stirred at r.t. for 12 h at which time it was washed with H2O (5 × 100 mL) and concentrated to afford 3a (4.2 g, 100%). 1H NMR matches the literature data.28 Hydroximoyl chloride 3b–d were prepared following analogous procedures.
5-Amino-3-(4-fluorophenyl)isoxazole-4-carboxamide (4a)
A freshly prepared NaOEt in EtOH solution, made at r.t. from Na metal (977 mg, 42.5 mmol) in abs. EtOH (150 mL), was added to a stirred solution of 2-cyanoacetamide (3.57 g, 42.5 mmol) in abs. EtOH (50 mL) at 50 °C. To the resulting clear solution cooled to 0 °C was added dropwise a solution of hydroximoyl chloride 3a (7.38 g, 42.5 mmol) in abs. EtOH (100 mL). The resulting suspension was stirred at r.t. for 30 min and then refluxed overnight. EtOH was removed in vacuo, and the resulting residue was washed with H2O and recrystallized from MeOH to yield 4a as light yellow crystals (2.91 g, 31%).29a Carboxamides 4b–d were prepared following analogous procedures.
3-(4-Fluorophenyl)isoxazolo[5,4-d]pyrimidin-4(5H)-one (5a)
A mixture of 4a (2.88 g, 13 mmol), triethyl orthoformate (2.16 mL, 13 mmol), and Ac2O (15 mL) was refluxed overnight. The solution was cooled on ice, and the resulting precipitate was collected by filtration, washed with H2O, and air dried to yield 5a as an off white powder (2.05 g, 68%).29b Pyrimidinones 5b–d were prepared following analogous procedures refluxing for from 2.5–12 h.
4-Chloro-3-(4-fluorophenyl)-4,5-dihydroisoxazolo[5, 4-d]pyrimidine (6a)
POCl3 (167 µL, 1.79 mmol) was added to a solution of 5a (0.54 g, 2.16 mmol), N, N-dimethylaniline (411 µL, 3.24 mmol), and Et3N·HCl (0.6 g, 4.33 mmol) in dry MeCN (1.7 mL) under N2 in a sealable tube. The mixture was refluxed for 3 h, cooled, and the MeCN was removed in vacuo. The resulting residue was subjected to flash column chromatography purification (hexane–EtOAc = 9:1) to provide 6a as off-white crystals (480 mg, 92%).29c Pyrimidines 6b–d were prepared following analogous procedures.
N-(2,3-Dimethylphenyl)-3-(4-fluorophenyl)-4,5-dihydroisoxazolo[ 5,4-d]pyrimidin-4-amine (7)
A mixture of 6a (111 mg, 0.443 mmol), 2,3-dimethylbenzenamine (108 µL, 0.885 mmol), and catalytic HCl (g) in 2-PrOH (2 mL) was sealed in a tube and refluxed overnight. Cooling on ice produced white crystals which were collected by filtration and washed with cold 2-PrOH to afford 7 (112 mg, 76%).29d Isoxazolopyrimidines 8–26 and 29 were prepared following analogous procedures.
N-(2,3-dimethylphenyl)-3-(4-fluorophenyl)-4,5-dihydroisoxazolo[5,4-d]pyrimidin-4-amine (28)
To a solution of 7 (95 mg, 0.284 mmol) in DMF (3 mL) was added 60% NaH (26 mg, 0.643 mmol) in mineral oil. The suspension was stirred at r.t. for 30 min; MeI (40 µL, 0.643 mmol) was added and the mixture stirred at r.t. overnight. The resulting mixture was washed with H2O (3 × 10 mL), extracted with EtOAc (10 mL), washed with brine, dried over anhyd Na2SO4, and filtered. Removal of EtOAc afforded crude 28 which was subjected to flash chromatography (hexanes–EtOAc = 9:1 → 4:1) to deliver pure 28 (58 mg, 61%).29e
Acknowledgement
The authors thank the Tara K. Telford Fund for Cystic Fibrosis Research at UC Davis, the National Institutes of Health (DK072517, GM076151, and HL073856), and the National Science Foundation [CHE-0910870; and CHE-0443516, CHE-0449845, and CHE-9808183 for NMR spectrometers] for their generous support.
