Abstract
Objective:
To establish criteria for the diagnosis of progressive multifocal leukoencephalopathy (PML).
Methods:
We reviewed available literature to identify various diagnostic criteria employed. Several search strategies employing the terms “progressive multifocal leukoencephalopathy” with or without “JC virus” were performed with PubMed, SCOPUS, and EMBASE search engines. The articles were reviewed by a committee of individuals with expertise in the disorder in order to determine the most useful applicable criteria.
Results:
A consensus statement was developed employing clinical, imaging, pathologic, and virologic evidence in support of the diagnosis of PML. Two separate pathways, histopathologic and clinical, for PML diagnosis are proposed. Diagnostic classification includes certain, probable, possible, and not PML.
Conclusion:
Definitive diagnosis of PML requires neuropathologic demonstration of the typical histopathologic triad (demyelination, bizarre astrocytes, and enlarged oligodendroglial nuclei) coupled with the techniques to show the presence of JC virus. The presence of clinical and imaging manifestations consistent with the diagnosis and not better explained by other disorders coupled with the demonstration of JC virus by PCR in CSF is also considered diagnostic. Algorithms for establishing the diagnosis have been recommended.
Interest in progressive multifocal leukoencephalopathy (PML) has increased considerably since its observation in association with natalizumab treatment for Crohn disease and multiple sclerosis in 2005.1–3 Publications on PML have increased fivefold in the 30 years from 1980 to 2010. Other monoclonal therapies and other drugs have also been reported to be associated with an increased risk of PML4 and prognosis has improved considerably. Therefore, establishing the diagnosis of PML has assumed a greater importance than when it was considered a universally fatal complication of an oftentimes underlying lymphoproliferative malignancy.
The approach to diagnosis of PML has evolved considerably since its initial description in 1958.5 Initially, the diagnosis of PML was predicated on brain histopathology as there were no clinical, laboratory, or radiographic features that would unequivocally establish the diagnosis. The histopathology was characterized by a classic triad of demyelination, bizarre astrocytes, and oligodendroglial nuclear inclusions. The uniqueness of the concurrence of these histopathologic findings alerted Astrom et al.5 to the novelty of the disorder. The subsequent demonstration of the causative polyomavirus, JC virus, in 1971,6 permitted the use of electron microscopy or immunohistochemical techniques to demonstrate the virus in tissue specimens.7,e1 The next advance occurred with the establishment of PCR to amplify JC virus DNA from brain and CSF.8,e2
The etiology of PML is a ubiquitous polyomavirus that infects 50% or more of the adult population throughout the world. PML remains an extraordinarily rare complication of this infection in otherwise normal persons and almost always occurs in the setting of predisposing immunosuppressive conditions. In the recent past, it has been recognized that PML is not the only brain disorder caused by JC virus. Other disorders that have been described include granule cell neuronopathy of the cerebellum9 and a fulminant JC virus encephalopathy involving cortical pyramidal neurons.10 On occasion, the pathologic findings in a patient with PML include features that are indistinguishable from these 2 disorders,11 suggesting that some overlap may exist and is likely the consequence of viral mutations.12 The virus has also been found in the brains of otherwise normal individuals (reviewed in White and Khalili13). Therefore, the simple demonstration of the virus, either in tissue or CSF, is insufficient to establish the diagnosis of PML.
No single criterion establishes the diagnosis of PML; rather, it requires clinical, imaging, and virologic evidence. Recently, a working group of German investigators with expertise in neurology, virology, hematology, and pharmacovigilance proposed a case definition for PML developing in association with monoclonal antibodies.14 Shortcomings in this proposed schema include 1) limitation to PML in the setting of monoclonal antibodies; 2) heavy reliance on the demonstration of JC virus DNA by PCR in CSF without addressing the sensitivity and specificity of the assay; 3) underemphasis of the value of cranial MRI abnormalities occurring before clinical symptoms become evident; and 4) liberal criteria for excluding PML with failure to account for patients having more than one neurologic disease concomitantly.
PATHOLOGIC, CLINICAL, AND RADIOGRAPHIC FEATURES OF PML
Pathology.
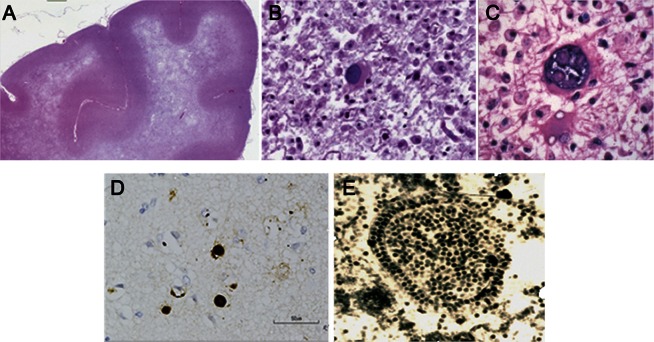
The cardinal feature of PML is demyelination, which is apparent both macroscopically and microscopically. Demyelination may, on rare occasions, be monofocal, but it typically occurs as a multifocal process, suggesting a hematologic spread of the virus. These lesions may occur in any location in the white matter and range in size from 1 millimeter to several centimeters5,e3; larger lesions are not infrequently the result of coalescence of multiple smaller lesions.15 The myelin loss may be very extensive, involving an entire hemisphere,15 and may result in atrophy of the affected structures. The histopathologic hallmarks of PML are a triad5,e3 of multifocal demyelination (figure 1A), hyperchromatic, enlarged oligodendroglial nuclei (figure 1B), and enlarged bizarre astrocytes with lobulated hyperchromatic nuclei (figure 1C). The latter may be seen to undergo mitosis and appear malignant, which has resulted in a mistaken diagnosis of glioma on occasion.3 Electron microscopic examination or immunohistochemistry reveals JC virus in the oligodendroglial cells. These virions measure 28–45 nm in diameter and appear singly or in dense crystalline arrays (figure 1E).5,e3 Less frequently, the virions are detected in reactive astrocytes and uncommonly in macrophages that are engaged in removing the affected oligodendrocytes.16,e4 JC virus antigens can be detected by immunostaining in oligodendrocytes and astrocytes, and, rarely, in cerebellar granule cell neurons17 and cortical pyramidal neurons.18 In situ hybridization and in situ PCR for JC virus DNA or immunostaining for JC virus antigen (figure 1D) allows for detection of the virus in the infected cells in formalin-fixed tissue.19 Historically, PML was regarded as being devoid of lymphocytic or plasma cell infiltration,15 but the presence of inflammatory cells was associated, even prior to the AIDS pandemic, with improved prognosis in the rare PML patient.20 The presence of an inflammatory infiltrate is typically a feature of relative immune preservation or immune reconstitution in the setting of PML.
