Abstract
Conservative management of medial compartment knee osteoarthritis (OA) is a misleading term used to describe the application of medical, orthotic, and/or rehabilitative therapies exclusive of surgical interventions. The implication of this nomenclature is that these therapies offer satisfactory symptom relief, alter disease progression, and have limited side effects. Unfortunately, conservative therapeutic options possesses few, if any, characteristics of an ideal treatment, namely one that significantly alleviates pain, improves knee function, and reduces medial compartmental loading without adverse side effects. As uncompensated mechanical loading is a primary culprit in the development and progression of knee OA, we propose that the therapeutic perspective of conservative treatment should shift from pharmacological treatments, which have no influence on joint loading, minimal potential to alter joint function, substantial associated risks, and significant financial costs, towards minimally invasive load absorbing therapeutic interventions. A safe and effective minimally invasive medical device specifically engineered for symptomatic relief of medial knee OA by limiting joint contact forces has the potential to reduce the clinical and economic knee OA burden. This review characterizes the current standard of care recommendations for conservative management of medial compartment knee OA with respect to treatment efficacy, risk profile, and economic burden.
Key words: knee, osteoarthritis, conservative care.
Introduction
Osteoarthritis (OA) is a disease characterized by progressive articular cartilage destruction, ultimately leading to disabling pain and joint dysfunction. The knee is the most commonly affected joint and knee OA represents the leading cause of disability in the adult population.1–3 More than 1 in 3 Americans over 60 years of age have radiographic evidence of knee OA and 1 in 8 have symptomatic knee OA.4 With the continued aging of the population and the alarming obesity epidemic, the prevalence of OA is expected to increase by 40% by 2025.5 OA is also responsible for a substantial economic burden, accounting for $128 billion per year in direct and indirect costs in the United States alone.6–8 Overall, the clinical and economic burden of OA is anticipated to increase and will remain a major medical problem for decades to come.
A wide variety of treatment options are available to the patient with knee OA, categorized as non-pharmacological, pharmacological, and surgical. Commonly utilized non-pharmacological treatments include weight loss, lateral wedge insoles, bracing, and physical therapy. Pharmacological treatments include analgesics, nonsteroidal anti-inflammatory drugs (NSAIDS), opioids, hyaluronic acid or corticosteroid injections, and various drugs purported as disease-modifying osteoarthritis drugs (DMOADs). Surgical options include arthroscopic debridement and lavage, high tibial osteotomy, and unicompartmental and total knee arthroplasty. Despite the fact that all of the 12 existing guidelines for knee OA management dictate that optimal management of OA requires a combination of non-pharmacological and pharmacological modalities,9 these conservative therapies have major limitations. Perhaps the most notable shortcoming of non-pharmacological and pharmacological treatment is a failure to successfully correct the underlying pathology - namely, abnormal joint loading resulting in continued disease progression. The purpose of this paper is to summarize the clinical evidence on conservative care for knee OA treatment and to identify the attributes of the ideal treatment regimen in this patient population.
The typical knee osteoarthritis conservative care regimen
Conservative options for knee OA treatment can be classified as orthotic joint unloading therapies, analgesics, anti-inflammatories, opioids, DMOADs, and hyaluronic acid injections. In general, joint unloading therapies such as weight loss, lateral wedge insoles, and bracing, are the preferred first-line treatments for symptomatic knee OA. If symptom improvement is not realized after an extended period, generally 3 to 6 months of use, add-on therapy utilizing analgesics such as acetaminophen is recommended. Topical NSAIDs and capsaicin are also recommended as alternatives to oral analgesics or in combination with them. If acetaminophen does not provide sufficient analgesia, oral NSAIDs at their lowest effective dose are recommended, with the caution that long-term use should be avoided whenever possible because of their association with gastrointestinal side effects. In patients with elevated gastrointestinal risk, COX-2 inhibitors or nonselective NSAIDs in combination with a proton pump inhibitor are recommended. If acetaminophen, nonselective NSAIDs, and COX-2 inhibitors all prove insufficient (or intolerable), DMAODs such as glucosamine sulfate, chondroitin sulfate, or diacerein may be attempted. Opioids, with or without acetaminophen, may be used if other oral analgesics fail, although stronger opioids are discouraged except when very severe pain is present due to concerns of dependency and complications. Intra-articular injections of hyaluronic acid are considered if oral medications fail to provide symptomatic relief.
