Abstract
Purpose
Chemotherapy improves breast cancer survival but is underused more often in black than in white women. We examined associations between patient-physician relationships and chemotherapy initiation and timeliness of initiation among black and white patients.
Methods
Women with primary invasive, non-metastatic breast cancer were recruited via hospitals (in Washington, DC and Detroit) and community outreach between July 2006 and April 2011. Data were collected via telephone interviews and medical records. Logistic regression models evaluated associations between chemotherapy initiation and independent variables. Since there were race interactions, analyses were race-stratified. Factors associated with time from surgery to chemotherapy initiation and delay of ≥ 90 days were evaluated with linear and logistic regressions, respectively.
Results
Among eligible women, 82.8% were interviewed and 359 (90.9%)of those had complete data. The odds of initiating chemotherapy were 3.26 times (95% CI: 1.51, 7.06) higher among black women reporting greater communication with physicians (vs. lesser), after considering covariates. In contrast, the odds of starting chemotherapy were lower for white women reporting greater communication (vs. lesser) (adjusted OR .22, 95% CI: .07, .73). The opposing direction of associations was also seen among the sub-set of black and white women with definitive clinical indications for chemotherapy. Among those initiating treatment, black women had longer mean time to the start of chemotherapy than whites (71.8 days vs. 55.0 days, p= .005), but race was not significant after considering trust in oncologists, where initiation time decreased as trust increased, controlling for covariates. Black women were also more likely to delay ≥ 90 days than whites (27% vs. 8.3%; p=.024), but this was not significant after considering religiosity.
Conclusion
The patient-physician dyad and sociocultural factors may represent leverage points to improve chemotherapy patterns in black women.
Keywords: chemotherapy initiation, disparities, patient-provider communication
Introduction
Black women have higher rates of breast cancer mortality than white women despite lower age-adjusted incidence and comparable mammography use [1–3]. Moreover, stage for stage, black women have lower breast cancer survival [4–6], and this disparity has widened over the last two decades [4]. More aggressive breast cancers and/or limitations in access to timely diagnosis and quality care may explain some of the differences in survival outcomes [7]. However, race disparities persist even after considering these factors [8–10].
Since systemic therapy can reduce mortality by up to 50% [11, 12], sub-optimal use of adjuvant chemotherapy by black women may contribute to survival disparities [13]. Current research on patterns of care suggests that black women are more likely to experience delays in chemotherapy initiation than whites and may even have lower rates of initiating adjuvant therapy [14–16]. Unfortunately, significant delays such as those 90 days or more have been associated with increased mortality [13, 17–19]. When black women receive appropriate systemic therapies, their survival outcomes are similar to their white counterparts [20–22].
Prescription of chemotherapy takes place within the patient-provider relationship, so components of this dyadic relationship may influence use. However, most studies have relied on secondary data or retrospective approaches and have had limited information about black women’s interactions with their providers [14–16, 18, 23]. Moreover, we do not know if factors related to black women’s therapy initiation differ from their white counterparts [24, 25]. To fill this gap, we conducted a study of black and white breast cancer patients to examine whether factors associated with chemotherapy initiation, days to initiation, and treatment delay differ by race. We hypothesized that black women would have lower chemotherapy initiation and greater delay, but that race differences could be diminished by good communication with and trust in oncologists. Results from this study are intended to inform interventions to improve the quality of breast cancer care and reduce treatment disparities.
Methods
The Adherence Model of Health Behavior [26] guided our study since it is unique in highlighting constructs relevant to initiation of cancer therapy among different race/ethnic groups. The model specifically posits that the “art of care” within the patient-provider interaction and sociocultural constructs predict cancer adherence behaviors [26, 27].
Setting and Population
A convenience sample of women was recruited via hospital in-reach and outreach between July 2006 and April 2011. In-reach occurred at three hospitals in Washington, DC (including one NCI-designated Cancer Center) and one NCI-designated Cancer Center in Detroit, MI. Hospital in-reach was supplemented by outreach efforts, including fliers, posters, web-postings, mailings, and e-mail. Study procedures were approved by Institutional Review Boards at all institutions.
We included women over age 21 that were diagnosed with invasive non-metastatic disease for whom systemic adjuvant therapy would be considered with curative intent. We oversampled black women to facilitate race comparisons and to investigate within race-group differences. Because we were interested in factors that would affect chemotherapy, we restricted the sample to women who were < 20 weeks past their definitive surgery.
Women with ductal and lobular carcinoma in-situ, distant metastasis, recurrent disease, second primaries, who were not English speakers, who were of other races, or who could not give informed consent were excluded.
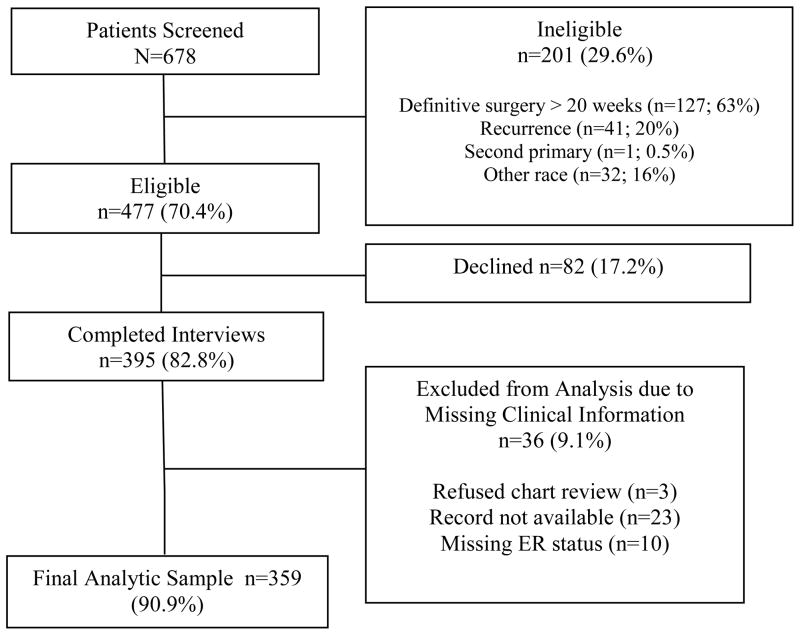
Among 678 potentially eligible patients screened for the study, 477 were eligible and 395 (82.8%) consented (Figure 1); 36 women were excluded from subsequent analyses due to missing clinical data. The final analytic data set includes 359 women (254 recruited via in-reach and 105 recruited via outreach).