Figure 1. Pathology of progressive multifocal leukoencephalopathy.
(A) Luxol fast blue staining with hematoxylin counterstain of the frontal lobe in a patient with progressive multifocal leukoencephalopathy (PML) shows extensive multifocal and confluent areas of demyelination. Small islands of demyelination coalesce to produce large confluent areas resulting in a “ground glass” bright appearance on T2-weighted MRI scan. (B) Enlarged oligodendrocyte with a large inclusion-bearing nucleus is characteristic of PML. No discrete intranuclear inclusion is seen. (C) A large bizarre astrocyte is depicted. (D) Immunostaining with polyclonal antibody to JC virus from Abcam Inc. shows dark brown staining of nuclei of several oligodendrocytes. (E) Electron micrograph of crystalline array of assembled JC virions in nuclei of infected oligodendrocyte in PML brain lesion. Virions measure 40 nm in diameter.
Clinical features.
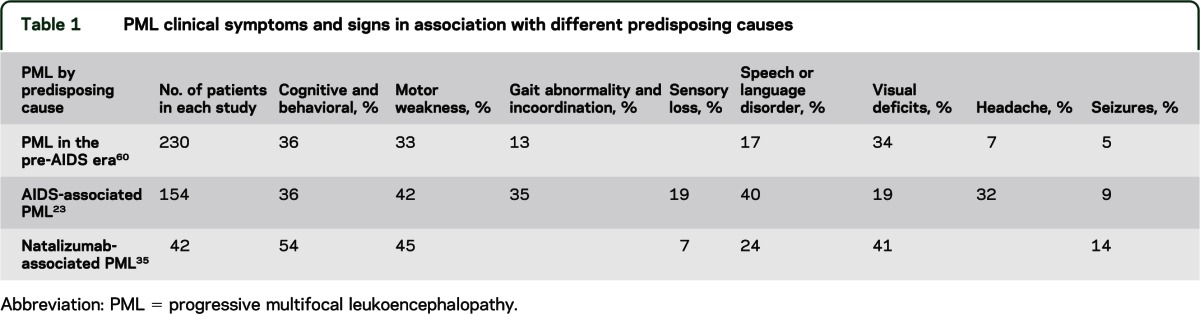
As virtually any area of the brain may be involved by PML, the clinical manifestations are quite diverse. Some variation in the frequency of clinical features appears to depend on the underlying predisposing cause of PML (table 1). Behavioral and cognitive abnormalities are seen in one-third to one-half of all patients. Among the common clinical findings are motor weakness, gait abnormalities, visual field deficits, speech and language disturbances, and incoordination. Sensory loss, seizures, headache, and diplopia occur less frequently. The predisposing condition for PML does not preclude any clinical abnormality. While multiple deficits may be observed, particularly in advanced disease, the disorder may present with but one salient objective neurologic abnormality at onset and may remain monofocal both clinically and radiographically.
Table 1.
PML clinical symptoms and signs in association with different predisposing causes
Optic nerve disease has not been reported with PML; visual deficits are usually due to involvement of the optic radiations. Although spinal cord involvement by PML has been reported in pathologic specimens, it is exceptionally rare and myelopathic clinical features have yet to be described.21,e5–e8 The failure to recognize myelopathic signs and symptoms may be the consequence of overwhelming brain involvement in these unusual patients. PML does not involve the peripheral nervous system.
Neuroimaging.
In the appropriate clinical context, brain imaging may strongly support the diagnosis of PML. CT of the brain in PML reveals hypodense lesions of the affected white matter. On CT scan, the lesions of PML exhibit no mass effect and infrequently contrast enhance. A “scalloped” appearance beneath the cortex is noted when there is involvement of the subcortical arcuate fibers.22 Cranial MRI is far more sensitive to the presence of the white matter lesions of PML than CT scan.22 MRI shows hyperintense lesions on T2-weighted images and fluid-attenuated inversion recovery (FLAIR) images in the affected regions (figure 2). On T1-weighted images, these lesions are hypointense. With CT scan, faint, typically peripheral, contrast enhancement may be observed in 5%–10% of cases.22,23 As many as 15% of patients with HIV-associated PML may exhibit gadolinium enhancement on MRI,23 and gadolinium enhancement has been observed in 40% of natalizumab-associated PML at the time of diagnosis.24–26 The lesions of PML may occur virtually anywhere in the brain and although characteristically multifocal, they need not be. Indeed, natalizumab-associated PML is often monofocal, with frontal lobe lesions predominating.27 In a review of the first 40 cases of natalizumab-associated PML, lesions were most frequently large (>3 cm), subcortical, and exhibited a sharp border toward the cortex and ill-defined border toward the white matter on T2-weighted image.26 A proposal in 2006 for distinguishing multiple sclerosis (MS) from PML lesions28 has recently been revised.26 In every radiographic series of PML, the frontal lobes and parieto-occipital regions are the regions that appear to be most commonly affected, presumably as a consequence of their volume. However, isolated or associated involvement of the basal ganglia, external capsule, and posterior fossa structures (cerebellum and brainstem) may be seen as well.22,e9
Figure 2. MRI in progressive multifocal leukoencephalopathy.