While the frequency of joint unloading modality use is unknown, the prevalence of analgesic use in symptomatic knee OA patients is staggering. Over 650,000 patients in the US chronically consume NSAIDS and over 350,000 have a chronic opioid prescription. Additionally almost 3 out of 4 knee OA patients, representing 3 million patients, have used analgesics in the last month.4
Limitations of conservative care for knee osteoarthritis treatment
Despite the liberal prescribing of conservative care for knee OA, three major therapeutic limitations warrant re-examining this treatment paradigm: unsatisfactory clinical efficacy for pain relief, potential for side effects with pharmacological options, and inability to delay disease progression.
Ineffectiveness of conservative care for symptomatic knee osteoarthritis
Conservative care for knee OA has unsatisfactory overall efficacy. The Osteoarthritis Research Society International (OARSI) Treatment Guidelines Committee estimated the effect sizes of common treatments for OA knee pain and collated the number of published knee OA treatment guidelines that voted for or against widespread use of the therapy (Figure 1).10 For reference, the term effect size refers to the standardized mean difference of effects between two therapies divided by the pooled standard deviation and can be interpreted as trivial (<0.2), small (0.2–0.49), moderate (0.5–0.79), or large (≥0.8) effects.11
Figure 1.
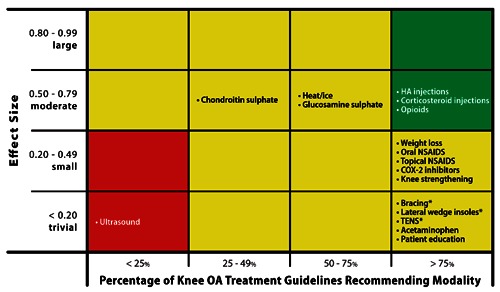
Relationship of effect size with degree of support from published guidelines for knee osteoarthritis (OA) treatments. Green shading represents characteristics of an ideal OA treatment, with moderate to large effect size and support from most (>75%) knee OA treatment guidelines. Red shading represents characteristics of an unsatisfactory OA treatment, with trivial to small effect size and support from few (<25%) knee OA treatment guidelines. Yellow shading represents characteristics of equivocal OA treatments, with effect size and knee OA treatment guideline support data not included in categories above. *Effect size not available.
Several noteworthy observations can be made from these data. First, many of the commonly prescribed knee OA treatments have poor efficacy. Second, of the five conservative treatments with moderate effect sizes, only HA injections and opioids are recommended by at least 75% of the knee OA treatment guidelines. Analgesics and NSAIDS are perhaps the most commonly utilized conservative care treatment with almost 3 out of 4 patients with symptomatic knee OA using them in the last month to manage pain.4 Unfortunately, the effect size of acetaminophen on knee OA pain is just 0.13, which implies a trivial clinical effect. The effectiveness of NSAIDS in pain amelioration is similarly poor with no statistical difference detected between subjects taking NSAIDS vs. placebo.12 The OARSI guidelines for viscosupplementation were guided by the results of a Cochrane review of 76 randomized trials published through 2006 that reported moderate effect sizes (range 0.54–0.61) at 1 to 4 weeks versus placebo for pain, function, and stiffness.13 However, clinical effectiveness diminished over time and, by 5 to 13 weeks, there was no evidence of benefit. Recently, a more comprehensive and timely analysis of the clinical effectiveness of viscosupplementation for knee OA has been published. Rutjes et al.14 identified 89 randomized controlled trials of viscosupplementation for knee OA published through January 2012. The authors concluded that viscosupplementation was associated with a short-term, clinically irrelevant benefit approximating a 0.9 cm improvement in pain severity on a 10-cm visual analogue scale. Additional observations with viscosupplementation for knee OA included even smaller effect sizes reported when outcome assessment was blinded and increased risks for serious adverse events (odds ratio 1.41).
The widespread adoption of these minimally effective conservative treatments should be a cause for concern. Although multimodal conservative therapy is widely advocated and unanimously supported by knee OA treatment position statements,15 this regimen is successful in less than 50% of patients after 12 weeks and,16 over 1 year, results in statistically significant, but practically minor improvements (effect size=0.3) in OA symptoms.17 Overall, there is a distinct mismatch between consensus knee OA treatment guidelines and best evidence data summaries derived from systematic reviews.
