This is a case report from a specialist point of view that includes a comprehensive review of the clinical course pre- and postconsultation along with a brief but pertinent review of the literature as it relates to this particular unusual and protracted case, which was ultimately successfully diagnosed and treated.
Abstract
A nonsmoking woman in her mid-70s presents to the allergist for consultation of a chronic cough of almost 3-years’ duration without a specific diagnosis as to etiology in spite of numerous diagnostic tests and therapeutic trials.
This is a case report from a specialist point of view that includes a comprehensive review of her clinical course pre- and postconsultation along with a brief but pertinent review of the literature as it relates to this particular unusual and protracted case, which was ultimately successfully diagnosed and treated.
Introduction
Cough is one of the most common reasons patients seek health care. A primary complaint of cough accounts for an estimated 30 million office visits every year in the US. Chronic cough of at least 2 months’ duration is less common. Accurate diagnosis of the underlying cause(s) and effective treatment of chronic cough can be a perplexing and challenging problem for primary care physicians (PCPs). Much has been published in recent decades about the underlying causes of chronic cough.1–10 In some cases of chronic cough, confirmation of a diagnosis is significantly delayed or never occurs. The majority of cases of chronic cough can be attributed to one or more of 5 etiologies:
- Gastroesophageal reflux disease (GERD)
- Reflux laryngitis (laryngopharyngeal reflux)20
Angiotensin-converting enzyme inhibitor
Nonasthmatic (nonobstructive) eosinophilic bronchitis21
Rarer causes of chronic cough include congestive heart failure, foreign body aspiration, interstitial lung diseases including sarcoidosis, and primary or secondary cancer of the lung.
Bronchiectasis, as noted, can be associated with chronic obstructive pulmonary disease. Among the causes of bronchiectasis are chronic infections associated with aspiration pneumonia related to GERD, common variable immunodeficiency disease, cystic fibrosis, tuberculosis, Mycobacterium avium-complex disease, and allergic bronchopulmonary aspergillosis (ABPA).22–25 The major diagnostic criteria for ABPA include a history of asthma (see Sidebar: Diagnostic criteria for allergic bronchopulmonary aspergillosis).26,27
Diagnostic criteria for allergic bronchopulmonary aspergillosis.
Rosenberg-Patterson criteria1,2
Major Criteria (“ARTEPICS”)
Asthma
Roentgenographic fleeting opacities
Test positive for Aspergillus (evidence of sp-IgE sensitization) (skin test included)
Eosinophilia (>500/dL)
Precipitating antibodies in serum
IgE in serum is elevated (>1000 IU/mL)
Central bronchiectasis
Serum A fumigatus-specific IgG and IgE (looking for >2 times the value from pooled serum samples of patients with asthma sans ABPA)
Minor Criteria
Presence of Aspergillus in sputum
Producing sputum with brownish-black mucus plugs
Delayed (type III) skin test reaction to Aspergillus antigen
Minimal Diagnostic Criteria for ABPA3
- ABPA-CB (ABPA with Central Bronchiectasis)
- Asthma
- Detectable IgE antibody to Aspergillus (skin and/or serologic testing)
- Central bronchiectasis
- Elevated total serum IgE (>1000 IU/mL)
- High A fumigatus-specific IgG and IgE
- ABPA-S (ABPA that is seropositive sans CB)
- Asthma
- Detectable IgE antibody to Aspergillus (skin and/or serologic testing)
- Transient pulmonary infiltrates on chest radiographs
- Elevated total serum IgE (>1000 IU/mL)
- High A fumigatus-specific IgG and IgE
References
- 1.Rosenberg M, Patterson R, Mintzer R, Cooper BJ, Roberts M, Harris KE. Clinical and immunologic criteria for diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977 Apr;86(4):405–14. doi: 10.7326/0003-4819-86-4-405. [DOI] [PubMed] [Google Scholar]
- 2.Patterson R, Greenberger PA, Halwig JM, Liotta JL, Roberts M. Allergic bronchopulmonary aspergillosis: natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med. 1986 May;146(5):916–8. doi: 10.1001/archinte.146.5.916. DOI: http://dx.doi.org/10.1001/archinte.1986.00360170130020. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz HJ, Greenberger PA. The prevalence of allergic bronchopulmonary aspergillosis in patients with asthma, determined by serologic and radiologic criteria in patients at risk. J Lab Clin Med. 1991 Feb;117(2):138–42. [PubMed] [Google Scholar]
We present an unusual case of ABPA in an elderly patient who presented with chronic cough, even though asthma was never confirmed.