References and Notes
- 1.Riordan JR. Annu. Rev. Biochem. 2008;77:701. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 2.Kunzelmann K, Nitschke R. Exp. Nephrol. 2000;8:332. doi: 10.1159/000020687. [DOI] [PubMed] [Google Scholar]
- 3.Kleizen B, Braakman I, de Jonge HR. Eur. J. Cell Biol. 2000;79:544. doi: 10.1078/0171-9335-00078. [DOI] [PubMed] [Google Scholar]
- 4.Tarran R, Button B, Boucher RC. Annu. Rev. Physiol. 2006;68:543. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 5.Verkman AS, Song Y, Thiagarajah JR. Am. J. Physiol. Cell Physiol. 2003;284:C2. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- 6.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu P. Proc. Natl. Acad. Sci. U.S.A. 2009;106:18825. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verkman AS, Galietta LJ. Nat. Rev. Drug Discov. 2009;8:153. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Goor F, Straley KS, Cao D, González J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings LR, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack JH, Tung R, Grootenhuis PD, Negulescu P. Am. J. Physiol. 2006;290:L1117. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 9.Verkman AS, Lukacs GL, Galietta LJV. Curr. Pharm. Des. 2006;12:2235. doi: 10.2174/138161206777585148. [DOI] [PubMed] [Google Scholar]
- 10.Rosser MFN, Grove DE, Cry DM. Curr. Chem. Biol. 2009;3:420. [Google Scholar]
- 11.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJV, Verkman AS. J. Clin. Invest. 2005;115:2564. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedemonte N, Sonawane ND, Taddei A, Hu J, Zegarra-Moran O, Suen YF, Robins LI, Dicus CW, Willenbring D, Nantz MH, Kurth MJ, Galietta LJV, Verkman AS. Mol. Pharmacol. 2005;67:1797. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 13.Yoo CL, Yu GJ, Yang B, Robins LI, Verkman AS, Kurth MJ. Bioorg. Med. Chem. Lett. 2008;18:2610. doi: 10.1016/j.bmcl.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu GJ, Yoo CL, Yang B, Lodewyk MW, Meng L, El-Idreesy TT, Fettinger JC, Tantillo DJ, Verkman AS, Kurth MJ. J. Med. Chem. 2008;51:6044. doi: 10.1021/jm800533c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burch HA, Benjamin LE, Russell HE, Freedman R. J. Med. Chem. 1974;17:451. doi: 10.1021/jm00250a017. [DOI] [PubMed] [Google Scholar]
- 16.Frietze WE. WO 2000011003. Chem. Abstr. 2000;132:180596. 2000;
- 17.Hynes JB, Gratz RF, Ashton WT. J. Med. Chem. 1972;15:1332. doi: 10.1021/jm00282a034. [DOI] [PubMed] [Google Scholar]
- 18.Taylor EC, Patel HH. Tetrahedron. 1992;48:8089. [Google Scholar]
- 19.Thokchom HS, Nongmeikapam AD, Laitonjam WS. Can. J. Chem. 2005;83:1056. [Google Scholar]
- 20.Adhikari VA, Savalgi VP, Badiger VV. Curr. Sci. 1988:703. [Google Scholar]
- 21.Kappe CO, Flammang R, Wentrup C. Heterocycles. 1994;63:1615. [Google Scholar]
- 22.Quintela JM, Peinador C. Trends Heterocycl. Chem. 2006;11:33. [Google Scholar]
- 23.Chem. Abstr. 1984;101:110940. JP 59036683, 1984; [Google Scholar]
- 24.Dixon SM, Milinkevich KA, Fujii J, Liu R, Yao N, Lam KS, Kurth MJA. J. Comb. Chem. 2007;9:143. doi: 10.1021/cc060090p. [DOI] [PubMed] [Google Scholar]
- 25.Rajagopalan P, Talaty CN. Tetrahedron. 1967;23:3541. [Google Scholar]