Risk of pharmacological treatments for symptomatic knee osteoarthritis
In addition to clinical ineffectiveness, use of pharmacological treatments is associated with an elevated risk of complications. The safety profile of acetaminophen is inconclusive,15 although some RCTs have reported associations with gastrointestinal bleeding,18 gastrointestinal-related hospitalization,19 and renal failure.20 Oral NSAIDS are associated with elevated risk of gastrointestinal side effects,21 gastrointestinal-related hospitalization,19 and myocardial infarction in comparison to placebo or no treatment.22 Opioids are strongly associated with deleterious effects including constipation, nausea, vomiting, dizziness, somnolence, dependence and risk of any complication,23,24 and, therefore, are generally reserved for patients with severe pain who do not respond to NSAIDS. Although COX-2 inhibitors result in fewer serious gastrointestinal side effects compared to NSAIDS,25 the risk of cardiovascular events is greater.22,26–28 Intra-articular HA injections have no known systemic risks although local adverse events such as pain and swelling are common.13,29 The risks associated with pharmacological treatment are particularly concerning in the elderly OA patient with several comorbidities who may be vulnerable to side effects from drug interactions.
Inability of conservative care to delay disease progression
The so-called joint-offloading therapies that are mainstays of symptomatic OA treatment paradoxically have the poorest clinical benefit (all effect sizes £0.2). In theory, weight loss, lateral wedge insoles, and bracing would be hypothesized to improve knee OA symptoms by reducing overall knee joint loading forces or by correcting joint malalignment. In practice, however, these modalities have limited usefulness. Intensive weight loss regimens in obese knee OA patients have resulted in lower peak knee forces on the order of 2 kg for every 1 kg body weight lost.30 However, weight loss is exceedingly difficult to maintain over the long-term with only 12% of participants maintaining at least 75% of lost weight and 40% actually gaining more weight than was lost 3 years following an intensive weight loss intervention.31 A 12-week study of multimodal therapy that encouraged knee OA patients with a body mass index >28 kg/m2 to lose at least 5% of body weight reported that only 14% of patients achieved this goal.16 Similarly, lateral wedge insoles and knee bracing provide no demonstrable clinical benefit on knee pain or disease progression, which may be partially attributable to poor compliance and patient discomfort.32–38
Pain amelioration accomplished with analgesics or anti-inflammatories has been shown to have no benefit on delaying OA progression and, paradoxically, some studies have reported that these therapies may actually encourage OA progression since presumably patients may be more active with higher resulting forces across the knee joint.39–42 This phenomenon, termed analgesic arthropathy, may be associated with any pain-relieving modality and is caused because pain is a protective mechanism that causes compensatory changes in gait patterns in order to reduce mechanical loading at the knee joint. Similar to findings with acetaminophen and NSAIDS, HA injections may actually increase medial compartment loading and accelerate joint deterioration.39 Corticosteroid injections are associated with reductions in knee pain, but no change in knee function, over 2 weeks; however, these clinical improvements disappear by 4 weeks.10 Considering their short therapeutic window and that corticosteroids can be safety injected up to just 4 times per year, intra-articular corticosteroids are not a viable treatment for reliable knee OA symptom relief.
Disease-modifying osteoarthritis drugs are those purported to modify joint structure, not just to alleviate pain. However, the results of DMOAD clinical trials are inconclusive, demonstrating the full range of positive and negative results.43 To date, no DMOAD has been approved by the FDA for the treatment of OA, which requires a demonstration of a structural and clinical benefit. Since articular cartilage is aneural and avascular, the symptoms of OA are generated by structures other than cartilage and, therefore, the primary target of DMAOD therapy appears to be misdirected.44 Even if DMOAD were to demonstrate successful reversal of cartilage degradation, the clinical meaningfulness of this finding is questionable given that OA affects all joint tissues including meniscus, synovium, and subchondral bone, not just articular cartilage.
Long-term consequences of chronic conservative care
Clinical implications of long-term conservative care
Long-term conservative care of knee OA results in little, if any, meaningful improvement in pain relief. Furthermore, no conservative care modality reliably retards OA progression. Regardless of which conservative measures are utilized, knee OA patients will variably, but predictably, experience disease progression, enter into a protracted treatment gap,45 and undergo TKA with the same frequency and at the same rate as if these measures were not employed.