Case Presention
The patient was a woman in her early 70s when she presented in the summer of 2008, with a cough that had started 8 days earlier. Previously she had been healthy, with no history of asthma or recurrent lung or sinus disease. She had had mild, intermittent rhinitis at least since the age of 66 years, controlled with an over-the-counter antihistamine and/or intermittent use of intranasal steroid therapy. Cough was her only initial bothersome complaint; she had no wheezing, shortness of breath, or sputum production.
A physician assistant in primary care first saw the patient 1 week after cough onset and recommended an over-the-counter cough medicine for what was believed to be a viral infection. She was seen by her PCP 9 days later, when normal results of a chest x-ray were documented and a tuberculosis skin test (purified protein derivative) was administered. Results of the skin test were negative 2 days later. Despite lack of evidence of a bacterial infection, she was given a course of amoxicillin, 500 mg 3 times daily for 10 days. The cough was unchanged at follow-up 19 days later. She was then given a prescription cough medication, but no improvement was noted after an additional 10 days. This prompted the ordering of spirometry and a pulmonary medicine consultation.
The patient was 9 weeks into her cough when she was first seen in pulmonary medicine. It was noted that results of the pulmonary function tests administered 3 weeks earlier were consistent with mild obstruction. She had a normal forced vital capacity, 2.12 L (86% predicted); a mildly reduced forced expiratory volume in 1 s (FEV1), 1.52 L (76% predicted); and a normal residual volume, at 93% predicted. Diffusing capacity of the lung for carbon monoxide was slightly reduced, at 79% predicted. There was no documentation of reversible airway obstruction, which is essential in ruling out classic asthma, because bronchodilator with before-and-after FEV1 tests had not been ordered. No further pulmonary function tests were administered until she was seen almost 3 years later on consultation in the Allergy Department. Thus, an opportunity to diagnose asthma was missed early in the course of her disease. During the entire course leading up to a final diagnosis, however, she experienced no attacks of coughing, wheezing, or dyspnea that appeared to require a short-acting bronchodilator.
On further evaluation of chronic cough, the pulmonologist ordered ImmunoCAP Environmental Panel screening for IgE specific to the major inhaled allergens. Results were positive for a variety of pollens, dust mites, and molds. Most notable was a class V (on a scale of 0-VI) level of IgE antibody to Aspergillus fumigatus (65.6 kUA/L, scale of 0.10 to >100). Test results for serum IgG antibodies to Coccidioides, Cryptococcus, and Histoplasma were negative.
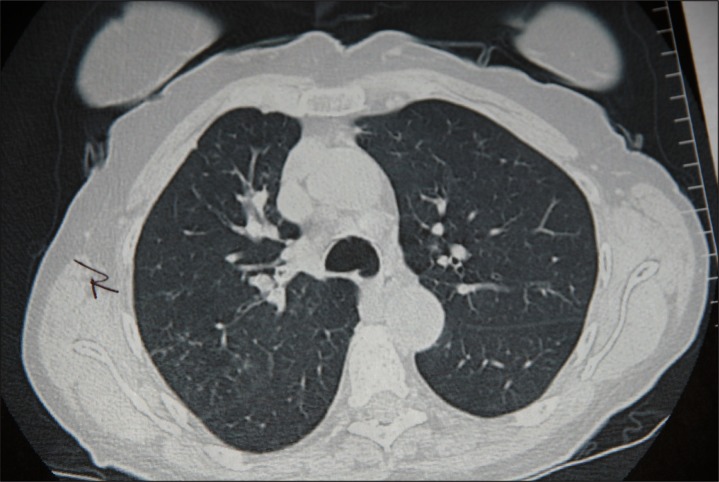
A chest computed tomography (CT) scan obtained 13 weeks into her course of chronic cough, revealed thick intrabronchial densities, especially in the right upper lobe (RUL), along with increased markings in the RUL and the presence of a few mediastinal nodes. In addition, there were soft tissue densities extending into several of the bronchi in the right hemithorax associated with some distal atelectatic changes (some of the abnormalities can be seen in Figure 1). At this time, ABPA, hypersensitivity pneumonitis, infection, and cancer should have been given strong consideration in the differential diagnosis of this patient’s chronic cough.
Figure 1.
High resolution computed tomography scan of the chest 13 weeks post-onset of chronic cough.
