Abstract
Electrical activation of an excitatory reflex between sensory fibers in the pudendal nerve and the bladder has been demonstrated in cats and is a potential means of restoring micturition function in persons with spinal cord injury. We investigated the clinical feasibility of activating this reflex to restore bladder function in persons with spinal cord injury by using intraurethral electrical stimulation to activate pudendal sensory fibers innervating the urethra. Excitatory bladder responses (contractions) were evoked by trains of electrical pulses applied to either the proximal (29.7 ± 11.6 cmH2O) or distal (30.2 ± 11.6 cmH2O) segment of the urethra. The results indicate that an excitatory reflex between pudendal nerve afferents and the bladder exists in humans with spinal injury and may provide a substrate for restoring micturition function.
I. Introduction
Spinal cord injury (SCI) results in lower urinary tract symptoms that include detrusor overactivity and detrusor sphincter dyssynergia. If left untreated, these pathological factors can cause urinary tract infections, bladder trabeculation, ureteral reflux, and kidney damage. Despite effective bladder management methods (anti-cholinergic medication plus clean intermittent catheterization), significant morbidity and diminished quality of life remain a problem for persons with SCI.
Electrical activation of pudendal nerve afferents that mediate inhibitory and excitatory bladder reflexes has been investigated recently as a potential means of restoring bladder function. In both spinal intact and chronic SCI cats, electrical pudendal nerve stimulation produces bladder responses that are strongly dependent on the stimulation frequency: bladder inhibition (3 to 15 Hz) [1-4], and bladder excitation (> 20 Hz) [1, 3, 5, 6].
Although an analogous bladder inhibitory reflex has been confirmed in persons with SCI [7-9], the demonstration of an excitatory pathway has been limited to transient or low pressure increases in detrusor pressure [10, 11]. These latter results were due, in part, to a limited understanding of the anatomical innervation of the lower urinary tract by pudendal nerve afferents. This motivated further study in cats [12, 13], where we identified two pudendal afferent pathways that could mediate excitatory bladder responses evoked by electrical stimulation. These pathways were labeled the cranial sensory (CSN) and dorsal genital (DGN) nerves, and were found to innervate the proximal and distal segments of the urethra, respectively.
In this study, we examined the feasibility of clinically translating the results from the cat to individuals with chronic SCI. We used intraurethral stimulation with a catheter electrode to activate selectively the pudendal afferents innervating the proximal and distal segments of the urethra.
II. Methods
The experimental protocol was approved by the Institutional Review Board of Duke University Medical Center, and written informed consent was obtained prior to each experiment. The study involved 7 individuals (6 male, 1 female, age = 26 to 66 years) with chronic SCI (C5 to T12).
A.Instrumentation
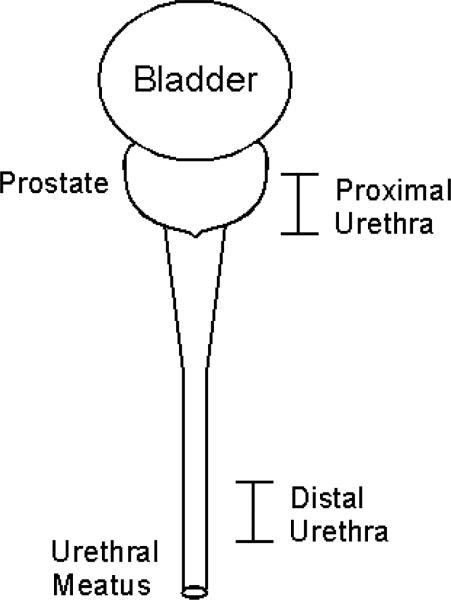
All experiments began with a urodynamic evaluation of the bladder. Measurement of bladder and abdominal pressures was consistent with previous experiments [11]. Electromyographic (EMG) activity from the perineum was recorded by pairs of percutaneously inserted stainless steel wires. Both pressure and EMG signals were filtered (DC to 40 Hz, 3 Hz to 1 kHz), amplified (both gain = 1000, ETH-255, iWorx/CB Sciences, Inc., Dover, NH), and digitally stored at 5 kHz on a computer. An 8-electrode stimulating catheter (diameter = 2 mm, electrode length = 2 mm) was inserted into the urethra, such that the active electrode contact was positioned at specific segments within the proximal and distal urethra (Figure 1). In males, this corresponded to positions that were 4-6 cm distal to the bladder neck (proximal urethra) and 3-5 cm from the urethral meatus (distal urethra). In females, the proximal and distal segments were defined by locations 1 cm away from the bladder neck and urethral meatus, respectively.