A patient with knee OA and mild radiographic evidence of joint space narrowing will experience disease progression to a severity indicative of the need of TKA over a mean of 12 years, with over 75% of these patients advancing to this stage after 18 years.46 With an average age of OA symptom onset of approximately 60 years and a mean U.S. life expectancy of 78 years,47–50 over 75% of knee OA patients will eventually meet the radiographic criteria to warrant TKA. These patients will be forced to choose between undergoing TKA or, more commonly, refusing TKA at the expense of mobility and pain-free ambulation.51–53
In patients who elect to undergo arthroplasty for end-stage knee OA, the procedure reliably restores joint function and improves health-related quality of life.9 However, the procedure suffers from limitations. First, use of arthroplasty in elderly (>70 years) is associated with greater risk for perioperative complications versus their younger counterparts.54 Second, the lifespan of a TKA implant is poor in relation to the typical age of a TKA patient. From 1980 to 2006, the incidence of TKA has exponentially increased in patients aged 50 to 59 years.55 In fact, over 4.5 million Americans currently have an intact total knee prosthesis, including 5% of those 50 years and older.56 Given the earlier onset of knee OA and performance of TKA, the typical 10–15 year survival of a TKA implant, the continued aging of the population, the soaring rates of obesity, and the greater levels of residual pain after TKA in younger patients,57 it can be anticipated that many younger TKA patients will ultimately require revision surgery, a procedure with greater complication rates and lower treatment success rates compared to first-time TKA. Clearly, employing therapeutic strategies that delay or obviate the need for TKA would result in enormous clinical, economic, and societal benefits.
Economic implications of long-term conservative care
The economic burden of long-term conservative care is enormous. The estimated cost effectiveness of typical conservative modalities for knee OA treatment is listed in Table 1.58–62 Overall, knee OA treatments must be particularly cost effective in order for the typical patient to consider utilizing the therapy. Unfortunately, no known conservative treatment for knee OA has a cost per quality adjusted life year (QALY) below the commonly cited willingness to pay threshold of $1200 to $5700 per QALY in knee OA patients.58 This conclusion is confirmed by a systematic review reporting only limited evidence of cost effectiveness for conservative treatment of knee OA.63
Table 1. Cost effectiveness of selected knee osteoarthritis conservative modalities.
| Conservative modality | Cost effectiveness (Cost/QALY) |
| Maximum willingness to pay for knee osteoarthritis patients | $ 1200-570058 |
| Knee bracing | $ 600059 |
| Primary care weight loss program | $ 11,00059 |
| Intra-articular hyaluronic acid | $ 14,00060 |
| Nonsteroidal anti-inflammatory drugs | $ 15,00059 |
| COX-2 inhibitors | $ 71,00061 |
| Oxycodone | $ 76,00062 |
Unavailable cost-effectiveness data for acetaminophen, intra-articular corticosteroids, opioids, DMAOD, and lateral wedge insoles.
Direct costs attributable to conservative care modalities are considerable. OA patients spend $173 per year on medications and average 3.3 office visits per year.64 Viscosupplementation treatments cost $1700 to $3700 annually.65 As OA progresses, the cost of treatments concomitantly rises. The 3.6 million patients in the United States with end-stage knee OA spend almost $4000 per year on associated conservative treatments,45–58,63–66 resulting in $14.4 billion in annual costs. Despite the considerable costs of conservative care, these therapies arguably result in mediocre pain amelioration with no demonstrable change in disease progression. An ideal knee OA treatment would utilize the principle of reducing knee joint loading forces and would be initiated when early signs of radiographically confirmed knee OA are first identified. If such a therapy were developed, disease progression could be slowed, the need for joint arthroplasty could be delayed or obviated, and billions of dollars in annual costs for conservative therapies with limited clinical usefulness could be avoided.
Characteristics of the ideal knee osteoarthritis treatment
Unfortunately, no therapeutic option is available that possesses the characteristics of an ideal knee OA treatment, namely one that alleviates knee pain, improves knee function, reduces affected joint compartment loading without load transfer to adjacent joint surfaces, enjoys high patient acceptance, and is cost effective.67 A widely held position is that abnormal mechanical loading is the main culprit in the development of OA and, therefore, no drug can feasibly encourage healing until the underlying aberrant biomechanical malalignment issues are addressed.68–70 The fact that knee joint malalignment is an independent risk factor for knee OA in numerous studies is supportive of this hypothesis.71 Minimally invasive medical devices are widely used across many therapeutic areas and could potentially serve to fill this therapeutic void.70
OA onset and progression is largely influenced by excessive loading forces across the knee joint.72,73 Furthermore, unloading the knee of these forces may heal damaged cartilage.74 Therefore, it is intriguing to envision a minimally invasive implant that would improve patient symptoms, slow disease progression, and, ultimately, delay or obviate the need for TKA. Some have argued that adequate joint unloading may even renew or restore damaged tissue to normal.70,74 Such a therapy could reduce the reliance on TKA, with concomitant reductions in the associated clinical and economic burden of end-stage OA. A primary advantage of a minimally invasive implant over conservative unloading therapies is that the limitation of poor patient compliance is obviated.