During the one-month follow-up appointment to the pulmonologist, chronic cough related to bronchiectasis was diagnosed, but the underlying etiology was not specified. Tests that may have yielded definitive results were not performed, including total serum IgE to rule out ABPA and tests for IgG antibodies specific to Aspergillus, quantitative immunoglobulin tests to rule out humoral immunodeficiency, and bronchoscopy to detect and identify pathogenic material in the bronchi.
She had a total of 10 visits with her pulmonologist. Initially she was empirically treated with a trial of mometasone furoate, one puff of 220 mcg twice a day from the date of her initial consultation for a total of 8 months when she discontinued therapy on her own. About 5 months after the onset of her cough she was treated with a course of prednisone at 20 mg daily, which she discontinued on her own after a week. She was seen by her pulmonologist 6 weeks after stopping the mometasone furoate inhaler and reported an increase in chest congestion and some difficulty breathing. It was noted that the mometasone had improved her cough, but the extent of improvement was not documented. The record shows no change in diagnosis at this visit, and she was switched to fluticasone/salmeterol, 250/50 twice a day for about 2 months. The fluticasone/salmeterol was stopped after 2 months because of chest discomfort and sore throat. However it was noted again that her cough had lessened to an unspecified extent. In neither trial of asthma controller medication did the cough remit. More coughing was observed on a follow-up visit 1 year postpulmonary consultation, prompting the initiation of another trial of cough suppressants, starting with benzonatate, 100 mg 3 times a day. No other medications were recommended.
Sputum culture was ordered at this time and when seen again by her pulmonologist 2 months later, the patient was informed that her culture was positive for the fungus Aspergillus fumigatus. At this 14 month follow-up visit, persistent cough was again noted, productive of thick mucus that occasionally appeared black but without hemoptysis. Historical wheezing was noted for the first time in the physician narrative, but her chest was clear on physical examination. Spirometry and additional lab tests were not ordered at this time. The diagnosis remained unspecified bronchiectasis. She refused another course of oral steroid. Instead, she was started on an antifungal preparation targeting Aspergillus: itraconazole, 100 mg twice a day, was continued for 13 months. She was also prescribed hydrocodone and chlorpheniramine cough syrup, 1 to 2 teaspoons daily, as needed. The hydrocodone cough syrup prescription was refilled over a period of 17 months for a total dispensed volume of 3.24 liters of medication. Again, the cough failed to remit.
During the ensuing 10 weeks she visited her pulmonologist 6 more times, and no significant change in overall status of persistent cough and mucus production was noted. There was no hemoptysis or fever and no mention of wheezing from her 23rd month of follow-up care onward, with historical “occasional wheezing” recorded but never documented on physical examination during the previous 4 visits. It was noted that the hydrocodone cough syrup improved her cough to some degree.
Almost three years into her course, she began to experience intense chest pain radiating front to back, prompting an Emergency Department visit. She revisited the Emergency Department again three days later because of chest pain, but was not hospitalized. Cardiology consultation and testing at this time revealed evidence of pericarditis as the probable cause of chest pain, which rapidly improved with ibuprofen and colchicine. Around this same time she had been switched to omeprazole and scheduled for upper endoscopy about two weeks later to evaluate dysphagia. That examination revealed a normal esophagus with mild gastritis and no evidence of stenosis.
In the interim, at almost three years into her course of chronic cough with documented bronchiectasis, she was given a referral to the Allergy Department for further evaluation. Upon allergy consultation, further history did not elicit recurrent fevers, but she did report occasional chills and night sweats. The cough was noted to be chronically productive from the onset, with occasional thick green or black plugs of mucus. She again denied hemoptysis. The cough was present day and night, frequently disturbing sleep, but not accompanied by vomiting. She considered her cough to be severe and reported recent onset of dyspnea on exertion interfering with daily activities. It was confirmed that there was no history of acute wheezing attacks and that she had not used or been prescribed a short-acting bronchodilator.
She recalled that before onset of chronic cough she had experienced about 2 weeks of recurrent exposure to an old washing machine in the garage of her house that subsequently was discovered to be infested with mold. The washing machine was removed. No mold survey was done. She continued to live in the same home where she had lived for 30 years. This exposure at the time was believed to be a seminal event, but it must be noted that for the majority of patients first diagnosed with ABPA, a clear, identifiable exposure to mold is not likely to be discovered in a history.28
Pertinent history was positive for hypertension treated with lisinopril for about 5 months then switched to hydrochlorothiazide 2 years before the onset of her chronic cough. She had not noted coughing with the angiotensin-converting enzyme inhibitor. She initially noted GERD 2 years before the development of her chronic cough. She had been treated with famotidine, 20 mg twice daily, for GERD. There was no history of upper airway cough syndrome or sinus disease. She had not undergone laryngoscopy during the course of her disease.
