Use of propofol has increased manifold over the last decade, most commonly in intensive care settings, in patients critically ill or injured requiring prolonged sedation. Most of the deaths reported were at propofol levels greater than 4 mg/kg/hour, and many of these for greater than 48 hours. Metabolic acidosis is among the earliest signs and can occur within hours. Adequate carbohydrate intake will prevent the body from switching to fat metabolism.
Abstract
Background:
Propofol is a popular anesthetic and sedative. Use of propofol has increased manifold in this country over the last decade, and it is most commonly used in intensive care settings. Its rapid action with short half-life, decreased cerebral oxygen consumption, and reduction of intracranial pressure are properties that have made it a favorite in the intensive care unit. Many of these patients are critically ill or injured and require prolonged sedation. Propofol has been associated with morbidity and mortality, and in such cases the question often arises regarding the role propofol plays in these complications.
Objective:
To address the issue of propofol-related infusion syndrome and its management.
Method:
A hypothetical clinical vignette was created to give a classic presentation of propofol-related infusion syndrome.
Conclusion:
It is hoped that this short report will bring more awareness of this entity so that it will be considered in the differential diagnosis in sedated critical care patients.
Introduction
Propofol is a popular anesthetic and sedative. Use of propofol has increased manifold in this country over the last decade, and it is most commonly used in intensive care settings. Its rapid action with short half-life, decreased cerebral oxygen consumption, and reduction of intracranial pressure are properties that have made it a favorite in the intensive care unit (ICU). Many of these patients are critically ill or injured and require prolonged sedation. Propofol has been associated with morbidity and mortality, and in such cases the question often arises regarding the role propofol plays in these complications.
A hypothetical clinical vignette is presented to illustrate this problem.
Case Report
A man, age 50 years, was admitted for observation after a motor vehicle accident. His only complaint was some neck and chest pain. His full-body scan only revealed fracture of the right fourth rib. Results of a complete blood panel and baseline chemistry panel were within normal limits. That evening he experienced respiratory distress. A repeated chest radiograph showed pneumothorax and a small zone of haziness on the right lower side suggesting aspiration pneumonia. A chest tube was inserted and a regimen of antibiotics was started.
The next day the patient had to be intubated. Propofol therapy was initiated for sedation and, because of his severe agitation, the dosage of propofol was increased to 85 μg/kg/minute (5.1 mg/ kg/hour). The usual adult maintenance sedation dosage is 5 to 50 μg/kg/minute (0.3 to 3 mg/kg/hour). He remained sedated on a ventilator in the ICU and on the sixth day was noted to have a pulse rate of 50 beats per minute, blood pressure of 100/47 mm Hg, and temperature of 37.0°C. Investigations revealed mild pulmonary edema, severe lactic acidosis, total white blood cell count of 9550/μL, creatine kinase level of 51,000 IU/L, and new-onset acute kidney injury. Increasing doses of norepinephrine and dobutamine were needed, although he received adequate intravenous fluids compared with his fluid losses. The next day he went into ventricular fibrillation and died after resuscitation efforts failed.
Discussion
Deaths related to propofol infusion were initially reported in the 1990s in children, and in 1998, Bray1 coined the term propofol infusion syndrome. The original presentations included refractory bradycardia along with one or more complications of metabolic acidosis, rhabdomyolysis, hepatomegaly, and hyperlipidemia.2 Other complications include cardiac and renal failure (see Sidebars: Clinical Presentation of Propofol-Related Infusion Syndrome and Abnormal Investigation Findings Associated with Propofol-Related Infusion Syndrome). Rhabdomyolysis is the major cause of acute kidney injury in such cases. In 2001, the US Food and Drug Administration placed a warning against use of propofol for long-term sedation in pediatric patients.
Clinical Presentation of Propofol-Related Infusion Syndrome.
Cardiac failure and arrhythmias (bradyarrhythmias, ventricular fibrillation)
Metabolic acidosis
Rhabdomyolysis
Acute kidney injury
Hyperlipidemia
Hepatomegaly
Abnormal Investigation Findings Associated with Propofol-Related Infusion Syndrome.