Figure 1.
Study Schema
Data Collection
Potentially eligible hospital patients were identified from surgery logs, pathology reports, and electronic appointment systems; patients responding to outreach recruitment self-referred to the study. Clinical research assistants confirmed eligibility and obtained consent for interviews and chart reviews. Interviews were conducted centrally by trained staff using a standardized computer-assisted telephone survey. On average, women were interviewed 3 months past their definitive surgery and interviews lasted about 50 minutes. Treatment and clinical variables were abstracted from medical records 12–18 months after interviews. Participants received a $25 incentive.
Measures
Study outcomes were chemotherapy initiation, time to initiation (in days), and chemotherapy delay (≥ 90 days). Initiation (yes vs. no) was defined as having initiated any chemotherapy regimen [23]. Additionally, we examined initiation among women for whom chemotherapy would be regarded as clinically indicated in accordance with NCCN practice guidelines during the study period (e.g., positive nodes and/or estrogen receptor [ER] negative) [28, 29]. Days to chemotherapy initiation was measured among patients who initiated therapy as the number of days between a patient’s last definitive surgery and her first cycle of adjuvant chemotherapy. The third outcome, initiation delay, was defined as ≥ 90 days from surgery to start of chemotherapy in accordance with reports [17, 30] that have linked this length of delay with decrements in survival [17].
Race was based on self-identification. Factors related to patient-centered interactions with physicians were collected via self-report and included communication, trust, medical mistrust, and perceived discrimination. The Makoul Communication Scale (7-items) was adapted to assess self-reported communication with oncologists (Cronbach’s alpha: overall= .83; blacks= .82; whites= .85) [31]. The scale includes key dimensions of communication such as information-giving (e.g. “the doctor fully explained the risks of chemotherapy”) and physicians’ solicitation behaviors (e.g. “the doctor did not ask your opinion about taking chemotherapy”). Scores ranged from 8 to 41 and were dichotomized at the median; scores above the median reflect self-reported perceptions of greater communication.
To measure patients’ level of trust in their oncologist, we adapted items from the Primary Care Assessment Survey, which has shown good reliability (.86) in cancer settings and was reliable in our sample (Cronbach’s alpha overall=.81, black= .81, white = .80); higher scores indicate higher trust [33–35]. Perceived healthcare discrimination was assessed using the Race-Based Experiences scale that includes 7 questions about healthcare discrimination and was categorized as any versus none [36]. The suspicion subscale of the Group-Based Medical Mistrust Scale measured the perceived level of group distrust in healthcare systems and practices [37] with higher scores indicating more mistrust (Cronbach’s alpha overall= .84; blacks=.77; whites=.87).
Sociocultural factors included religiosity and chemotherapy attitudes. Religiosity was measured using nine items from Lukwago and colleagues (e.g., “I talk openly about my faith”) (Cronbach’s alpha overall= .95, blacks = .94, whites = .94) and was dichotomized at the median with higher scores indicating high religiosity [38]. To measure attitudes about chemotherapy, we expanded a two-item measure [24] to seven items that captured women’s perceptions about the efficacy of therapy (“chemotherapy does not help you live longer”) and about side effects (“the side effects of chemotherapy are worse than the disease”) (Cronbach’s alpha overall= .60, blacks = .59, whites = .50). Scores above the median reflected positive attitudes and those below were negative. Additionally, women were asked whether they received information about breast cancer treatment by radio/TV and internet (yes vs. no).
Clinical factors that were used to control for chemotherapy outcomes included estrogen receptor (ER) status (positive vs. negative), surgery type (lumpectomy or mastectomy), nodal status (positive or negative), pathological tumor size, and human epidermal growth factor receptor (Her2/neu), which was categorized similar to other reports as positive, negative, or unknown [39]. Comorbidity was measured using the Charlson comorbidity index score [40]. Body mass index was calculated from data in the medical charts and categorized as either obese (kg/m2 ≥30) or non-obese (kg/m2 < 30) [41]. Demographic variables were age, education, marital status, and employment status.
Statistical Analysis
We used t-tests and chi-square tests to assess bivariate relationships between chemotherapy initiation and delay and study variables. Multivariable logistic regression was employed to model initiation and delay. Selection of variables for inclusion in regression models was based on bivariate significance (P <.05). We tested for the presence of interactions between variables of interest and race. Because we found significant interactions by race with regards to chemotherapy initiation, we conducted race-stratified analyses. No interactions were found for ≥ 90 day delay, so those analyses include all women and control for race. We evaluated the goodness-of-fit and the predictive capability of the logistic models using the Hosmer-Lemeshow test and the C-statistic measure.