Early interpositional devices utilized free-floating technology with anteroposterior motion of the implant and rotation during flexion and extension. However, the lack of appropriate fixation resulted in an unacceptably high number of device dislocations and revision surgeries.75–77 Extra-capsular (non-articular) medial compartment knee load absorber implants are currently under evaluation in clinical trials that require only a subcutaneous incision and provide device fixation at the medial distal femoral cortex and the medial proximal tibial cortex to achieve significant offloading of the medical compartment.78,79 Continued advancements in minimally invasive, joint offloading medical devices for knee OA treatment are intriguing, especially given the overall failure of conservative management to improve symptoms or halt disease progression.
Conclusions
Conservative care for knee OA is neither clinically effective for pain or disease progression nor cost effective within the constraints of the typical patient's willingness to pay. The decade-long search for a safe and efficacious DMOAD continues with disappointing results and does not address the fundamental causative factor of abnormal joint loading. New treatment modalities for knee OA should be pursued given the stagnation in new efficacious offerings. The minimally invasive medical device market is an ideal arena to explore the concept of a joint unloading device that meets all of the characteristics of an ideal knee OA treatment.
Acknowledgements:
the authors thank Mr. Randy Asher for graphical assistance.
References
- 1.Felson DT, Naimark A, Anderson J, et al. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 2.van Saase JL, van Romunde LK, Cats A, et al. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis. 1989;48:271–80. doi: 10.1136/ard.48.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91–7. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271–9. [PubMed] [Google Scholar]
- 5.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 6.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;427(Suppl):S6–15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 7.Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage degeneration: the impact in the new millennium. Clin Orthop Relat Res. 2001;391(Suppl):S14–25. [PubMed] [Google Scholar]
- 8.Samson DJ, Grant MD, Ratko TA, et al. Treatment of primary and secondary osteoarthritis of the knee. Evid Rep Technol Assess Full Rep. 2007;157:1–157. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–62. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–99. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 12.Scott DL, Berry H, Capell H, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford) 2000;39:1095–101. doi: 10.1093/rheumatology/39.10.1095. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD005321.pub2. CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutjes AW, Juni P, da Costa BR, et al. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180–91. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15:981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Snijders GF, den Broeder AA, van Riel PL, et al. Evidence-based tailored conservative treatment of knee and hip osteoarthritis: between knowing and doing. Scand J Rheumatol. 2011;40:225–31. doi: 10.3109/03009742.2010.530611. [DOI] [PubMed] [Google Scholar]
- 17.Patel S, Hossain FS, Paton B, Haddad FS. The effects of a non-operative multimodal programme on osteoarthritis of the knee. Ann R Coll Surg Engl. 2010;92:467–71. doi: 10.1308/003588410X12664192076052a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia Rodriguez LA, Hernandez-Diaz S. Relative risk of upper gastrointestinal complications among users of acetaminophen and nonsteroidal anti-inflammatory drugs. Epidemiology. 2001;12:570–6. doi: 10.1097/00001648-200109000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Rahme E, Barkun A, Nedjar H, et al. Hospitalizations for upper and lower GI events associated with traditional NSAIDs and acetaminophen among the elderly in Quebec, Canada. Am J Gastroenterol. 2008;103:872–82. doi: 10.1111/j.1572-0241.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- 20.Fored CM, Ejerblad E, Lindblad P, et al. Acetaminophen, aspirin, and chronic renal failure. N Engl J Med. 2001;345:1801–8. doi: 10.1056/NEJMoa010323. [DOI] [PubMed] [Google Scholar]