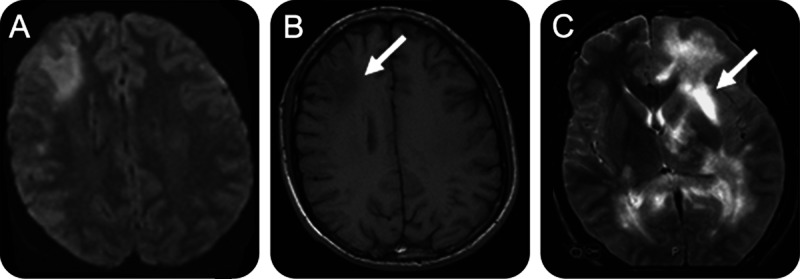
(A) Fluid-attenuated inversion recovery image with large subcortical lesion of right frontal lobe. A smaller lesion is observed posterior to this lesion. (B) T1-weighted image shows hypointense lesion (arrow) in the right frontal lobe. (C) T2-weighted image from another patient with extensive high signal intensity lesions in the white matter sparing the cortex. An area of hyperintensity similar to that of CSF suggests an area of cavitation.
In HIV infection, other diseases may affect the white matter in a similar manner, including AIDS dementia. Radiographic distinctions between AIDS dementia and PML include a greater propensity for the latter to involve the subcortical white matter, hypointensity on T1-weighted imaging, and occasional contrast enhancement.22 Cytomegalovirus lesions are typically located in the periventricular white matter and centrum semiovale.29,e10 Subependymal enhancement is sometimes observed as a consequence of cytomegalovirus infection29 although it is not often detected on imaging.30 Other potentially HIV-associated disorders that may result in hyperintense signal abnormalities of the white matter resembling PML include varicella-zoster leukoencephalitis,31 an MS-like illness,32 acute disseminated encephalomyelitis,33,e11 CNS vasculitis,34 a reversible leukoencephalopathy associated with nucleoside analogue antiretrovirals,35 and white matter edema associated with primary or metastatic brain tumors. Almost always, the clinical features, laboratory findings, and associated radiographic features enable the correct diagnosis.
Distinguishing white matter lesions of PML from those of MS can also be difficult. MRI FLAIR images are most sensitive in detecting the lesions of PML, even in the posterior fossa, in contradistinction to MS. Unlike MS, PML often occurs at the gray–white junction, is often monofocal (typically affecting the frontal lobes), and may be large, whereas MS lesions generally predominate periventricularly and are smaller. A Dawson finger pattern (sausage-shaped hyperintensity oriented peripendicular to the ventricular surface) is classic for MS, but not observed in PML. Diffusion-weighted imaging (DWI) may be helpful in determining the relative age of a lesion and DWI lesions are frequently observed with PML. These lesions may be mistaken for an acute cerebrovascular insult. On T2-weighted imaging, the affected tissue of PML has a “ground glass” appearance. About 50% of PML associated with natalizumab, where inflammation is common with PML, exhibits gadolinium enhancement that is a faint rim of enhancement or a speckled interior enhancement.
The role of other neuroimaging techniques, such as magnetization transfer MRI, magnetic resonance spectroscopy, SPECT, and PET, is limited. Magnetization transfer MRI studies have been suggested for monitoring the degree of demyelination in PML.36 Magnetic resonance spectroscopy reveals a decrease in N-acetylaspartate and creatine and increased choline products, myo-inositol, and lactate in the lesions of PML.37,e12-13 These changes likely reflect neuronal loss and cell membrane and myelin breakdown.37 Cerebral angiography is not routinely performed, but exhibited arteriovenous shunting and a parenchymal blush in the absence of contrast enhancement on MRI in 4 of 6 patients in one study.38 Pathologic studies suggested that small-vessel proliferation and perivascular inflammation explained these unexpected angiographic features.38 Thallium-201 SPECT (Tl201 SPECT) generally reveals no uptake in the lesions of PML,39 but not invariably. A patient with contrast-enhancing MRI lesions and a positive Tl201 SPECT40 and 2 patients with both positive Tl201 and gallium SPECT mistakenly diagnosed as CNS lymphoma41 have been reported. PET imaging with 18F fluorodeoxyglucose typically demonstrates hypometabolic lesions.42 In one instance, 18F PET at conventional (60 minutes after injection) and delayed (300 minutes after injection) were unable to distinguish pathologically proven PML from a malignant brain lesion.43
Laboratory studies.
The vast majority of HIV-infected patients with PML have CD4 lymphocyte counts <200 cells/mm3. In 3 separate series of AIDS-related PML,23,44,e7 the mean CD4 count ranged from 84 to 104 cells/mm3. However, in the largest series of HIV-associated PML,23 more than 10% of patients had CD4 lymphocyte counts in excess of 200 cells/mm3. In the era of highly active antiretroviral therapy (HAART), the percentage of HIV-associated PML with CD4 counts exceeding 200 cells/mm3 may be higher.
CSF examination is very helpful in excluding other diagnoses. Cell counts are usually less than 20 cells/mm3.23 In one large study, the median cell count was 2 cells/mm3 and the mean was 7.7 cells/mm3.23 In that same study, 55% had an abnormally elevated CSF protein23 with the highest recorded value being 208 mg/dL (2.08 g/L); however, the mean value was 66.5 mg/dL. Hypoglycorrhachia was observed in less than 15%. These abnormalities are not inconsistent with those previously reported to occur with HIV infection alone45,e14–e15 and may not necessarily be attributable to PML.