- 26.Connolly DJ, Lacey PM, McCarthy M, Saunders CP, Carroll A-M, Goddard R, Guiry PJ. J. Org. Chem. 2004;69:6572. doi: 10.1021/jo049195+. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes C, Oliveira C, Gano L, Bourkoula A, Pirmettis I, Santos I. Bioorg. Med. Chem. 2007;15:3974. doi: 10.1016/j.bmc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Di Nunno L, Vitale P, Scilimati A, Simone L, Capitelli F. Tetrahedron. 2007;63:12388. [Google Scholar]
- 29.Representative Spectral Data (a) Compound 4a: 1H NMR (600 MHz, DMSO): δ = 7.65 (br s, 2 H), 7.63–7.58 (m, 2 H), 7.38–7.32 (m, 2 H). 13C NMR (15 MHz, DMSO): δ = 171.70, 164.11, 163.85, 162.22, 159.78, 131.09, 131.04, 125.46, 125.44, 115.93, 115.79, 86.79. ESI-MS: m/z = 222.08 [M + H]+. (b) Compound 5a: 1H NMR (300 MHz, DMSO): δ = 13.17 (br s, 1 H), 8.46 (s, 1 H), 8.38 (m, 2 H), 7.42 (m, 2 H). ESI-MS: m/z = 232.06 [M + H]+. (c) Compound 6a: 1H NMR (600 MHz, CDCl3): δ = 9.02 (s, 1 H), 7.92–7.77 (m, 2 H), 7.28 (m, 2 H). 13C NMR (150 MHz, CDCl3): δ = 175.19, 165.60, 164.12, 158.07, 157.03, 156.72, 132.17, 132.11, 122.57, 116.39, 116.24, 110.62, 77.37, 77.16, 76.95. (d) Compound 7: 1H NMR (600 MHz, CDCl3): δ = 8.59 (s, 1 H), 7.85–7.69 (m, 2 H), 7.45 (d, J = 7.9 Hz, 1 H), 7.38–7.29 (m, 2 H), 7.16 (t, J = 7.7 Hz, 1 H), 7.10 (d, J = 7.5 Hz, 1 H), 6.81 (br s, 1 H), 2.31 (s, 3 H), 2.03 (s, 3 H). 13C NMR (150 MHz, CDCl3): δ = 176.12, 165.31, 163.63, 159.45, 157.18, 156.18, 138.28, 134.79, 131.06, 130.70, 130.64, 128.85, 126.29, 124.84, 123.31, 117.45, 117.30, 95.73, 20.71, 14.21. ESI-MS: m/z = 335.10 [M + H]+. (e) Compound 28: 1H NMR (600 MHz, CDCl3): δ = 8.67 (s, 1 H), 6.79 (m, 7 H), 6.56 (br s, 1 H), 3.35 (s, 3 H), 2.00 (s, 2 H), 1.96 (s, 3 H). 13C NMR (150 MHz, CDCl3): δ = 176.07, 163.97, 162.25, 160.25, 157.52, 144.82, 139.36, 133.03, 130.43, 129.88, 127.08, 123.56, 114.55, 96.77, 40.97, 20.28, 15.68. ESI-MS: m/z = 349.12 [M + H]+.
- 30.General Procedure for Bioassays – Δ508-CFTR Corrector Activity Assay Assays were performed by utilizing FRT epithelial cells stably coexpressing human ΔF508-CFTR and the high-sensitivity halide-sensing fluorescent protein YFP-H148Q/I152L used as described previously.11 Cells were grown at 37 °C (95% air/5% CO2) for 24 h and then incubated for 16–20 h with 50 µL of medium containing the test compound. At the time of the assay, cells were washed with PBS and then incubated with PBS containing forskolin (20 µM) and genistein (50 µM) for 20 min. Measurements were carried out using FLUOstar fluorescence plate readers (Optima; BMG LABTECH Gmbh), each equipped with 500 ± 10 nm excitation and 535 ± 15 nm emission filters (Chroma Technology Corp.). Each well was assayed individually for I− influx by recording fluorescence continuously (200 ms per point) for 2 s (baseline) and then for 12 s after rapid (<1 s) addition of 165 µL PBS in which 137 mM Cl− was replaced by I−. I− influx was computed by fitting the final 11.5 s of the data to an exponential for extrapolation of initial slope All experiments contained negative control (DMSO vehicle) and positive control corr-4a11 ({N-[2-(5-chloro-2-methoxyphenylamino)-4′-methyl-4,5′-bithiazol-2′-yl]benzamide}). Background I− influx (from DMSO control) was subtracted to report the increase in I− influx in Table 1.