- 21.Ofman JJ, MacLean CH, Straus WL, et al. A metaanalysis of severe upper gastrointestinal complications of nonsteroidal antiinflammatory drugs. J Rheumatol. 2002;29:804–12. [PubMed] [Google Scholar]
- 22.Hernandez-Diaz S, Varas-Lorenzo C, Garcia Rodriguez LA. Non-steroidal antiin-flammatory drugs and the risk of acute myocardial infarction. Basic Clin Pharmacol Toxicol. 2006;98:266–74. doi: 10.1111/j.1742-7843.2006.pto_302.x. [DOI] [PubMed] [Google Scholar]
- 23.Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 2007;15:957–65. doi: 10.1016/j.joca.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–80. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Hooper L, Brown TJ, Elliott R, et al. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. BMJ. 2004;329:948. doi: 10.1136/bmj.38232.680567.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juni P, Nartey L, Reichenbach S, et al. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364:2021–9. doi: 10.1016/S0140-6736(04)17514-4. [DOI] [PubMed] [Google Scholar]
- 27.Caldwell B, Aldington S, Weatherall M, et al. Risk of cardiovascular events and celecoxib: a systematic review and meta-analysis. J R Soc Med. 2006;99:132–40. doi: 10.1258/jrsm.99.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aldington S, Shirtcliffe P, Weatherall M, Beasley R. Increased risk of cardiovascular events with parecoxib/valdecoxib: a systematic review and meta-analysis. N Z Med J. 2005;118:U1755. [PubMed] [Google Scholar]
- 29.Reichenbach S, Blank S, Rutjes AW, et al. Hylan versus hyaluronic acid for osteoarthritis of the knee: a systematic review and meta-analysis. Arthritis Rheum. 2007;57:1410–8. doi: 10.1002/art.23103. [DOI] [PubMed] [Google Scholar]
- 30.Aaboe J, Bliddal H, Messier SP, et al. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis Cartilage. 2011;19:822–8. doi: 10.1016/j.joca.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Grodstein F, Levine R, Troy L, et al. Three-year follow-up of participants in a commercial weight loss program. Can you keep it off? Arch Intern Med. 1996;156:1302–6. [PubMed] [Google Scholar]
- 32.Baker K, Goggins J, Xie H, et al. A randomized crossover trial of a wedged insole for treatment of knee osteoarthritis. Arthritis Rheum. 2007;56:1198–203. doi: 10.1002/art.22516. [DOI] [PubMed] [Google Scholar]
- 33.Maillefert JF, Hudry C, Baron G, et al. Laterally elevated wedged insoles in the treatment of medial knee osteoarthritis: a prospective randomized controlled study. Osteoarthritis Cartilage. 2001;9:738–45. doi: 10.1053/joca.2001.0470. [DOI] [PubMed] [Google Scholar]
- 34.Pham T, Maillefert JF, Hudry C, et al. Laterally elevated wedged insoles in the treatment of medial knee osteoarthritis. A two-year prospective randomized controlled study. Osteoarthritis Cartilage. 2004;12:46–55. doi: 10.1016/j.joca.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Bennell KL, Bowles KA, Payne C, et al. Lateral wedge insoles for medial knee osteoarthritis: 12 month randomised controlled trial. BMJ. 2011;342:d2912. doi: 10.1136/bmj.d2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warden SJ, Hinman RS, Watson MA, Jr, et al. Patellar taping and bracing for the treatment of chronic knee pain: a systematic review and meta-analysis. Arthritis Rheum. 2008;59:73–83. doi: 10.1002/art.23242. [DOI] [PubMed] [Google Scholar]
- 37.Brouwer RW, Jakma TS, Verhagen AP, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD004020.pub2.CD004020 [DOI] [PubMed] [Google Scholar]
- 38.Giori NJ. Load-shifting brace treatment for osteoarthritis of the knee: a minimum 2 1/2-year follow-up study. J Rehabil Res Dev. 2004;41:187–94. doi: 10.1682/jrrd.2004.02.0187. [DOI] [PubMed] [Google Scholar]
- 39.Briem K, Axe MJ, Snyder-Mackler L. Medial knee joint loading increases in those who respond to hyaluronan injection for medial knee osteoarthritis. J Orthop Res. 2009;27:1420–5. doi: 10.1002/jor.20899. [DOI] [PubMed] [Google Scholar]