The greatest value of the CSF is demonstrating the presence of JC virus by PCR. Several studies44,46,47 have demonstrated a high sensitivity and specificity of CSF PCR for JC virus in PML. Many authorities regard the demonstration of JC viral DNA coupled with the appropriate clinical and radiologic features sufficient to be diagnostic of PML, thus obviating the need for brain biopsy.48 Quantitative PCR techniques for JC virus in biological fluids continue to be refined.49 Prior to the development of ultrasensitive PCR techniques for JC virus, the sensitivity of this test was on the order of 75%.44 However, the sensitivity with newer ultrasensitive techniques is >95%. Although amplification of the virus from the CSF in the absence of PML has been considered very unlikely, a low copy number of JC virus was reported in 2 of 515 CSF samples from patients without PML using these ultrasensitive techniques.50 Since JC virus viremia can occur in healthy individuals, any contamination of the CSF with blood has the potential for providing a false-positive result. Despite the high sensitivity of the PCR assay, a negative PCR does not rule out PML, and in some cases biopsy of the brain with PCR amplification from the brain tissue has been employed to establish the diagnosis51; however, reliance on JC virus PCR alone to demonstrate the presence of the virus in tissue remains investigational and should be viewed cautiously.
Most diagnostic laboratories are able to detect >200 copies of JC virus DNA/mL of CSF. In HIV-infected patients, the viral load is usually high, and this is sufficient for the diagnosis; however, the ability to detect JC virus declines substantially following exposure to HAART and in the presence of higher CD4 counts,52 and may render laboratory-confirmed diagnosis difficult. This is particularly problematic in patients started on HAART who develop PML–immune reconstitution inflammatory syndrome as the first manifestation of PML. In patients with MS and other autoimmune diseases, the viral loads are often lower and the sensitivity of the assay can be critical in establishing the diagnosis. The laboratory at the NIH is able to reliably detect up to 10 copies/mL of JC virus using primers from the N terminal of large T antigen, which is a conserved region of the virus.53 Extraction of DNA from at least 200 μL of CSF prior to amplification helps increase the sensitivity. In light of the rare occurrence of detection of low viral copy numbers of JC virus in CSF in the absence of PML,50 this finding in isolation, i.e., without clinical or imaging findings to suggest PML, must be interpreted cautiously.
Diagnostic measures.
Many authorities considered the demonstration of JC viral DNA coupled with the appropriate clinical and imaging features to be diagnostic of PML, therefore obviating the need for a brain biopsy.48 Occasionally, either the clinical or imaging features are unconvincing or CSF PCR is negative or has not been performed. A matrix to assist in determining diagnostic certainty in these circumstances in the absence of brain biopsy is provided (table 2).
Table 2.
Establishing the diagnosis with clinical, radiographic, and laboratory dataa
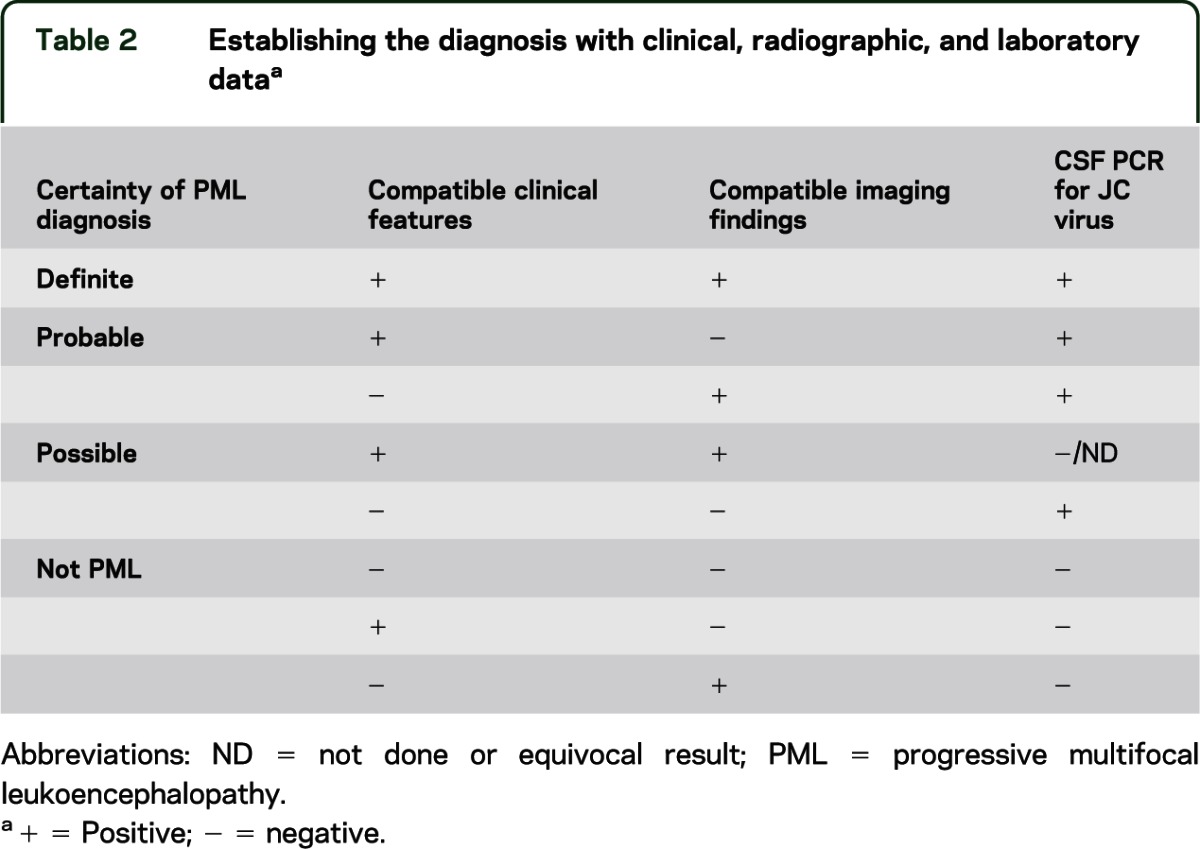
The histopathologic triad5 is rather convincing evidence of the disorder as this unique cluster is not observed in other neurologic disorders. Nonetheless, there are examples in which PML has been misdiagnosed at the time of biopsy as a glioma.3 Furthermore, although historically PML lesions were not associated with inflammation, an inflammatory response may be observed, particularly in individuals with natalizumab-associated PML54,e16 and increasingly in other circumstances,55,e17 including in HIV infection following HAART.56,e18 The occurrence of PML in the setting of MS has added to the complexity of the diagnosis due to the underlying demyelination observed with each, resulting in radiologic and pathologic findings that may overlap.