To examine relationships between indepdent variables and time to initiation, we used Pearson’s correlation tests and ANOVA. Significant variables (P < .05) were included in a series of linear regression models: race and time to interview were entered first, followed by demographic factors, and then by patient-provider factors. We evaluated whether each block of independent variables changed the race effect and explained more outcome variability using changes in R2 and corresponding F tests Log transformation of the time outcome was performed to satisfy the normality assumption. Since the results using the log transformed and original data were similar, for ease of interpretation we only present the original data. Since there were no race interactions, stratified analyses were not considered.
To account for potential clustering by treating hospital we also run corresponding logistic and linear regression models with generalized estimating equations (GEE). Since the results were virtually unchanged, we only present the results of the models without GEE. All analyses were conducted using IBM SPSS Software Version 19.
Results
Participants’ Characteristics
The study sample was 58% black and 42% white. Women’s ages ranged from 25 to 89 years (m= 54.8; SD = 11.7) and virtually all patients were insured (99.4%). As shown in Table 1, compared to whites, black women were more likely to be unmarried (65% vs. 28%; p<. 001) and obese (50% vs. 22%; p<. 001). More than half of black women (54%) had tumor sizes ≥ 2cm compared to about one-third of white women (35%, p= .001). There was a non-significant trend towards more ER negative tumors in black patients (27% vs. 20% for whites; p=.114). Racial differences in additional sources of information (i.e., internet) were noted (p=.014). In terms of the interactions with providers, black women reported higher medical mistrust (12.8 ± 3.3 vs. 9.6 ± 3.1; p<.001), more discrimination (44% vs. 16%; p<.001), and less trust in oncologists than whites (8.9 ± 1.8 vs. 9.2 ± 1.1; respectively; p<.05). No differences were noted in patients’ ratings of patient-physician communication about chemotherapy (p ≥ .05).
Table 1.
Demographic, Clinical, Psychosocial, and Healthcare Characteristics of Breast Cancer Patients by Race and Chemotherapy Initiation Status (N=359)
| Total N=359 | Black N=210 | White N=149 | p-value | Chemotherapy Initiated | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes N=141 | No N=218 | |||||||||||
| n | % | n | % | n | % | n | % | n | % | |||
| Demographic Characteristics | ||||||||||||
| Age: Mean (SD) | 54.8(11.7) | 54.1(12.0) | 55.7(11.2) | .197 | 52.2 (11.3) | 56.4 (11.6) | .001 | |||||
| Race | ||||||||||||
| Black | 210 | 58.5 | -- | -- | -- | -- | 97 | 46.2 | 113 | 53.8 | .001 | |
| White | 149 | 41.5 | -- | -- | -- | -- | 44 | 29.5 | 105 | 70.5 | ||
| Education | ||||||||||||
| No college education | 78 | 21.7 | 65 | 31.0 | 13 | 8.7 | <.001 | 45 | 57.7 | 33 | 42.3 | <.001 |
| Some college | 106 | 29.5 | 74 | 35.2 | 32 | 21.5 | 42 | 39.6 | 64 | 60.4 | ||
| Bachelors and above | 175 | 48.7 | 71 | 33.8 | 104 | 69.8 | 54 | 30.9 | 121 | 69.1 | ||
| Marital Status | ||||||||||||
| Married/Living as Married | 182 | 50.7 | 74 | 35.2 | 108 | 72.5 | <.001 | 68 | 37.4 | 149 | 62.6 | .452 |
| Currently Single | 177 | 49.3 | 136 | 64.8 | 41 | 27.5 | 73 | 41.2 | 104 | 58.8 | ||
| Employment | ||||||||||||
| Full Time Employed | 127 | 37.7 | 64 | 33.7 | 63 | 42.9 | .085 | 55 | 43.3 | 72 | 56.7 | .391 |
| Other | 210 | 62.3 | 126 | 66.3 | 84 | 57.1 | 81 | 38.6 | 129 | 61.4 | ||
| Clinical Characteristics | ||||||||||||
| Estrogen Receptor Status | ||||||||||||
| ER positive | 274 | 76.3 | 154 | 73.3 | 120 | 80.5 | .114 | 92 | 33.6 | 182 | 66.4 | |
| ER Negative | 85 | 23.7 | 56 | 26.7 | 29 | 19.5 | 49 | 57.6 | 36 | 42.4 | < .001 | |
| Surgery | ||||||||||||
| Mastectomy | 127 | 35.6 | 68 | 32.5 | 59 | 39.9 | .154 | 54 | 42.5 | 73 | 57.5 | .385 |
| Lumpectomy | 230 | 64.4 | 141 | 67.5 | 89 | 60.1 | 87 | 37.8 | 143 | 62.2 | ||
| Nodal Status | ||||||||||||
| Positive | 128 | 38.8 | 77 | 39.7 | 51 | 37.5 | .688 | 77 | 60.2 | 51 | 39.8 | < .001 |
| Negative | 202 | 61.2 | 177 | 60.3 | 85 | 62.5 | 64 | 31.7 | 138 | 68.3 | ||