Lactate
Creatine kinase
Triglycerides
ST-segment elevation in right precordial leads
It is complicated to determine the incidence of propofol-related infusion syndrome (PRIS) because many of the PRIS-associated clinical manifestations may reflect either pharmacologic manifestations of propofol (eg, bradycardia) or manifestations of critical illness (eg, metabolic acidosis). In the first large prospective study involving more than 1000 patients,3 PRIS was defined as development of metabolic acidosis and cardiac dysfunction after the initiation of propofol along with at least one of the following: rhabdomyolysis, hypertriglyceridemia, or renal failure. Although the study had many limitations, the reported incidence of PRIS was 1.1%. However, the actual number of cases in the US is likely substantial given that 5 million patients are admitted to the ICU and propofol is used by 80% of clinicians. This study reported a mortality of rate of 18%, but an analysis of the Food and Drug Administration’s MedWatch system noted 30% mortality in cases of PRIS.4
Various mechanisms have been proposed for the pathophysiology of PRIS.2,5 To summarize, propofol impairs function of the mitochondrial respiratory chain. The impaired production of mitochondrial adenosine triphosphate and presence of soya bean (added to enhance solubility of propofol) increase triglyceride load. There is impaired fatty acid oxidation and buildup of toxic fatty acid intermediates. This, along with cellular hypoxia and impaired hepatic handling of lactate, worsens acidosis. Impairment of lipid metabolism in cardiac and skeletal muscles, along with impaired synthesis of adenosine triphosphate, contributes to myocytolysis, leading to myocardial failure, arrhythmia, and rhabdomyolysis. Vasile et al5 proposed other triggering factors, which include excess catecholamines (both endogenous and exogenous) and corticosteroid therapy in critically ill patients. These factors can cause myocyte injury and lipolysis, causing an increase in circulating free fatty acids.
Preventive measures can be taken to lower risk of PRIS (see Sidebars: Risk Factors for Propofol-Related Infusion Syndrome and Prevention of Propofol-Related Infusion Syndrome). Most of the deaths reported were at propofol levels greater than 4 mg/kg/hour, and many of these patients with PRIS were given propofol for greater than 48 hours, although PRIS has been reported after only a few hours. Therefore, a lower dose for a shorter duration is preferable. Adequate carbohydrate intake will prevent the body from switching to fat metabolism, because carbohydrates are preferentially metabolized and hence lessen fat mobilization. As noted earlier, this will lessen the load of toxic fatty acids. More concentrated propofol formulations should also lessen lipid load. Lorazepam and midazolam are effective alternatives. The American College of Critical Care Medicine advises clinicians to consider alternative sedatives in patients requiring increasing doses of vasopressors and in those in cardiac failure.6
Risk Factors for Propofol-Related Infusion Syndrome.
Critical illness
Propofol dosage > 4 mg/kg/hour
Duration of therapy > 48 hours
Exogenous catecholamines and corticosteroids
Poor intake of carbohydrate
Prevention of Propofol-Related Infusion Syndrome.
Use lowest dose of propofol with shortest duration
Minimize lipid load (concentrating propofol drip and adjusting parenteral nutrition)
Provide adequate amount of carbohydrate
Stop propofol infusion at earliest sign of abnormal laboratory results or ECG changes
ECG = electrocardiographic.
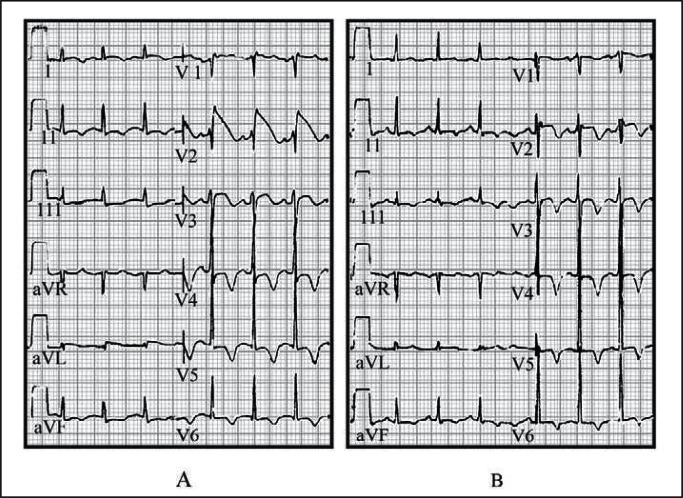
Clinicians can detect PRIS at its early stages. Metabolic acidosis is among the earliest signs and can occur within hours. Electrocardiographic changes (Figure 1) showing convex-curved (coved) ST-segment elevation (as in Brugada syndrome) in V1 to V3 may precede malignant arrhythmia and can alert the physician.7 The utility of monitoring creatine kinase has been questioned and hypertriglyceridemia can occur within hours in otherwise healthy patients receiving propofol. Despite this, the American College of Critical Care Medicine6 recommends checking triglyceride levels after 2 days, and European regulatory authorities suggest monitoring for metabolic acidosis, rhabdomyolysis, hyperkalemia, and heart failure.8
Figure 1.