Relying on tissue diagnosis (table 3) requires brain biopsy, which is not without potential sampling errors and complications. Brain biopsy for focal lesions in the AIDS population was associated with a 93%57–96%58 sensitivity, a 12% perioperative morbidity, and a mortality of 2%.57 Light microscopy and immunohistochemistry techniques alone may be insufficient in establishing the etiology and PCR enhances the yield when tissues obtained by stereotactic biopsy are nondiagnostic.59
Table 3.
Establishing the diagnosis with histopathology
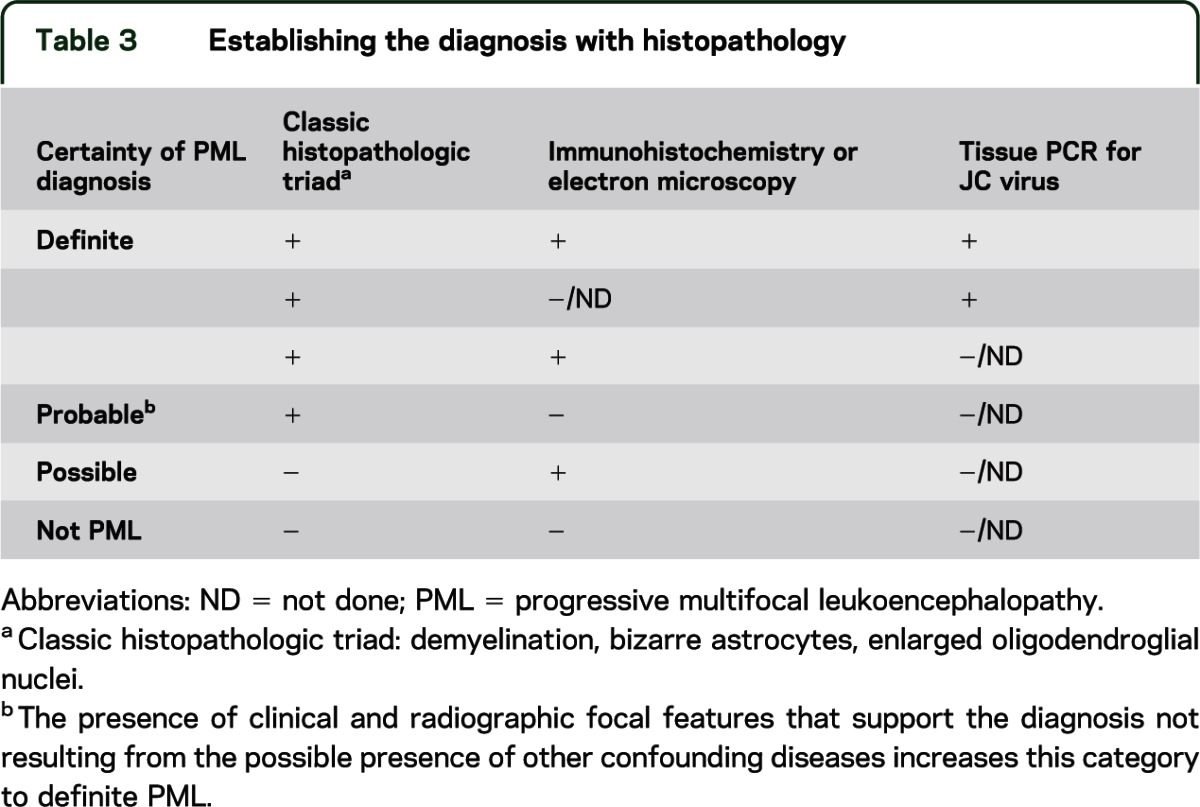
Therefore, there are 2 approaches to establishing the diagnosis of PML: namely, securing the diagnosis with tissue or, as is more commonly practiced, establishing the diagnosis with clinical or radiographic criteria coupled with demonstrating the presence of the virus in the CSF compartment.