Figure 1.
Location of active electrode contact positioned along the urethra.
B.Urodynamic Evaluation
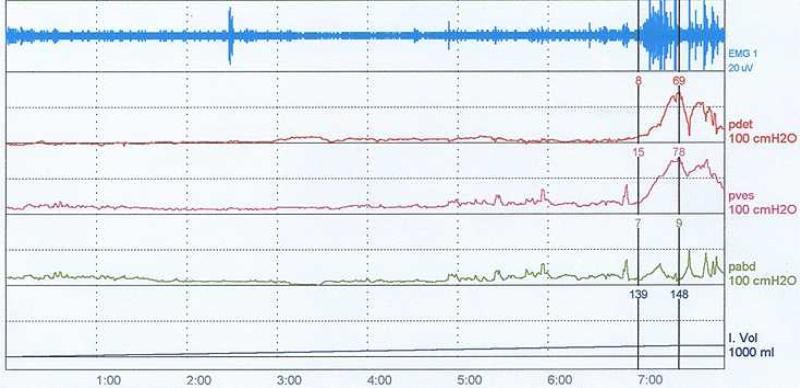
The detrusor pressure (Pdet = Pbladder – Pabdomen) and EMG activity (perineal or EAS) were recorded continuously as the bladder was filled with room temperature saline at 20-40 ml/min (Figure 2). The volume threshold at which a distension-evoked bladder contraction occurred (i.e., bladder capacity, Vth) was defined by the infused bladder volume at which the Pdet exceeded 30 cmH2O for at least 10 seconds. The bladder was emptied and the stimulation threshold (T) for eliciting a reflex EMG response (bulbocavernosus reflex (BCR)) was determined by intraurethral (IU) stimulation delivered individually to the proximal (Tp) and distal (Td) urethra. Within each IU segment, the amplitude of single current pulses was increased until triggered EMG traces or anal twitches were verified visually. The bladder was then filled to approximately 80 % of Vth, and 30-second trains of current pulses were applied to each segment of the urethra. The protocol involved a randomized sequence of 6 trials that combined two stimulus amplitudes (2 and 4 times Tp or Td) and three stimulation frequencies (2, 10 and 35 Hz). If no BCR was elicited, a default threshold of 15 mA was used.
Figure 2.
Urodynamic evaluation of bladder. [EMG = perineal EMG, pdet = detrusor pressure, pves = bladder pressure, pabd = abdominal pressure, and I.Vol = infused volume]
C.Data Analysis
An electrically-evoked excitatory bladder response was defined by a minimum increase in detrusor pressure of 10 cmH2O above baseline during stimulation. The baseline was the Pdet averaged over 2 seconds prior to electrical stimulation. An evoked response was characterized qualitatively by the peak Pdet, normalized with respect to the maximum Pdet measured during distension evoked bladder contractions. All data are expressed as the mean ± standard error, unless otherwise specified. Statistical analysis used an initial one-way ANOVA followed by a Tukey multiple comparison test (S-PLUS, Insightful Corp.).
III. Results
Overactive bladder and spastic EMG activity was commonly observed during urodynamic fills in all participants (Figure 2). A distension evoked (DE) bladder contraction was observed in 6 of 7 participants, with considerable inter-individual variability in Vth = 19 to 300 ml and Pdet = 34 to 113 cmH2O). Residual effects of anti-cholinergic medication were observed in one individual (experiment 7), as this participant failed to exhibit any DE bladder activity.
