Abstract
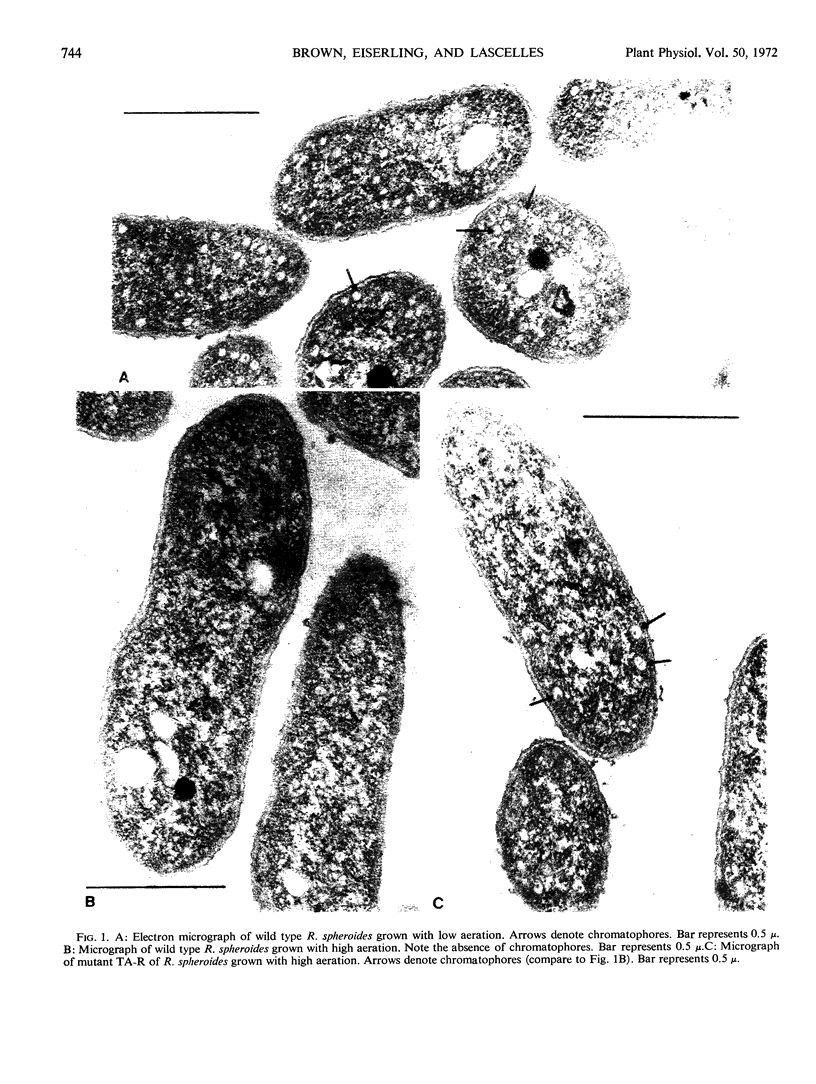
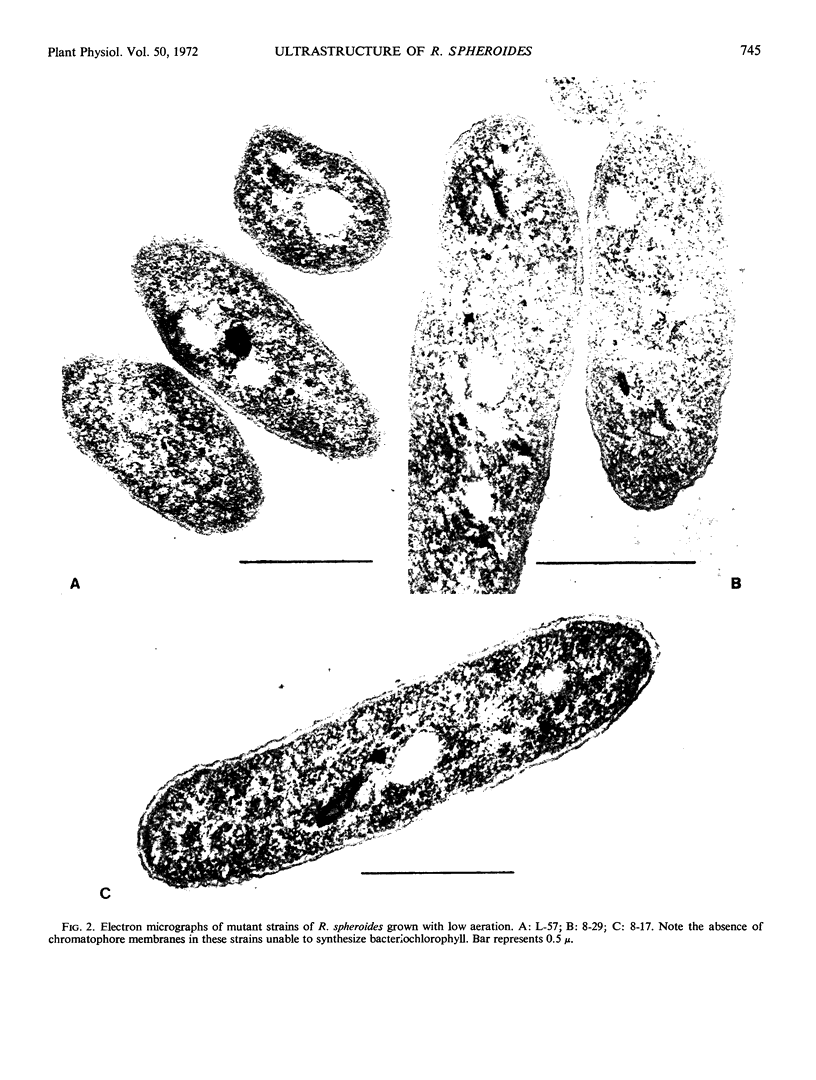
The ultrastructure of sectioned cells of mutant and wild type Rhodopseudomonas spheroides has been examined by electron microscopy. The characteristic vesicles associated with the presence of bacteriochlorophyll were found in wild type cells grown with low aeration. These were also found in mutant TA-R which forms bacteriochlorophyll under high aeration. None of the mutants with blocks in bacteriochlorophyll synthesis contained intracytoplasmic membrane. These included mutant 8-17 which accumulates bacteriochlorophyllide but fails at the phytolation step. We conclude that the intact bacteriochlorophyll molecule, or some particular membrane protein associated with it, is needed for the development of the characteristic intracytoplasmic membrane system in R. spheroides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Fukami A., Adachi K. A new method of preparation of a self-perforated micro plastic grid and its application. J Electron Microsc (Tokyo) 1965;14(2):112–118. [PubMed] [Google Scholar]
- Gibson K. D. Electron microscopy of chromatophores of Rhodopseudomonas spheroides. J Bacteriol. 1965 Oct;90(4):1059–1072. doi: 10.1128/jb.90.4.1059-1072.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T. Chloroplast structure and biogenesis. Annu Rev Biochem. 1971;40:161–196. doi: 10.1146/annurev.bi.40.070171.001113. [DOI] [PubMed] [Google Scholar]