| Tumor Size | ||||||||||||
| <2cm | 178 | 53.9 | 89 | 46.1 | 89 | 65.0 | .001 | 54 | 30.3 | 124 | 69.7 | <. 001 |
| ≥2cm | 152 | 46.1 | 104 | 53.9 | 48 | 35.0 | 86 | 56.6 | 66 | 43.4 | ||
| HER-2 | ||||||||||||
| Positive | 42 | 11.7 | 24 | 11.4 | 18 | 12.1 | .900 | 26 | 61.9 | 16 | 38.1 | <.001 |
| Negative | 248 | 69.1 | 144 | 68.6 | 104 | 69.8 | 103 | 41.5 | 145 | 58.5 | ||
| Unknown* | 69 | 19.2 | 42 | 20.0 | 27 | 18.1 | 12 | 17.4 | 57 | 82.6 | ||
| Chemotherapy Indicated** | ||||||||||||
| Indicated | 187 | 52.1 | 117 | 55.7 | 70 | 47.0 | .103 | 108 | 57.8 | 79 | 42.2 | <.001 |
| Considered | 172 | 47.9 | 93 | 44.3 | 79 | 53.0 | 33 | 19.2 | 139 | 80.8 | ||
| Comorbidities | ||||||||||||
| No comorbid disease | 125 | 34.8 | 63 | 30.0 | 62 | 41.6 | .023 | 37 | 29.6 | 88 | 70.4 | .006 |
| ≥1 comorbid diseases | 234 | 65.2 | 147 | 70.0 | 87 | 58.4 | 104 | 44.4 | 130 | 55.6 | ||
| Body Mass Index (BMI) | ||||||||||||
| Obese (≥30 kg/m2) | 128 | 37.6 | 96 | 50.3 | 32 | 21.5 | <.001 | 59 | 46.1 | 69 | 53.9 | .090 |
| Not obese (<30 kg/m2) | 212 | 62.4 | 95 | 49.7 | 117 | 78.5 | 78 | 36.8 | 134 | 63.2 | ||
| Patient Attitudinal Factors | ||||||||||||
| Chemotherapy Attitude | ||||||||||||
| Positive | 176 | 49.4 | 99 | 47.4 | 77 | 52.4 | .352 | 89 | 50.6 | 87 | 49.4 | < .001 |
| Negative | 180 | 50.6 | 110 | 52.6 | 70 | 47.6 | 51 | 28.3 | 129 | 71.7 | ||
| Medical Mistrust scale: Mean (SD) | 11.5 (3.6) | 12.8(3.3) | 9.6(3.1) | <.001 | 11.8 (3.4) | 11.2 (3.7) | .116 | |||||
| Perceived Discrimination | ||||||||||||
| Any | 116 | 32.3 | 92 | 43.8 | 24 | 16.1 | <.001 | 54 | 45.6 | 62 | 53.4 | .051 |
| None | 243 | 67.7 | 118 | 56.2 | 125 | 83.9 | 87 | 35.8 | 156 | 64.2 | ||
| Religiosity | ||||||||||||
| High | 168 | 49.4 | 126 | 66.0 | 42 | 28.2 | <.001 | 75 | 44.6 | 93 | 55.4 | .106 |
| Low | 172 | 50.6 | 65 | 34.0 | 107 | 71.8 | 62 | 36.0 | 110 | 64.0 | ||
| Patient-MD Relationship | ||||||||||||
| Trust in Medical Oncologist: | ||||||||||||
| Mean (SD) | 9.0 (1.5) | 8.9(1.8) | 9.2(1.1) | .048 | 9.1 (1.3) | 9.0 (1.7) | .455 | |||||
| Chemotherapy Communication | ||||||||||||
| High | 180 | 50.4 | 98 | 47.1 | 82 | 55.0 | .140 | 86 | 47.8 | 94 | 52.2 | .001 |
| Low | 177 | 49.6 | 110 | 52.9 | 67 | 45.0 | 54 | 30.5 | 123 | 69.5 | ||
| Provider Gender§ | ||||||||||||
| Female | 275 | 85.7 | 153 | 84.5 | 122 | 87.1 | .508 | 118 | 42.9 | 157 | 57.1 | .189 |
| Male | 46 | 14.3 | 28 | 15.5 | 18 | 12.9 | 15 | 32.6 | 31 | 67.4 | ||
| Information Sources | ||||||||||||
| Internet | ||||||||||||
| Yes | 203 | 63.6 | 106 | 57.9 | 97 | 71.3 | .014 | 77 | 37.9 | 126 | 62.1 | .132 |
| No | 116 | 36.4 | 77 | 42.1 | 39 | 28.7 | 54 | 46.6 | 62 | 53.4 | ||
| Radio/TV | ||||||||||||
| Yes | 91 | 30.6 | 69 | 38.3 | 22 | 18.8 | <.001 | 131 | 40.3 | 194 | 59.7 | .999 |
| No | 206 | 69.4 | 111 | 61.7 | 95 | 81.2 | 4 | 40.0 | 6 | 60.0 | ||
| Treatment Site | ||||||||||||
| NCI-designated Cancer Center | 157 | 43.7 | 86 | 41.0 | 70 | 47.0 | .256 | 62 | 39.5 | 95 | 60.5 | .941 |
| Non-NCI designated | 202 | 56.3 | 124 | 59.0 | 79 | 53.0 | 79 | 39.1 | 123 | 60.9 | ||
P-values are obtained from Chi square tests and t-tests.
Percentages add up to 100 along the rows for chemotherapy initiation and along columns for the “total” and race categories.
Abbreviations: NCI =National Cancer Institute; ER= Estrogen Receptor; SD= Standard Deviation
No information regarding testing or test results in patients’ medical record.
Based on NCCN clinical guidelines
Oncologist or Surgeon
Chemotherapy Initiation
The overall rate of chemotherapy initiation was 39%: 30% in whites and 46% in blacks. Because of significant interactions between race and age (p=.035) and race by communication (p=.002), stratified analyses were performed. These models revealed differences by race in the direction and significance level of study variables (Table 2). The largest difference in effect was for communication, where greater patient-provider communication (vs. less) was associated with higher odds of initiation among blacks (OR: 3.26, 95% CI: 1.51, 7.06), while greater communication was associated with lower initiation in whites (OR: 0.22, 95% CI: 0.07, 0.73).