DISCUSSION
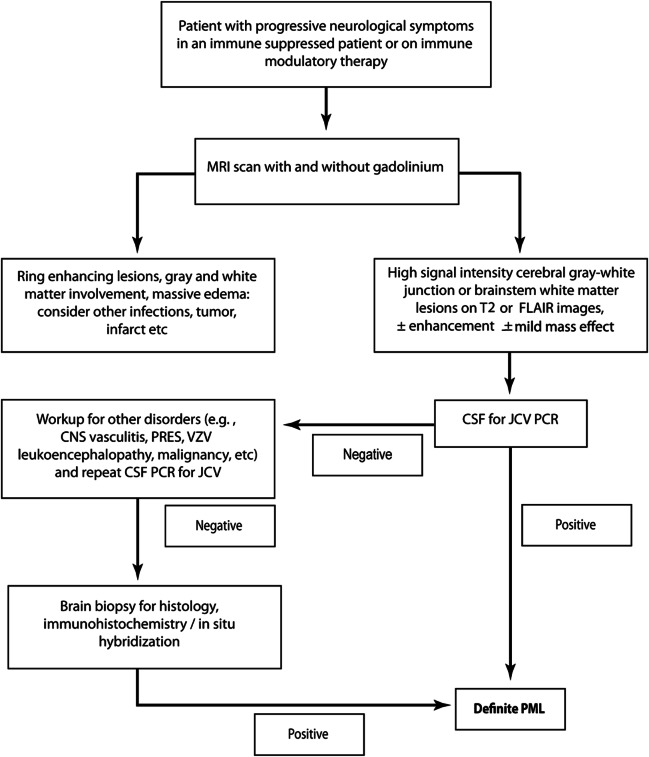
We provide a framework for the diagnosis of PML (tables 2 and 3). The algorithm in figure 3 demonstrates that a secure diagnosis of PML can be established in 2 fashions. In one, the characteristic histopathologic features of PML are coupled with evidence of the presence of JC virus by electron microscopy, immunohistochemistry, or PCR. Alternatively, the presence of classic radiographic findings and clinical features consistent with the diagnosis coupled with a positive CSF JC virus PCR is also sufficient for the unequivocal diagnosis of PML. The diagnosis of definite PML is predicated on irrefutable evidence. Relegation to probable PML is based on a missing critical feature, either the failure to demonstrate JC virus when histopathologic criteria are employed or the absence of either clinical or imaging features despite CSF JC virus PCR positivity when the diagnosis is based on clinical features. Patients with probable PML should be managed as PML. The diagnosis of PML remains less certain in the possible PML category; however, this category is particularly helpful when attempting to assess the chance of PML in at-risk patient groups. Management of possible PML is a clinical decision.
Figure 3. Algorithm for diagnosing progressive multifocal leukoencephalopathy.
FLAIR = fluid-attenuated inversion recovery; JCV = JC virus; PML = progressive multifocal leukoencephalopathy; PRES = posterior reversible encephalopathy syndrome; VZV = varicella-zoster virus.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Maria Barhams, MHSA, at the NHLBI for providing a comprehensive search of the relevant medical literature for the AAN Neuroinfectious Disease Committee.
GLOSSARY
- DWI
diffusion-weighted imaging
- FLAIR
fluid-attenuated inversion recovery
- HAART
highly active antiretroviral therapy
- MS
multiple sclerosis
- PML
progressive multifocal leukoencephalopathy
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
All the authors are members of the PML Guidelines Committee and contributed to the study concept and design. Dr. Berger drafted the first and final versions of the manuscript. Drs. Aksamit, Clifford, Davis, Koralnik, Sejvar, Bartt, Major, and Nath provided critical revisions of the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
J. Berger serves on Data Safety Boards for Millennium and Amgen; has served on scientific advisory boards or as a consultant for Amgen, Bayer, BiogenIdec, Eisai, Genzyme, GlaxoSmithKline, Genentech, Novartis, and Pfizer; has received speaking fees from CMSC, AAN, Bayer, and BiogenIdec; and receives research support from the PML Consortium. A. Aksamit reports no disclosures. D. Clifford is funded by NIH grants U10 NS077384, MH22005, AI25903, and the Alzheimer Association. He serves on Data Safety Boards for Biogen, GSK, Millennium, Genzyme, Genentech, and Pfizer; has been a consultant to Amgen, Brinker, Biddle, Reath (PML Consortium), Genentech, Genzyme, Bristol-Myers Squibb, Millennium, Biogen Idec, and Pfizer; has received research support from Biogen Idec, NeurogesX, Tibotec, and Pfizer; and has received speaking fees from University of Kentucky and CMSC/ACTRIMS and ECTRIMS. L. Davis reports no disclosures. I. Koralnik is funded by NIH grants R56-NS041198, R01-NS047029, R01-NS074995, and K24-NS060950; has received a research grant from Biogen Idec and the National MS Society; served on scientific advisory boards for Hoffmann La-Roche, GlaxoSmithKline, and Merck Serono; received consulting fees from Bristol-Myers Squibb, Ono Pharmaceuticals, Merck Serono, Hoffmann La Roche, GlaxoSmithKline, Perseid Therapeutics, Vertex Pharmaceutical, and Johnson & Johnson; and receives royalties from Up-To-Date for topics on the management of HIV and CNS mass lesions and on PML. J. Sejvar, R. Bartt, E. Major, and A. Nath report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 2005;353:369–374 [DOI] [PubMed] [Google Scholar]
- 2.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005;353:375–381 [DOI] [PubMed] [Google Scholar]
- 3.Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med 2005;353:362–368 [DOI] [PubMed] [Google Scholar]
- 4.Berger JR. Progressive multifocal leukoencephalopathy and newer biological agents. Drug Saf 2010;33:969–983 [DOI] [PubMed] [Google Scholar]
- 5.Astrom KE, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain 1958;81:93–111 [DOI] [PubMed] [Google Scholar]
- 6.Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1971;1:1257–1260 [DOI] [PubMed] [Google Scholar]
- 7.Aksamit AJ, Sever JL, Major EO. Progressive multifocal leukoencephalopathy: JC virus detection by in situ hybridization compared with immunohistochemistry. Neurology 1986;36:499–504 [DOI] [PubMed] [Google Scholar]
- 8.Arthur RR, Dagostin S, Shah KV. Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol 1989;27:1174–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koralnik IJ, Wuthrich C, Dang X, et al. JC virus granule cell neuronopathy: a novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol 2005;57:576–580 [DOI] [PubMed] [Google Scholar]