She had never been a smoker, nor did she have a significant history of second-hand exposure. Results of physical examination were blood pressure, 136/84 mm Hg; temperature 36.7°C; pulse 95 beats/minute; height, 152.4 cm; weight, 52.2 kg; and body mass index, 22.46 kg/m2. Saturation of peripheral oxygen was 94% (normal on room air, at rest). Forced vital capacity was 75% (1.73 L). FEV1 was 56% (1.02 L).
She was a petite woman appearing well developed, well nourished, younger than the stated age, and in no distress, but with frequent bouts of spontaneous, spasmodic coughing throughout the examination. There were no significant head, eyes, ears, nose, and throat findings and no upper respiratory secretions. She had an increased heart rate, normal rhythm, and no gallop or murmur. There were no wheezes, rales, or rhonchi, with fair air exchange throughout.
Laboratory findings at this time revealed the total serum IgE level to be markedly elevated, at 10,003 IU/mL (age-adjusted normal value <114 IU/mL). Aspergillus-specific IgG precipitating antibodies were negative to A flavus and A niger but positive to A fumigatus 1, 2, 3, and 6. Blood count revealed 8900 white blood cells with 801 eosinophils/dL. Quantitative immunoglobulin results were normal. The result of a repeat test for A fumigatus-specific IgE was class V (85 KUA/L), still greatly elevated.
ABPA was diagnosed after the test results were reviewed. Asthma was not spirometrically ruled out and was not suspected because of her inadequate clinical responses to oral and inhaled corticosteroids, including a combination product with a long-acting bronchodilator.
The principal diagnosis of ABPA prompted a discussion with the patient and her husband regarding the risks and benefits of another trial of oral corticosteroid therapy, this time combined with antifungal medication that would be expected to bring about remission of her ABPA. A lower than usual dose of oral corticosteroid was advised because of her fear of oral corticosteroids, her osteoporosis, and hypertension.
Primarily because of clinical presentation and failure to adequately respond to previous trials of inhaled asthma controller medications, therapy for asthma was not believed to be indicated. This view would soon be justified by her responsiveness to therapy for ABPA alone.
ABPA-specific therapy was initiated almost 3 years into her course of chronic cough: methylprednisolone, 16 mg once daily; and itraconazole, 100 mg twice daily. She was seen on follow-up 3 weeks later and reported marked improvement in her cough. Her spirometry results were improved: forced vital capacity was 88% (1.93 L), and FEV1 was 74% (1.34 L).
This degree of improvement showed what appears to be significant reversibility of airway disease and perhaps asthma itself, but the patient’s previous and subsequent course suggests otherwise regarding an asthma diagnosis, a point to be discussed briefly at the end of the Discussion. She estimated her primary symptom of cough was 40% to 50% better, and she was no longer experiencing nocturnal awakening related to cough. She also was able to reduce the hydrocodone and chlorpheniramine cough syrup to half the previous dose.
However, while on the combination therapy for ABPA she was starting to notice swelling in both legs, and her weight had increased from 52.2 kg to 54.4 kg during a period of about 6 weeks. In addition, her blood pressure was now elevated, at 186/80 mm Hg. She did not appear cushingoid, her lungs were clear, and there was significant swelling localized to the lower extremities. Her lung function was also significantly improved. In addition, during a period of about 6 weeks, there was a 40% reduction in her total serum IgE level to 6083 KUA/L, consistent with a very good response to the regimen. A 35% to 50% reduction in total IgE is often associated with remission of the immunologic processes of ABPA.26
The patient was advised at this time to begin reducing the oral corticosteroid dosage to 12 mg daily, in an attempt to reduce side effects. She was also advised to resume the diltiazem 120 mg daily that was prescribed by her cardiologist. She had stopped taking the diltiazem on her own accord around the time she began the regimen for ABPA.
At subsequent follow-up by remote access while a scheduled allergy follow-up was pending, continued reduction of IgE level to 3129 KUA/L was observed; 12 mg of methylprednisolone was maintained, along with itraconazole, 100 mg twice a day.