Table 2.
Adjusted Odds Ratios of Chemotherapy Initiation in Breast Cancer Patients by Race
| Variable | All Patients, Eligible for Chemotherapy (N=359) | Chemotherapy Indicated* (N=187) | ||
|---|---|---|---|---|
|
| ||||
| Black (n=210) | White (n= 149) | Black (n=117) | White (n= 70) | |
| OR; 95% CI | OR; 95% CI | OR; 95% CI | OR; 95% CI | |
| Age (per one year increase) | .99 (.95, 1.03) | .90 (.84, .95) ‡ | 1.00 (.95, 1.04) | .86 (.79,94) |
| Education | ||||
| Some college (vs. ≤ HS) | .44 (.17, 1.09) | .13 (.02, 1.04) | .65 (.21, 2.00) | .03 (.01, .61) |
| Bachelor’s degree+ (vs.≤ HS) | .25(.09, .68) ‡ | .26 (.05, 1.49) | .27 (.09, .85) | .31 (.03, 2. |
| ER negative (vs. ER positive) | 4.22 (1.70, 10.47) ‡ | 1.54 (.44, 5.41) | ---- | ---- |
| Positive nodes (vs. negative) | 4.35 (1.93, 9.79) ‡ | 3.93 (1.34, 11.48) † | ---- | ---- |
| ≥2cm Tumor size (vs. <2cm) | 2.65 (1.21, 5.80) † | 4.44 (1.53, 12.85) ‡ | 2.06 (.79, 5.39) | 4.54 (1.06,19. |
| ≥1 Comorbid diseases (vs. 0) | 1.93 (.73, 5.09) | 2.02 (.64, 6.43) | 1.67 (.54, 5.14) | 1.26 (.27, 6. |
| Positive attitude (vs. negative) | 2.58 (1.16, 5.71) † | 2.99 (1.05, 8.48) † | 1.58 (.61, 4.07) | 1.33 (.29, 6. |
| Communication – greater (vs. less) | 3.26 (1.51, 7.06) ‡ | .22(.07, .73) † | 3.25 (1.26, 8.39) | .17 (.03, .95) |
|
| ||||
| C statistic | .85 | .87 | .78 | .88 |
| H-L Goodness of fit | P=.467 | P=.755 | P=.402 | P=.384 |
Models controlled for treatment site, time from diagnosis, and HER2 status.
indicated per NCCN guidelines
p-value < .05;
p-value < .01 HS= high school
Age effects were somewhat different within each race group, with each one year of increasing age significantly associated with lower chemotherapy initiation in whites (OR: 0.90, 95% CI: .84, .95) but not in blacks (OR: .99, 95% CI: .95, 1.03). Attitudes about chemotherapy were also associated with initiation in both race groups, with positive (vs. negative) attitudes being associated with higher odds of initiation for blacks (OR: 2.58, 95% CI: 1.16 to 5.71) and whites (OR: 2.99, 95% CI: 1.05 to 8.48).
Analysis among the sub-group of patients with more definitive indications for chemotherapy (n=187) revealed higher uptake of chemotherapy (58%: 65.8% in blacks vs. 44.3% in whites; p< .001). In multivariable analyses, we found the same significant interactions by race as in the overall sample. Thus, we constructed race-stratified models for the indicated group as we did for the overall sample. Race-stratified multivariable analyses among women with more definitive indications for chemotherapy revealed a similar pattern of associations with chemotherapy initiation as in the overall group (Table 2). For example, among black women with indications for chemotherapy, those with greater chemotherapy communication (vs. less) were more likely to initiate chemotherapy (OR: 3.25, 95% CI: 1.26, 8.39). Among whites with indications for chemotherapy, those who reported greater communication were again less likely to initiate therapy than those reporting less chemotherapy communication (OR: 0.17, 95% CI: .03, .95). Finally, the results were similar for women regardless of recruitment modality (data not shown).
Time to Chemotherapy Initiation
Among those who started chemotherapy, time from last definitive surgery to initiation of chemotherapy ranged from 19 to 180 days (66.3 ± 32.9 days). Black women had a greater mean number of days to initiation than whites (71.8 days vs. 55.0 days, p=. 005). In bivariate analyses, two other variables were positively associated with greater time to initiation: single status (73.5 days vs. 58.0 days, p=. 013) and lower trust in oncologists (r= −0.30; p=. 004). In multivariable analyses, race was no longer significantly related to time to initiation after controlling for marital status and trust. This model (Table 3) explained 19.6% of the variability in time to chemotherapy initiation.
Table 3.
Associations of Characteristics with Time to Chemotherapy Initiation and ≥ 90 Days Chemotherapy Delay
| Characteristic | Days to Chemotherapy Initiation Estimated Coefficients | ≥ 90 days delay Odds Ratio, 95% CI |
|---|---|---|
| Race | ||
| Black | 7.6 | 1.15 (.25, 5.40) |
| White | Ref | Ref |
| Marital Status | 14.9 † | |
| Unmarried | Ref | 6.54 (1.81, 23.55) ‡ |
| Married/living as married | Ref | |
| Trust in Oncologist (per one point increase) | −7.9 ‡ | ---- |
|
| ||
| Religiosity | 4.94 (1.29, 18.99) † | |
|
| ||
| High (vs. low) | ---- | |
| Model F statistic | F (4,85)=6.4; p<. 001 | H-L Goodness of fit, p= .300 |
| Adjusted R2 (%) | 19.6 | C statistic = .78 |
Models controlled for treatment site and time since diagnosis.
p<.05;
p<.01
----Variable not significant in the bivariate analysis.