- Lascelles J. The accumulation of bacteriochlorophyll precursors by mutant and wild-type strains of Rhodopseudomonas spheroides. Biochem J. 1966 Jul;100(1):175–183. doi: 10.1042/bj1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles J., Wertlieb D. Mutant strains of Rhodopseudomonas spheroides which form photosynthetic pigments aerobically in the dark. Growth characteristics and enzymic activities. Biochim Biophys Acta. 1971 Mar 2;226(2):328–340. doi: 10.1016/0005-2728(71)90100-9. [DOI] [PubMed] [Google Scholar]
- Oelze J., Schroeder J., Drews G. Bacteriochlorophyll, fatty-acid, and protein synthesis in relation to thylakoid formation in mutant strains of Rhodospirillum rubrum. J Bacteriol. 1970 Mar;101(3):669–674. doi: 10.1128/jb.101.3.669-674.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards W. R., Lascelles J. The biosynthesis of bacteriochlorophyll. The characterization of latter stage intermediates from mutants of Rhodopseudomonas spheroides. Biochemistry. 1969 Aug;8(8):3473–3482. doi: 10.1021/bi00836a051. [DOI] [PubMed] [Google Scholar]
- Stevenson L. H., Socolofsky M. D. Cyst formation and poly-beta-hydroxybutyric acid accumulation in Azotobacter. J Bacteriol. 1966 Jan;91(1):304–310. doi: 10.1128/jb.91.1.304-310.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]