Between Allergy Department visits, treatment was complicated by a prolonged hospitalization spanning a period of 11 days, to manage necrotizing pneumonia, along with hyperglycemia, hypoalbuminemia with generalized edema, and hypertension. Chest CT performed within 2 weeks of hospital discharge, revealed a persistent, small left pleural effusion; a cavitary lesion in the lingual lobe with air-fluid level and patchy air spaces in the right middle lobe and RUL and several nodular opacities in the right lung. Also noted were prominent but stable paratracheal nodes in the carina region, consistent with reactive lymphadenopathy. After about 8 weeks of initiation of therapy for ABPA, at the time of this hospitalization, the total serum IgE level had decreased to 2576 KUA/L. About this time, an infectious disease consultant recommended voriconazole, 200 mg twice daily, to replace itraconazole. Voriconazole was continued for about 3 months. The prednisone was stopped 1-month posthospital discharge. She was on oral corticosteroid therapy for a total of 14 weeks.
Her lowest total serum IgE level obtained about 7 weeks off oral steroid therapy was at 1182 KUA/L. A follow-up chest x-ray about 14 weeks posthospital discharge, showed some residual disease. At that point, she was off all therapy for ABPA but was continuing ongoing follow-up visits and monitoring of total serum IgE level, a major marker of disease activity.
At her last appointment with the Allergy Department, about 5 months off all therapy for ABPA, the patient was in clinical remission and was not taking medication for chronic cough. She remained able to walk up a flight of stairs without dyspnea on exertion. She had no complaint of wheezing. Spirometry results, however, had worsened: forced vital capacity was 72% (1.66 L), and FEV1 was 68% (1.23 L). Her total serum IgE level had increased to 2815 KUA/L, more than double its nadir. These parameter changes prompted resumption of prednisone at 30 mg daily for 2 weeks followed by 20 mg a day. A repeat total serum IgE 2 months postinitiation of therapy once again showed a good response, this time reduced to a level of 1644 KUA/L. Further spirometry was not done.
Discussion
ABPA is a well-recognized disease entity that is usually associated with cough. Three considerations set this case apart from the usual ABPA cases:
Late onset of disease (diagnosis is most commonly made in the third or fourth decades of life)
No existing documentation of asthma, which is considered a major diagnostic criterion
Initial lack of associated wheezing, which generally accompanies ABPA because of asthma.
It is possible that asthma could have been diagnosed early in the course of her chronic cough if spirometry had been performed before and after bronchodilator to look for an increase in FEV1 of at least 12% or 200 mL. On the other hand, any demonstrated improvement in lung function in this patient after the diagnosis of ABPA could be attributed to response to a combination therapeutic approach directed solely at the pathogenesis of ABPA, irrespective of an asthma diagnosis. More will be said in this regard at the conclusion of this section.
A recent comprehensive review of ABPA lists the diagnostic criteria for ABPA (see Sidebar: Diagnostic critera for allergic bronchopulmonary aspergillosis).26 The diagnostic criteria for ABPA were partially fulfilled early on in this case. Specifically, the strong allergic sensitivity specific to Aspergillus fumigatus, sputum culture positive for Aspergillus fumigatus, and radiologic findings consistent with ABPA might be considered red flags. The clincher was a markedly elevated total serum IgE level, which was undocumented until 3 years into her course. Elevated total serum IgE level is very important: not only is it a major diagnostic criterion, it is also a marker that is helpful in assessing responsiveness to therapy as well as in predicting the likelihood of relapse, especially in patients with an initial IgE level of >2500 IU/mL.29,30
Importantly, bronchiectasis was demonstrated early on in the course of our patient’s disease and was shown to be an underlying factor in 5.5% of a series of 91 patients with cough lasting more than 3 weeks.21 However, the cause of bronchiectasis was unspecified in the latter report. In the same study, a subgroup of patients without bronchiectasis but responsive to inhaled corticosteroids was identified. Eosinophilic bronchitis without asthma was found in 13% of those 91 patients. In a more recent study, 8% of patients with chronic cough evaluated in a specialty clinic were found to have associated bronchiectasis, but once again the cause of bronchiectasis was not established.31
Bronchiectasis is a well-known hallmark of ABPA and has already been noted to be a major diagnostic criterion. In one study using high-resolution chest CT, 95% (42/45) of patients with ABPA had bronchiectasis, 93% had centrilobular nodules, and 67% had mucoid impaction; all of these were significantly more common in the ABPA patients than in the control group of asthmatics without clinical evidence of ABPA.32
A more recent study presented a pictorial essay of ABPA and found that 20% of patients with ABPA had high-attenuation mucus, which was hinted at by the radiologist who assessed the first chest CT scan in our case, shortly after initial pulmonary consultation as noted in the history.33 According to the authors of the latter report, who have a broad experience with this disease, this finding is pathognomonic of ABPA. They also indicated that ABPA with high-attenuation mucus is the most severe form of ABPA. As such, it not only reflects immunologic severity, but also predicts the risk of recurrent relapses.