- 10.Wuthrich C, Dang X, Westmoreland S, et al. Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann Neurol 2009;65:742–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson E., Jr Progressive multifocal leukoencephalopathy. N Engl J Med 1961;265:815–823 [DOI] [PubMed] [Google Scholar]
- 12.Dang X, Koralnik IJ. A granule cell neuron-associated JC virus variant has a unique deletion in the VP1 gene. J Gen Virol 2006;87:2533–2537 [DOI] [PubMed] [Google Scholar]
- 13.White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy: revisited. J Infect Dis 2011;203:578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mentzer D, Prestel J, Adams O, et al. Case definition for progressive multifocal leukoencephalopathy following treatment with monoclonal antibodies. J Neurol Neurosurg Psychiatry 2012;83:927–933 [DOI] [PubMed] [Google Scholar]
- 15.Richardson EP, Jr, Webster HD. Progressive multifocal leukoencephalopathy: its pathological features. Prog Clin Biol Res 1983;105:191–203 [PubMed] [Google Scholar]
- 16.Mazlo M, Tariska I. Are astrocytes infected in progressive multifocal leukoencephalopathy (PML)? Acta Neuropathol 1982;56:45–51 [DOI] [PubMed] [Google Scholar]
- 17.Wuthrich C, Cheng YM, Joseph JT, et al. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol 2009;68:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wuthrich C, Koralnik IJ. Frequent infection of cortical neurons by JC virus in patients with progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol 2012;71:54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samorei IW, Schmid M, Pawlita M, et al. High sensitivity detection of JC-virus DNA in postmortem brain tissue by in situ PCR. J Neurovirol 2000;6:61–74 [DOI] [PubMed] [Google Scholar]
- 20.Kepes JJ, Chou SM, Price LW., Jr Progressive multifocal leukoencephalopathy with 10-year survival in a patient with nontropical sprue: report of a case with unusual light and electron microscopic features. Neurology 1975;25:1006–1012 [DOI] [PubMed] [Google Scholar]
- 21.Bernal-Cano F, Joseph JT, Koralnik IJ. Spinal cord lesions of progressive multifocal leukoencephalopathy in an acquired immunodeficiency syndrome patient. J Neurovirol 2007;13:474–476 [DOI] [PubMed] [Google Scholar]
- 22.Whiteman ML, Post MJ, Berger JR, Tate LG, Bell MD, Limonte LP. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology 1993;187:233–240 [DOI] [PubMed] [Google Scholar]
- 23.Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol 1998;4:59–68 [DOI] [PubMed] [Google Scholar]
- 24.Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 2010;9:438–446 [DOI] [PubMed] [Google Scholar]
- 25.Tan IL, McArthur JC, Clifford DB, Major EO, Nath A. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology 2011;77:1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yousry TA, Pelletier D, Cadavid D, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2012;72:779–787 [DOI] [PubMed] [Google Scholar]
- 27.Harrison DM, Newsome SD, Skolasky RL, McArthur JC, Nath A. Immune reconstitution is not a prognostic factor in progressive multifocal leukoencephalopathy. J Neuroimmunol 2011;238:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006;354:924–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller RF, Lucas SB, Hall-Craggs MA, et al. Comparison of magnetic resonance imaging with neuropathological findings in the diagnosis of HIV and CMV associated CNS disease in AIDS. J Neurol Neurosurg Psychiatry 1997;62:346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clifford DB, Arribas JR, Storch GA, Tourtellote W, Wippold FJ. Magnetic resonance brain imaging lacks sensitivity for AIDS associated cytomegalovirus encephalitis. J Neurovirol 1996;2:397–403 [DOI] [PubMed] [Google Scholar]
- 31.Gray F, Belec L, Lescs MC, et al. Varicella-zoster virus infection of the central nervous system in the acquired immune deficiency syndrome. Brain 1994;117:987–999 [DOI] [PubMed] [Google Scholar]
- 32.Berger JR, Scott G, Albrecht J, Belman AL, Tornatore C, Major EO. Progressive multifocal leukoencephalopathy in HIV-1-infected children. AIDS 1992;6:837–841 [DOI] [PubMed] [Google Scholar]
- 33.Bhigjee AI, Patel VB, Bhagwan B, Moodley AA, Bill PL. HIV and acute disseminated encephalomyelitis [letter]. S Afr Med J 1999;89:283–284 [PubMed] [Google Scholar]
- 34.Scaravilli F, Daniel SE, Harcourt-Webster N, Guiloff RJ. Chronic basal meningitis and vasculitis in acquired immunodeficiency syndrome: a possible role for human immunodeficiency virus [see comments]. Arch Pathol Lab Med 1989;113:192–195 [PubMed] [Google Scholar]
- 35.Church JA. Reversible leukoencephalopathy in a patient with nucleoside analogue-associated mitochondrial DNA depletion and metabolic disease. AIDS 2002;16:2366–2367 [DOI] [PubMed] [Google Scholar]
- 36.Brochet B, Dousset V. Pathological correlates of magnetization transfer imaging abnormalities in animal models and humans with multiple sclerosis. Neurology 1999;53:S12–S17 [PubMed] [Google Scholar]
- 37.Chang L, Ernst T, Tornatore C, et al. Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology 1997;48:836–845 [DOI] [PubMed] [Google Scholar]