- 40.Henriksen M, Simonsen EB, Alkjaer T, et al. Increased joint loads during walking--a consequence of pain relief in knee osteoarthritis. Knee. 2006;13:445–50. doi: 10.1016/j.knee.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Hurwitz DE, Sharma L, Andriacchi TP. Effect of knee pain on joint loading in patients with osteoarthritis. Curr Opin Rheumatol. 1999;11:422–6. doi: 10.1097/00002281-199909000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Ding C, Cicuttini F, Jones G. Do NSAIDs affect longitudinal changes in knee cartilage volume and knee cartilage defects in older adults? Am J Med. 2009;122:836–42. doi: 10.1016/j.amjmed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Brandt KD, Mazzuca SA. Lessons learned from nine clinical trials of disease-modifying osteoarthritis drugs. Arthritis Rheum. 2005;52:3349–59. doi: 10.1002/art.21409. [DOI] [PubMed] [Google Scholar]
- 44.Felson DT. The sources of pain in knee osteoarthritis. Curr Opin Rheumatol. 2005;17:624–8. doi: 10.1097/01.bor.0000172800.49120.97. [DOI] [PubMed] [Google Scholar]
- 45.London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses. 2011;76:887–92. doi: 10.1016/j.mehy.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 46.Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol. 2002;29:139–46. [PubMed] [Google Scholar]
- 47.Bachmeier CJ, March LM, Cross MJ, et al. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cartilage. 2001;9:137–46. doi: 10.1053/joca.2000.0369. [DOI] [PubMed] [Google Scholar]
- 48.Desmeules F, Dionne CE, Belzile E, et al. Waiting for total knee replacement surgery: factors associated with pain, stiffness, function and quality of life. BMC Musculoskelet Disord. 2009;10:52. doi: 10.1186/1471-2474-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kauppila AM, Kyllonen E, Mikkonen P, et al. Disability in end-stage knee osteoarthritis. Disabil Rehabil. 2009;31:370–80. doi: 10.1080/09638280801976159. [DOI] [PubMed] [Google Scholar]
- 50.Heron M, Hoyert DL, Murphy SL, et al. National vital statistics reports: deaths: final data for 20062009. 57((14)) Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf. [PubMed] [Google Scholar]
- 51.Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54:3212–20. doi: 10.1002/art.22146. [DOI] [PubMed] [Google Scholar]
- 52.Hawker GA, Wright JG, Badley EM, Coyte PC. Perceptions of, and willingness to consider, total joint arthroplasty in a population-based cohort of individuals with disabling hip and knee arthritis. Arthritis Rheum. 2004;51:635–41. doi: 10.1002/art.20524. [DOI] [PubMed] [Google Scholar]
- 53.Hawker GA, Wright JG, Coyte PC, et al. Determining the need for hip and knee arthroplasty: the role of clinical severity and patients' preferences. Med Care. 2001;39:206–16. doi: 10.1097/00005650-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 54.National Institutes of Health. NIH Consensus Statement on total knee replacement December 8-10, 2003. J Bone Joint Surg Am. 2004;86A:1328–35. doi: 10.2106/00004623-200406000-00031. [DOI] [PubMed] [Google Scholar]
- 55.Leskinen J, Eskelinen A, Huhtala H, et al. The incidence of knee arthroplasty for primary osteoarthritis grows rapidly among baby boomers: a population-based study in Finland. Arthritis Rheum. 2012;64:423–8. doi: 10.1002/art.33367. [DOI] [PubMed] [Google Scholar]
- 56.Weinstein AM, Rome B, Reichmann WM, et al., editors. How many Americans are currently living with total knee replacement? San Francisco, CA: American Academy of Orthopaedic Surgeons; 2012. [Google Scholar]
- 57.Bonnin MP, Basiglini L, Archbold HA. What are the factors of residual pain after uncomplicated TKA? Knee Surg Sports Traumatol Arthrosc. 2011;19:1411–7. doi: 10.1007/s00167-011-1549-2. [DOI] [PubMed] [Google Scholar]
- 58.Byrne MM, O'Malley K, Suarez-Almazor ME. Willingness to pay per quality-adjusted life year in a study of knee osteoarthritis. Med Decis Making. 2005;25:655–66. doi: 10.1177/0272989X05282638. [DOI] [PubMed] [Google Scholar]