A review of the literature uncovered a similar case of ABPA presenting in a 76-year-old man with cough as the primary symptom and without history of asthma or wheezing, but also without associated bronchiectasis.28 Absence of bronchiectasis in that case may have been related to earlier diagnosis and appropriate treatment.
Because of unrecognized disease and lack of treatment, ABPA can result in significant irreversible pulmonary changes, including bronchiectasis that could otherwise be forestalled or attenuated upon early recognition and appropriate treatment.34 However, it must be pointed out that, in the vast majority of patients, chronic cough is not an indication for a CT scan of the chest. This determination should be left to a pulmonary or allergy specialist, and if the chest CT findings are positive, they should be followed up to determine etiology, as previously noted.
The effect of poorly controlled chronic cough, when it occurs day and night, especially over months or years, can have a significant impact on the patient’s quality of life as well as family members living with the patient. French and coworkers identified a minimum of eight ways that chronic cough adversely affected their patient’s lifestyle and overall health. They concluded, “On the basis of our results, it is inappropriate to minimize a patient’s complaint of chronic cough and/or advise him/her ‘to live with it … since it can be successfully treated in most patients who adhere to treatment.”35
Early diagnosis of ABPA may require the involvement of one or more specialists to identify the cause(s) of chronic cough. In addition, proper management of the underlying condition may require ongoing follow-up by an experienced ABPA specialist. With a disease like ABPA, such follow-up care can span decades. In these difficult cases, specialist care is in the best interest of the patient and promotes sustained quality of care and quality of life.
Finally, it could be argued that the patient indeed did have at least a cough-variant form of asthma that went undiagnosed. On the basis of the patient’s three-year history of adequate trials of inhaled controller medications as well as short courses of oral corticosteroid but inadequate response, cough-variant asthma is highly unlikely.11,36 Spirometry results for patients with cough-variant asthma are often within the normal range, with less evidence of reversibility than seen in classic asthma.11 Any reversibility in this case after ABPA treatment alone could be attributed to the anti-inflammatory effect of the oral corticosteroid-itraconazole combination therapy, because there was no use of asthma controller or reliever medication to explain spirometric improvement as well as concomitant clinical and serologic improvement.37 Furthermore, she remained free from symptoms of asthma even without oral anti-inflammatory medications.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990 Mar;141(3):640–7. doi: 10.1164/ajrccm/141.3.640. DOI: http://dx.doi.org/10.1164/ajrccm/141.3.640. [DOI] [PubMed] [Google Scholar]
- 2.Smyrnios NA, Irwin RS, Curley FJ, French CL. From a prospective study of chronic cough: diagnostic and therapeutic aspects in older adults. Arch Intern Med. 1998 Jun 8;158(11):1222–8. doi: 10.1001/archinte.158.11.1222. DOI: http://dx.doi.org/10.1001/archinte.158.11.1222. [DOI] [PubMed] [Google Scholar]
- 3.Pratter MR, Brightling CE, Boulet LP, Irwin RS. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006 Jan;129(1 Suppl):222S–31S. doi: 10.1378/chest.129.1_suppl.222S. DOI: http://dx.doi.org/10.1378/chest.129.1_suppl.222S. [DOI] [PubMed] [Google Scholar]
- 4.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006 Jan;129(1 Suppl):1S–23S. doi: 10.1378/chest.129.1_suppl.1S. DOI: http://dx.doi.org/10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008 Apr 19;371(9621):1364–74. doi: 10.1016/S0140-6736(08)60595-4. DOI: http://dx.doi.org/10.1016/S0140-6736(08)60595-4. [DOI] [PubMed] [Google Scholar]
- 6.Benich JJ, 3rd, Carek PJ. Evaluation of the patient with chronic cough. Am Fam Physician. 2011 Oct 15;84(8):887–92. [PubMed] [Google Scholar]
- 7.Dalal B, Geraci SA. Office management of the patient with chronic cough. Am J Med. 2011 Mar;124(3):206–9. doi: 10.1016/j.amjmed.2010.05.005. DOI: http://dx.doi.org/10.1016/j.amjmed.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Birring SS. New concepts in the management of chronic cough. Pulm Pharmacol Ther. 2011 Jun;24(3):334–8. doi: 10.1016/j.pupt.2011.01.005. DOI: http://dx.doi.org/10.1016/j.pupt.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Birring SS. Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med. 2011 Mar 15;183(6):708–15. doi: 10.1164/rccm.201007-1017CI. DOI: http://dx.doi.org/10.1164/rccm.201007-1017CI. [DOI] [PubMed] [Google Scholar]
- 10.Weinberger SE, Silvestri RC. Evaluation of subacute and chronic cough in adults [monograph on the Internet] UpToDate. 2012. Oct, [cited 2012 Nov 16]. Available from: http://uptodate.com/contents/evaluation-of-subacute-and-chronic-cough-in-adults.