Delay in Chemotherapy Initiation of ≥90 days
Twenty-one percent of participants who initiated chemotherapy had ≥ 90 day delay: 27% of blacks vs. 8.3% of whites (p= .024). In bivariate analyses, only three factors were associated with a ≥ 90 day delay: race, marital status, and religiosity. About one-third (32.2%) of the single women delayed treatment compared to only 7.8% of those married (p=.002). More women with high religiosity delayed initiation (vs. low) (31.6% vs. 8.2%; p=.003). Table 3 displays results from multivariable models for ≥ 90 day delay; race was not significant after considering covariates, but marital status and religiosity remained significant.
Discussion
This study is among the few that reports across- and within-race variations in the use and timeliness of initiation of chemotherapy and how the “art of care” affects chemotherapy utilization. Measures of the patient-physician relationship were important for initiation, but varied in the direction of their effect by race group. Greater patient-physician communication was associated with higher odds of starting chemotherapy among black women but with lower odds of initiation among whites. This pattern was also true in the sub-set of black and white women with definitive clinical indications for chemotherapy. Black women were also more likely to have longer mean times to the start of chemotherapy than whites, but this race effect was moderated by trust in providers. Additionally, black women were more likely to have a ≥ 90 day initiation delay, but this was no longer significant after considering marital status and religiosity.
The differential impact of communication by race in chemotherapy initiation has not been studied before and could have several explanations. Black and white patients may have different needs and/or preferences for patient-physician communication [27]. Indeed, Ashton and colleagues noted that preferences for the style of communication can vary by race [42]. One study with primarily white breast cancer patients found that women who preferred to make their own decisions about chemotherapy were less likely to choose it [43]. Thus, black women may have preferred to rely on providers while whites may have preferred to make decisions with less input from providers. Reports regarding cancer patients’ information needs suggest that black patients report the need for more information from providers [44–46]. Manfredi and colleagues found that even when black breast cancer patients asked more questions than whites, they received less information [47]. Thus, if black women relied more on their physicians in making decisions and received desired communication, their decisions may have been positively impacted.
The association between greater chemotherapy communication and lower initiation in whites further underscores the complexities of patient-provider communication. In breast cancer patients, some data suggests that being white and having a higher income and education is associated with seeking information outside of the patient-provider relationship to inform decisions [48–50]. White women in our study reported receiving more information from the internet than blacks. Indeed, highly educated white women who reported having greater communication were the least likely to initiate therapy (data not shown). Our findings may also reflect differences in preferences for a self-efficacy decision style (emphasis on weighing options and controlling decisions) versus a medical expert style (more driven by physician’s provided information) [51]. Finally, despite high ratings of communication, there may have been differences in the content of information provided by race. For instance, black women may have received more messages about the severity of their disease and whites more information about side effects, so that whites made decisions to forego chemotherapy more often [43, 52]. Future research should include direct observation of encounters and mixed methods to better understand the differential effect of communication on chemotherapy uptake by race.
Even when women choose to receive chemotherapy, treatment delays can affect survival [17]. Similar to our results, others have found that black women tend to have longer initiation delay of systemic therapy than whites [30, 53–55]. Reasons for the observed delays have been underexplored. Our results suggest that the trust in providers may partly explain part these patterns. Women with less trust may delay care to seek additional opinions, or initially reject their physician’s recommendations [56–58]. Findings also suggest that being married decreases time to initiation and treatment delay, as noted by others. For instance, Lipscomb and colleagues found that the impact of race on completion of chemotherapy was influenced by women’s marital status [25]. Unmarried women may have less support to initiate therapy than their married counterparts.
Religiosity also appears to be associated with greater delay. This result may indicate that women who reported greater religiosity sought guidance through prayer or within their religious community before starting therapy, or they may have delayed their disclosure to others. Gullatte and colleagues found that when black women disclosed their breast symptoms to another person (vs. to God only), they were less likely to delay seeking initial care [59].
All participants would have been eligible for chemotherapy. Overall, it was reassuring that initiation rates were higher among the sub-set with the strongest clinical indications for therapy. However, based on guidelines [28, 29], use remained suboptimal, suggesting the need to better understand and address initiation among those with the clearest indications for chemotherapy. The lower use in whites was unexpected, and might be related to variations in tumor characteristics that we did not measure (e.g., tumor grade). Also, the sample size was too small to analyze all the sub-groups within the white sample.
The strengths of this study include the collection of primary data from patients, the high representation of black patients, that supported race-stratified analyses, consideration of two dimensions of initiation, treatment data from medical records, assessment of initiation by clinical subgroups, and ascertainment of psychosocial variables related to chemotherapy.
Despite these considerable strengths, there are some limitations to be considered. We relied on patient reports of patient-physician communication. We do not know the actual content of the interactions, including the amount or quality of information or the oncologist’s recommendations. Studies that observe encounters will be important to extend our results and determine if actual communication differs by race. The sample was not population-based and information about non-responders, particularly those who self-referred, is limited. The generalizability of our results may also be restricted as most participants were insured and recruited from cancer centers. These women may differ systematically from patients with low insurance coverage and/or cared for in community practices. Finally, we did not collect primary data about physicians (e.g., race, etc.); this will be important to further understand interactions between providers and patients about cancer care.
Overall, the differential impact of trust and communication in chemotherapy initiation and delay suggests that additional research is needed to understand this “communication paradox” to examine preferences for decision-making, actual interactions with providers, and information needs across race groups [43]. Until then, the robustness of our results suggests that the patient-physician dyad represents a good leverage point for interventions to improve chemotherapy patterns in black women and ultimately, to reduce race disparities in breast cancer mortality.