A) Electrocardiogram (ECG) of our hypothetical index patient at the time of diagnosis of propofol-related infusion syndrome. Note ST-segment elevation in right precordial lead V2, consistent with a typical Brugada-like ECG pattern. B) ECG of index patient 12 hours after withdrawal of propofol therapy. Note that, despite the still abnormal ST segments, inverted T waves, and prolonged QT interval, the ST segment in lead V2 is dramatically decreased compared with the previous ECG (panel A).
Reprinted from Vernooy K, Delhaas T, Cremer OL, et al. Electrocardiographic changes predicting sudden death in propofol-related infusion syndrome. Heart Rhythm 2006 Feb;3(2):131–7. DOI: http://dx.doi.org/10.1016/j.hrthm.2005.11.005, with permission from Elsevier.
Case studies2 have shown that stopping propofol administration can reverse impending PRIS at an early stage. Once in full swing, the hemodynamic instability is refractory to fluids and inotropes. Besides cessation of propofol therapy, the use of cardiac pacing, hemodialysis, or hemofiltration has been reported.2
It is hoped that this short report will bring more awareness of this entity and that it will be considered in the differential diagnosis in sedated critical care patients.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Creation
If Medicine is a trade, why should the doctor so often work for nothing? If it is an art, what works of art does he produce? None, says Claude Bernard, Le Médecin Artiste ne crée rien: but surely he is wrong. The doctor, so far from creating nothing, creates life: for he who saves or prolongs life, creates more life. If Miss X is 70, and the doctor, by an operation, enables her to live till she is 75, he has not prolonged the 70 years, for they were ended before he came; but he has created 5 brand-new years. If he had not been there, they would not be here: that is creation. He has not lengthened her past, nobody could; he has called into existence her present and her future: and they are she, therefore he has called into existence her. Not that he thinks much of that tremendous act.
—Confessio Medici, Chapter 5, Stephen Paget, 1855–1926, English surgeon
References
- 1.Bray RJ. Propofol infusion syndrome in children. Paediatr Anaesth. 1998;8(6):491–9. doi: 10.1046/j.1460-9592.1998.00282.x. DOI: http://dx.doi.org/10.1046/j.1460-9592.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 2.Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007 Jul;62(7):690–701. doi: 10.1111/j.1365-2044.2007.05055.x. DOI: http://dx.doi.org/10.1046/j.1460-9592.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 3.Roberts RJ, Barletta JF, Fong JJ, et al. Incidence of propofol-related infusion syndrome in critically ill adults: a prospective, multicenter study. Crit Care. 2009;3(5):R169. doi: 10.1186/cc8145. DOI: http://dx.doi.org/10.1186/cc8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong JJ, Sylvia L, Ruthazer R, Schumaker G, Kcomt M, Devlin JW. Predictors of mortality in patients with suspected propofol infusion syndrome. Crit Care Med. 2008 Aug;36(8):2281–7. doi: 10.1097/CCM.0b013e318180c1eb. DOI: http://dx.doi.org/10.1097/CCM.0b013e318180c1eb. [DOI] [PubMed] [Google Scholar]
- 5.Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003 Sep;29(9):1417–25. doi: 10.1007/s00134-003-1905-x. DOI: http://dx.doi.org/10.1007/s00134-003-1905-x. [DOI] [PubMed] [Google Scholar]
- 6.Jacobi J, Fraser GL, Coursin DB, et al. Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American society of Health-System Pharmacists (ASHP), American College of Chest Physicians Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002 Jan;30(1):119–41. doi: 10.1097/00003246-200201000-00020. DOI: http://dx.doi.org/10.1097/00003246-200201000-00020 Erratum in: Crit Care Med 2002 Mar;30(3):726. [DOI] [PubMed] [Google Scholar]
- 7.Vernooy K, Delhaas T, Cremer OL, et al. Electrocardiographic changes predicting sudden death in propofol-related infusion syndrome. Heart Rhythm. 2006 Feb;3(2):131–7. doi: 10.1016/j.hrthm.2005.11.005. DOI: http://dx.doi.org/10.1016/j.hrthm.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahlen K, Buckley CJ, Goodale DB, Pulsford AH. The ‘propofol infusion syndrome’: the facts, their interpretation and implications for patient care. Eur J Anaesthesiol. 2006 Dec;23(12):990–8. doi: 10.1017/S0265021506001281. DOI: http://dx.doi.org/10.1017/S0265021506001281. [DOI] [PubMed] [Google Scholar]