- 11.Niimi A. Cough and asthma. Curr Respir Med Rev. 2011 Feb;7(1):47–54. doi: 10.2174/157339811794109327. DOI: http://dx.doi.org/10.2174/157339811794109327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donnell AE. Bronchiectasis. Chest. 2008 Oct;134(4):815–23. doi: 10.1378/chest.08-0776. DOI: http://dx.doi.org/10.1378/chest.08-0776. [DOI] [PubMed] [Google Scholar]
- 13.Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005 Jul;12(4):205–9. DOI: http://dx.doi.org/10.1097/01.cpm.0000171422.98696.ed. [Google Scholar]
- 14.Loebinger MR, Wells AU, Hansell DM, et al. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J. 2009 Oct;34(4):843–9. doi: 10.1183/09031936.00003709. DOI: http://dx.doi.org/10.1183/09031936.00003709. [DOI] [PubMed] [Google Scholar]
- 15.Pratter MR. Chronic upper airway cough syndrome secondary to rhinosinus diseases (previously referred to as postnasal drip syndrome): ACCP evidence-based clinical practice guidelines. Chest. 2006 Jan;129(1 Suppl):63S–71S. doi: 10.1378/chest.129.1_suppl.63S. DOI: http://dx.doi.org/10.1378/chest.129.1_suppl.63S. [DOI] [PubMed] [Google Scholar]
- 16.Watelet JB, Van Zele T, Brusselle G. Chronic cough in upper airway diseases. Respir Med. 2010 May;104(5):652–7. doi: 10.1016/j.rmed.2009.11.020. DOI: http://dx.doi.org/10.1016/j.rmed.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Brooks SM, Bernstein IL. Irritant-induced airway disorders. Immunol Allergy Clin North Am. 2011 Nov;31(4):747–68. vi. doi: 10.1016/j.iac.2011.07.002. DOI: http://dx.doi.org/10.1016/j.iac.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest. 2005 May;127(5):1710–3. doi: 10.1378/chest.127.5.1710. DOI: http://dx.doi.org/10.1378/chest.127.5.1710. [DOI] [PubMed] [Google Scholar]
- 19.Sundar KM, Daly SE. Chronic cough and OSA: a new association? J Clin Sleep Med. 2011 Dec 15;7(6):669–77. doi: 10.5664/jcsm.1482. DOI: http://dx.doi.org/10.5664/jcsm.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saritas Yuksel E, Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease: cough, asthma, laryngitis, chest pain. Swiss Med Wkly. 2012 Mar 22;142:w13544. doi: 10.4414/smw.2012.13544. DOI: http://dx.doi.org/10.4414/smw.2012.13544. [DOI] [PubMed] [Google Scholar]
- 21.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999 Aug;160(2):406–10. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 22.Prince DS, Peterson DD, Steiner RM, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989 Sep 28;321(13):863–8. doi: 10.1056/NEJM198909283211304. DOI: http://dx.doi.org/10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 23.Teirstein AS. Help for the diagnosis of some, but not all cases of Mycobacterium avium-complex pulmonary disease. Am J Respir Crit Care Med. 2008 Apr 1;177(7):677–9. doi: 10.1164/rccm.200801-018ED. DOI: http://dx.doi.org/10.1164/rccm.200801-018ED Comment on: Kitada S, Kobayashi K, Ichiyama S, et al. Serodiagnosis of Mycobacterium avium-complex pulmonary disease using an enzyme immunoassay kit. Am J Respir Crit Care Med 2008 Apr 1;177(7):793–7. DOI: http://dx.doi.org/10.1164/rccm.200705-771OC. [DOI] [PubMed] [Google Scholar]
- 24.Heffner JE. A middle-aged woman with persistent cough. J Resp Dis. 2001;22(7):431–7. [Google Scholar]