Acknowledgments
This work was funded in part by grants from the American Cancer Society (Sheppard: PI MRSGT-06-132 CPPB), Komen for the Cure, Inc. (PI: Sheppard POP0503398), and the National Cancer Institute (Mandelblatt: RO1 CA124924, RO1 CA 127617 and KO5 CA96940). It was also supported by the Biostatistics and Bioinformatics Shared Resource (Luta) and the Nontherapeutic Subject Registry (NTSR) Shared Resource (Isaacs) at Lombardi Comprehensive Cancer Center under NCI Grant #P30CA51008. We thank the study participants and the research and clinical staff who helped to recruit women. We also acknowledge the support of Ms. Becky Montalvo, Ms. Nancy Muzeck, Dr. Susan Love, and Ms. Leah Wilcox.
Footnotes
Ethical Standards
The study complies with the current laws of the country in which it was performed.
Disclosures
Isaacs: Genetech and Glaxo Smith Kline (consultation)
All remaining authors have declared no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Howlander N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975 – 2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: 2012. http://seer.cancer.gov/csr/1975-2009pops09/ based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Peek MME, Han JH. Disparities in screening mammography Current status, interventions, and implications. Journal of general internal medicine: JGIM. 2004;19:184–194. doi: 10.1111/j.1525-1497.2004.30254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates. Cancer. 2003;97:2853–2860. doi: 10.1002/cncr.11411. [DOI] [PubMed] [Google Scholar]
- 5.Clegg LX, Li FP, Hankey BF, et al. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162:1985–1993. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 6.Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer. 2000;88:114–123. doi: 10.1002/(sici)1097-0142(20000101)88:1<114::aid-cncr16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Gerend MA, Pai M. Social determinants of Black-White disparities in breast cancer mortality: a review. Cancer Epidemiol Biomarkers Prev. 2008;17:2913–2923. doi: 10.1158/1055-9965.EPI-07-0633. [DOI] [PubMed] [Google Scholar]
- 8.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:90–96. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 9.Newman LA, Mason J, Cote D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94:2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 10.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97:134–147. doi: 10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 11.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 12.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 13.Lund MJ, Brawley OP, Ward KC, et al. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109:545–557. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 14.Bickell NA. Race, ethnicity, and disparities in breast cancer: victories and challenges. Womens Health Issues. 2002;12:238–251. doi: 10.1016/s1049-3867(02)00145-7. [DOI] [PubMed] [Google Scholar]
- 15.Griggs JJ, Sorbero ME, Stark AT, et al. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81:21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 16.Hershman D, Weinberg M, Rosner Z, et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst. 2003;95:1545–1548. doi: 10.1093/jnci/djg073. [DOI] [PubMed] [Google Scholar]
- 17.Hershman DL, Wang X, McBride R, et al. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006;99:313–321. doi: 10.1007/s10549-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 18.Fedewa SA, Ward EM, Stewart AK, et al. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. J Clin Oncol. 2010;28:4135–4141. doi: 10.1200/JCO.2009.27.2427. [DOI] [PubMed] [Google Scholar]
- 19.Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network Quality Measures. J Clin Oncol. 2008;26:3631–3637. doi: 10.1200/JCO.2008.16.5068. [DOI] [PubMed] [Google Scholar]
- 20.Dignam JJ. Differences in breast cancer prognosis among African-American and Caucasian women. CA Cancer J Clin. 2000;50:50–64. doi: 10.3322/canjclin.50.1.50. [DOI] [PubMed] [Google Scholar]
- 21.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001:36–43. doi: 10.1093/oxfordjournals.jncimonographs.a003458. [DOI] [PubMed] [Google Scholar]
- 22.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001:36–43. doi: 10.1093/oxfordjournals.jncimonographs.a003458. [DOI] [PubMed] [Google Scholar]
- 23.Hu C, Delclos GL, Chan W, et al. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol. 2011;28:1062–1074. doi: 10.1007/s12032-010-9644-7. [DOI] [PubMed] [Google Scholar]
- 24.Mandelblatt JS, Sheppard VB, Hurria A, et al. Breast cancer adjuvant chemotherapy decisions in older women: the role of patient preference and interactions with physicians. J Clin Oncol. 2010;1:3146–3156. doi: 10.1200/JCO.2009.24.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipscomb J, Gillespie TW, Goodman M, et al. Black-white differences in receipt and completion of adjuvant chemotherapy among breast cancer patients in a rural region of the US. Breast Cancer Res Treat. 2012;133:285–296. doi: 10.1007/s10549-011-1916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gritz ER, Bastani R. Cancer prevention--behavior changes: the short and the long of it. Prev Med. 1993;22:676–688. doi: 10.1006/pmed.1993.1061. [DOI] [PubMed] [Google Scholar]
- 27.Sheppard VB, Adams IF, Lamdan R, et al. The role of patient-provider communication for black women making decisions about breast cancer treatment. Psychooncology. 2011;20:1309–1316. doi: 10.1002/pon.1852. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network. Breast cancer treatment guidelines for patients NCCN v. VIII. 2006. [Google Scholar]
- 29.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology v. 2. 2008. [DOI] [PubMed] [Google Scholar]
- 30.Fedewa SA, Ward EM, Stewart AK, et al. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. J Clin Oncol. 2010;28:4135–4141. doi: 10.1200/JCO.2009.27.2427. [DOI] [PubMed] [Google Scholar]