- 38.Nelson PK, Masters LT, Zagzag D, Kelly PJ. Angiographic abnormalities in progressive multifocal leukoencephalopathy: an explanation based on neuropathologic findings. AJNR Am J Neuroradiol 1999;20:487–494 [PMC free article] [PubMed] [Google Scholar]
- 39.Iranzo A, Marti-Fabregas J, Domingo P, et al. Absence of thallium-201 brain uptake in progressive multifocal leukoencephalopathy in AIDS patients. Acta Neurol Scand 1999;100:102–105 [DOI] [PubMed] [Google Scholar]
- 40.Port JD, Miseljic S, Lee RR, et al. Progressive multifocal leukoencephalopathy demonstrating contrast enhancement on MRI and uptake of thallium-201: a case report. Neuroradiology 1999;41:895–898 [DOI] [PubMed] [Google Scholar]
- 41.Lee VW, Antonacci V, Tilak S, Fuller JD, Cooley TP. Intracranial mass lesions: sequential thallium and gallium scintigraphy in patients with AIDS. Radiology 1999;211:507–512 [DOI] [PubMed] [Google Scholar]
- 42.O'Doherty MJ, Barrington SF, Campbell M, Lowe J, Bradbeer CS. PET scanning and the human immunodeficiency virus-positive patient. J Nucl Med 1997;38:1575–1583 [PubMed] [Google Scholar]
- 43.Mertens K, Acou M, Van den Broecke C, et al. Progressive multifocal leukoencephalopathy (PML) mimicking high-grade glioma on delayed F-18 FDG PET imaging. J Clin Neurosci 2012;19:1167–1169 [DOI] [PubMed] [Google Scholar]
- 44.Fong IW, Britton CB, Luinstra KE, Toma E, Mahony JB. Diagnostic value of detecting JC virus DNA in cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy. J Clin Microbiol 1995;33:484–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall DW, Brey RL, Cahill WT, Houk RW, Zajac RA, Boswell RN. Spectrum of cerebrospinal fluid findings in various stages of human immunodeficiency virus infection. Arch Neurol 1988;45:954–958 [DOI] [PubMed] [Google Scholar]
- 46.McGuire D, Barhite S, Hollander H, Miles M. JC virus DNA in cerebrospinal fluid of human immunodeficiency virus-infected patients: predictive value for progressive multifocal leukoencephalopathy [erratum 1995;37:687]. Ann Neurol 1995;37:395–399 [DOI] [PubMed] [Google Scholar]
- 47.Weber T, Turner RW, Frye S, et al. Progressive multifocal leukoencephalopathy diagnosed by amplification of JC virus-specific DNA from cerebrospinal fluid. AIDS 1994;8:49–57 [DOI] [PubMed] [Google Scholar]
- 48.Cinque P, Koralnik IJ, Clifford DB. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J Neurovirol 2003;9(suppl 1):88–92 [DOI] [PubMed] [Google Scholar]
- 49.Drews K, Bashir T, Dorries K. Quantification of human polyomavirus JC in brain tissue and cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy by competitive PCR. J Virol Methods 2000;84:23–36 [DOI] [PubMed] [Google Scholar]
- 50.Iacobaeus E, Ryschkewitsch C, Gravell M, et al. Analysis of cerebrospinal fluid and cerebrospinal fluid cells from patients with multiple sclerosis for detection of JC virus DNA. Mult Scler 2009;15:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuhle J, Gosert R, Buhler R, et al. Management and outcome of CSF-JC virus PCR-negative PML in a natalizumab-treated MS patient. Neurology 2011;77:2010–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marzocchetti A, Di Giambenedetto S, Cingolani A, Ammassari A, Cauda R, De Luca A. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol 2005;43:4175–4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryschkewitsch CF, Jensen PN, Monaco MC, Major EO. JC virus persistence following progressive multifocal leukoencephalopathy in multiple sclerosis patients treated with natalizumab. Ann Neurol 2010;68:384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wuthrich C, Kesari S, Kim WK, et al. Characterization of lymphocytic infiltrates in progressive multifocal leukoencephalopathy: co-localization of CD8+ T cells with JCV-infected glial cells. J Neurovirol 2006;12:116–128 [DOI] [PubMed] [Google Scholar]
- 55.Huang D, Cossoy M, Li M, et al. Inflammatory progressive multifocal leukoencephalopathy in human immunodeficiency virus-negative patients. Ann Neurol 2007;62:34–39 [DOI] [PubMed] [Google Scholar]
- 56.Miralles P, Berenguer J, Lacruz C, et al. Inflammatory reactions in progressive multifocal leukoencephalopathy after highly active antiretroviral therapy. AIDS 2001;15:1900–1902 [DOI] [PubMed] [Google Scholar]
- 57.Antinori A, Ammassari A, De Luca A, et al. Diagnosis of AIDS-related focal brain lesions: a decision-making analysis based on clinical and neuroradiologic characteristics combined with polymerase chain reaction assays in CSF. Neurology 1997;48:687–694 [DOI] [PubMed] [Google Scholar]
- 58.Levy RM, Russell E, Yungbluth M, Hidvegi DF, Brody BA, Dal Canto MC. The efficacy of image-guided stereotactic brain biopsy in neurologically symptomatic acquired immunodeficiency syndrome patients. Neurosurgery 1992;30:186–189 [DOI] [PubMed] [Google Scholar]
- 59.Silver SA, Arthur RR, Erozan YS, Sherman ME, McArthur JC, Uematsu S. Diagnosis of progressive multifocal leukoencephalopathy by stereotactic brain biopsy utilizing immunohistochemistry and the polymerase chain reaction. Acta Cytol 1995;39:35–44 [PubMed] [Google Scholar]
- 60.Brooks BR, Walker DL. Progressive multifocal leukoencephalopathy. Neurol Clin 1984;2:299–313 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.