- 25.Marcotte GV, Essayan DM. Chronic productive cough and bronchiectasis in a 40-year-old woman. Ann Allergy Asthma Immunol. 1997 Jun;78(6):559–64. doi: 10.1016/S1081-1206(10)63215-2. DOI: http://dx.doi.org/10.1016/S1081-1206(10)63215-2. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R. Allergic bronchopulmonary aspergillosis. Chest. 2009 Mar;135(3):805–26. doi: 10.1378/chest.08-2586. DOI: http://dx.doi.org/10.1378/chest.08-2586. [DOI] [PubMed] [Google Scholar]
- 27.Patterson K, Strek ME. Allergic bronchopulmonary aspergillosis. Proc Am Thorac Soc. 2010 May;7(3):237–44. doi: 10.1513/pats.200908-086AL. DOI: http://dx.doi.org/10.1513/pats.200908-086AL. [DOI] [PubMed] [Google Scholar]
- 28.Krasnick J, Patterson R, Roberts M. Allergic bronchopulmonary aspergillosis presenting with cough variant asthma and identifiable source of Aspergillus fumigatus. Ann Allergy Asthma Immunol. 1995 Oct;75(4):344–6. [PubMed] [Google Scholar]
- 29.Imbeau SA, Nichols D, Flaherty D, Dickie H, Reed C. Relationships between prednisone therapy, disease activity, and the total serum IgE level in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1978 Aug;62(2):91–5. doi: 10.1016/0091-6749(78)90084-2. DOI: http://dx.doi.org/10.1016/0091-6749(78)90084-2. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal R, Gupta D, Aggarwal AN, et al. Clinical significance of decline in serum IgE levels in allergic bronchopulmonary aspergillosis. Respir Med. 2010 Feb;104(2):204–10. doi: 10.1016/j.rmed.2009.09.005. DOI: http://dx.doi.org/10.1016/j.rmed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Kastelik JA, Aziz I, Ojoo JC, Thompson RH, Redington AE, Morice AH. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J. 2005 Feb;25(2):235–43. doi: 10.1183/09031936.05.00140803. DOI: http://dx.doi.org/10.1183/09031936.05.00140803. [DOI] [PubMed] [Google Scholar]
- 32.Ward S, Heyneman L, Lee MJ, Leung AN, Hansell DM, Müller NL. Accuracy of CT in the diagnosis of allergic bronchopulmonary aspergillosis in asthmatic patients. Am J Roentgenol. 1999 Oct;173(4):937–42. doi: 10.2214/ajr.173.4.10511153. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal R, Khan A, Garg M, Aggarwal AN, Gupta D. Pictorial essay: allergic bronchopulmonary aspergillosis. Indian J Radiol Imaging. 2011 Oct;21(4):242–52. doi: 10.4103/0971-3026.90680. DOI: http://dx.doi.org/10.4103/0971-3026.90680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safirstein BH, D’Souza MF, Simon G, Tai EH, Pepys J. Five-year follow-up of allergic bronchopulmonary aspergillosis. Am Rev Respir Dis. 1973 Sep;108(3):450–9. doi: 10.1164/arrd.1973.108.3.450. [DOI] [PubMed] [Google Scholar]
- 35.French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998 Aug 10–24;158(15):1657–61. doi: 10.1001/archinte.158.15.1657. DOI: http://dx.doi.org/10.1001/archinte.158.15.1657. [DOI] [PubMed] [Google Scholar]
- 36.Doan T, Patterson R, Greenberger PA. Cough variant asthma: usefulness of a diagnostic-therapeutic trial with prednisone. Ann Allergy. 1992 Dec;69(6):505–9. [PubMed] [Google Scholar]
- 37.Wark PA, Hensley MJ, Saltos N, et al. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol. 2003 May;111(5):952–7. doi: 10.1067/mai.2003.1388. DOI: http://dx.doi.org/10.1067/mai.2003.1388. [DOI] [PubMed] [Google Scholar]