- 31.Makoul G, Arntson P, Schofield T. Health promotion in primary care: physician-patient communication and decision making about prescription medications. Soc Sci Med. 1995;41:1241–1254. doi: 10.1016/0277-9536(95)00061-b. [DOI] [PubMed] [Google Scholar]
- 32.Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998;36:728–739. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Fleming DA, Sheppard VB, Mangan PA, et al. Caregiving at the end-of-life: perceptions of healthcare quality and quality of life among patients and caregivers. J PainSymptom Manage. 2005;31:407–420. doi: 10.1016/j.jpainsymman.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Safran DG, Taira DA, Rogers WH, et al. Linking primary care performance to outcomes of care. J Fam Pract. 1998;47:213–220. [PubMed] [Google Scholar]
- 35.Sheppard VB, Wang J, Yi B, et al. Are health-care relationships important for mammography adherence in Latinas? J Gen Intern Med. 2008;23:2024–2030. doi: 10.1007/s11606-008-0815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bird ST, Bogart LM. Perceived race-based and socioeconomic status(SES)-based discrimination in interactions with health care providers. Ethn Dis. 2001;11:554–563. [PubMed] [Google Scholar]
- 37.Thompson HHS. The Group-Based Medical Mistrust Scale: psychometric properties and association with breast cancer screening. Prev Med. 2004;38:209–218. doi: 10.1016/j.ypmed.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 38.Lukwago SN, Kreuter MW, Bucholtz DC, et al. Development and validation of brief scales to measure collectivism, religiosity, racial pride, and time orientation in urban African American women. Fam Community Health. 2001;24:63–71. doi: 10.1097/00003727-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Rizzo M, Lund MJ, Mosunjac M, et al. Characteristics and treatment modalities for African American women diagnosed with stage III breast cancer. Cancer. 2009;115:3009–3015. doi: 10.1002/cncr.24334. [DOI] [PubMed] [Google Scholar]
- 40.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.Eknoyan G. Adolphe Quetelet (1796–1874)--the average man and indices of obesity. Nephrol Dial Transplant. 2008;23:47–51. doi: 10.1093/ndt/gfm517. [DOI] [PubMed] [Google Scholar]
- 42.Ashton CM, Haidet P, Paterniti DA, et al. Racial and ethnic disparities in the use of health services: bias, preferences, or poor communication? J Gen Intern Med. 2003;18:146–152. doi: 10.1046/j.1525-1497.2003.20532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandelblatt JS, Faul LA, Luta G, et al. Patient and Physician Decision Styles and Breast Cancer Chemotherapy Use in Older Women: Cancer and Leukemia Group B Protocol 369901. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.40.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawley ST, Fagerlin A, Janz NK, et al. Racial/ethnic disparities in knowledge about risks and benefits of breast cancer treatment: does it matter where you go? Health Serv Res. 2008;43:1366–1387. doi: 10.1111/j.1475-6773.2008.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jean-Pierre P, Fiscella K, Griggs J, et al. Race-based concerns over understanding cancer diagnosis and treatment plan: A. J Natl Med Assoc. 2010;102:184–189. doi: 10.1016/s0027-9684(15)30524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuyama RK, Grange C, Lyckholm LJ, et al. Cultural perceptions in cancer care among African-American and Caucasian patients. J Natl Med Assoc. 2007;99:1113–1118. [PMC free article] [PubMed] [Google Scholar]
- 47.Manfredi C, Kaiser K, Matthews AK, et al. Are racial differences in patient-physician cancer communication and information explained by background, predisposing, and enabling factors? J Health Commun. 2010;15:272–292. doi: 10.1080/10810731003686598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fogel J, Albert SM, Schnabel F, et al. Racial/ethnic differences and potential psychological benefits in use of the internet by women with breast cancer. Psychooncology. 2003;12:107–117. doi: 10.1002/pon.617. [DOI] [PubMed] [Google Scholar]
- 49.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005;23:5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 50.Levinson W, Kao A, Kuby A, et al. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med. 2005;20:531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber KM, Haunani Solomon D, Meyer BJ. A Qualitative Study of Breast Cancer Treatment Decisions: Evidence for Five Decision-Making Styles. Health communication (ahead-of-print) 2013:1–14. doi: 10.1080/10410236.2012.713775. [DOI] [PubMed] [Google Scholar]
- 52.Keating NL, Beth LM, Arora NK, et al. Cancer patients’ roles in treatment decisions: do characteristics of the decision influence roles? J Clin Oncol. 2010;28:4364–4370. doi: 10.1200/JCO.2009.26.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorin SS, Heck JE, Cheng B, et al. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166:2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 54.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 55.Lund MJ, Brawley OP, Ward KC, et al. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109:545–557. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 56.Gordon HS, Street RL, Jr, Sharf BF, et al. Racial differences in doctors’ information-giving and patients’ participation. Cancer. 2006;107:1313–1320. doi: 10.1002/cncr.22122. [DOI] [PubMed] [Google Scholar]
- 57.Hillen MA, Onderwater AT, van Zwieten MC, et al. Disentangling cancer patients’ trust in their oncologist: a qualitative study. Psychooncology. 2012;21:392–399. doi: 10.1002/pon.1910. [DOI] [PubMed] [Google Scholar]
- 58.O’Malley AS, Sheppard VB, Schwartz M, et al. The role of trust in use of preventive services among low-income African-American women. Prev Med. 2004;38:777–785. doi: 10.1016/j.ypmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 59.Gullatte MM, Brawley O, Kinney A, et al. Religiosity, Spirituality, and Cancer Fatalism Beliefs on Delay in Breast Cancer Diagnosis in African American Women. J Relig Health. 2009 doi: 10.1007/s10943-008-9232-8. [DOI] [PubMed] [Google Scholar]