- 59.Segal L, Day SE, Chapman AB, Osborne RH. Can we reduce disease burden from osteoarthritis? Med J Aust. 2004;180(Suppl 5):S11–7. doi: 10.5694/j.1326-5377.2004.tb05907.x. [DOI] [PubMed] [Google Scholar]
- 60.Torrance GW, Raynauld JP, Walker V, et al. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (Part 2 of 2): economic results. Osteoarthritis Cartilage. 2002;10:518–27. doi: 10.1053/joca.2001.0513. [DOI] [PubMed] [Google Scholar]
- 61.Elliott RA, Hooper L, Payne K, et al. Preventing non-steroidal anti-inflammatory drug-induced gastrointestinal toxicity: are older strategies more cost-effective in the general population? Rheumatology (Oxford) 2006;45:606–13. doi: 10.1093/rheumatology/kei241. [DOI] [PubMed] [Google Scholar]
- 62.Marshall DA, Strauss ME, Pericak D, et al. Economic evaluation of controlled-release oxycodone vs oxycodone-acetaminophen for osteoarthritis pain of the hip or knee. Am J Manag Care. 2006;12:205–14. [PubMed] [Google Scholar]
- 63.Pinto D, Robertson MC, Hansen P, Abbott JH. Cost-effectiveness of nonpharmacologic, nonsurgical interventions for hip and/or knee osteoarthritis: systematic review. Value Health. 2012;15:1–12. doi: 10.1016/j.jval.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Lanes SF, Lanza LL, Radensky PW, et al. Resource utilization and cost of care for rheumatoid arthritis and osteoarthritis in a managed care setting: the importance of drug and surgery costs. Arthritis Rheum. 1997;40:1475–81. doi: 10.1002/art.1780400816. [DOI] [PubMed] [Google Scholar]
- 65.Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Man Care Pharmacy. 2007;13:3–19. doi: 10.18553/jmcp.2007.13.s4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113–21. doi: 10.1001/archinternmed.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kon E, Filardo G, Drobnic M, et al. Non-surgical management of early knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20:436–49. doi: 10.1007/s00167-011-1713-8. [DOI] [PubMed] [Google Scholar]
- 68.Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93:1–24. doi: 10.1016/j.mcna.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Lafeber FP, Intema F, Van Roermund PM, Marijnissen AC. Unloading joints to treat osteoarthritis, including joint distraction. Curr Opin Rheumatol. 2006;18:519–25. doi: 10.1097/01.bor.0000240366.54960.a1. [DOI] [PubMed] [Google Scholar]
- 70.Waller C, Hayes D, Block JE, London NJ. Unload it: the key to the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19:1823–9. doi: 10.1007/s00167-011-1403-6. [DOI] [PubMed] [Google Scholar]
- 71.Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459–67. doi: 10.1002/art.24336. [DOI] [PubMed] [Google Scholar]
- 72.Block JA, Shakoor N. The biomechanics of osteoarthritis: implications for therapy. Curr Rheumatol Rep. 2009;11:15–22. doi: 10.1007/s11926-009-0003-7. [DOI] [PubMed] [Google Scholar]
- 73.Radin EL. Who gets osteoarthritis and why? An update. J Rheumatol. 2005;32:1136–8. [PubMed] [Google Scholar]
- 74.Radin EL, Burr DB. Hypothesis: joints can heal. Semin Arthritis Rheum. 1984;13:293–302. doi: 10.1016/0049-0172(84)90031-3. [DOI] [PubMed] [Google Scholar]
- 75.Sisto DJ, Mitchell IL. UniSpacer arthroplasty of the knee. J Bone Joint Surg Am. 2005;87:1706–11. doi: 10.2106/JBJS.D.02339. [DOI] [PubMed] [Google Scholar]
- 76.Scott RD. UniSpacer: insufficient data to support its widespread use. Clin Orthop Relat Res. 2003:164–6. doi: 10.1097/01.blo.0000093027.56370.a2. [DOI] [PubMed] [Google Scholar]
- 77.Bailie AG, Lewis PL, Brumby SA, et al. The Unispacer knee implant: early clinical results. J Bone Joint Surg Br. 2008;90:446–50. doi: 10.1302/0301-620X.90B4.20319. [DOI] [PubMed] [Google Scholar]
- 78.Clifford A, O'Connell M, Gabriel S, et al. The KineSpring load absorber implant: rationale, design and biomechanical characterization. J Med Eng Technol. 2011;35:65–71. doi: 10.3109/03091902.2010.535592. [DOI] [PubMed] [Google Scholar]
- 79.Allen MJ, Townsend KL, Bauer TW, et al. Evaluation of the safety of a novel knee load-bypassing device in a sheep model. J Bone Joint Surg Am. 2012;94:77–84. doi: 10.2106/JBJS.J.00918. [DOI] [PubMed] [Google Scholar]