A. Stimulation Threshold (T)
A BCR was elicited in 5 of 7 participants (no response in exp 2 and 7), where the activation thresholds of this reflex were relatively consistent at both ends of the urethra: proximal IU (14.4 ± 2.1 mA) vs. distal IU (12 ± 2 mA). Other than the typical ‘abdominal tapping’ sensation reported in those individuals with an incomplete SCI (Exp 3, 4 and 5), IU stimulation did not elicit any noxious sensations.
B. Excitatory Bladder Reflex
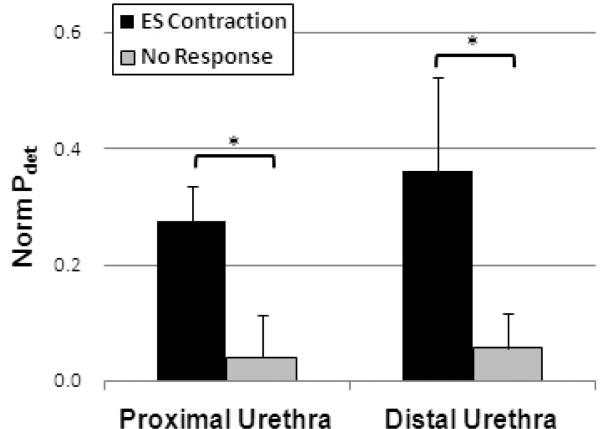
Electrical stimulation of the proximal urethra evoked sustained bladder activity in 3 of 7 participants. This excitatory reflex was elicited, regardless of the severity of spinal injury (Exp 1, 2 vs. Exp 3) or presence of a BCR (Exp 2 vs Exp 1, 3). High frequency, high amplitude (35 Hz and 4T) stimulation was effective at evoking these responses, but other stimulation parameters were also equally effective in one participant (Exp 1). This resulted in an overall average normalized peak Pdet of 0.27 ± 0.06 (range = 0.16 to 0.53) that was significantly greater than “non-responsive” bladder activity (*, p < 0.05, Figure 3).
Figure 3.
Comparison of normalized detrusor pressure (Norm Pdet) in response to selective electrical stimulation at proximal and distal segments of the urethra. [*, p < 0.05]
In 2 of the 7 participants, electrical stimulation of the distal urethra evoked excitatory bladder responses. This also resulted in significant increases in normalized Pdet (0.36 ± 0.16, range = 0.2 to 0.52, p < 0.05, Figure 3). Excitatory bladder responses were evoked regardless of the presence of a BCR (Exp 1 vs. Exp 4) or severity of spinal injury (Exp 1 vs. Exp 4). It is noted that there was no significant difference in bladder activity between trials evoked by proximal and distal IU stimulation (p = 0.55).
IV. Discussion
The results of this study expand our current understanding of the existence of an excitatory pudendal-to-bladder reflex in persons with SCI [10, 11]. We recorded sustained elevations in detrusor pressure (Pdet) in response to electrical stimulation applied individually with a catheter electrode in both the proximal and distal segments of the urethra. Excitatory bladder responses were evoked in individuals diagnosed with complete and also incomplete spinal injuries, suggesting that this reflex was part of a direct spinal micturition pathway.
Consistent with previous work in cats, both the physiological conditions and stimulation parameters used in this study support our hypothesis that the afferent limb of the reflex is mediated by the pudendal nerve. We found the ability to electrically evoke reflex bladder contractions depended strongly on the infused volume [12, 14, 15]. Volumes equal to at least 80% of the distension evoked volume threshold (Vth) were required to evoke a reflex contraction. We also found that excitatory bladder responses were evoked by stimulus pulses greater than the thresholds (multiples of T) required to elicit BCR responses [5, 6], indicating a requisite activation of smaller diameter myelinated fibers. Finally, it was clear that high frequency stimulation (f ≥ 20 Hz) was effective at evoking bladder contractions [1, 12, 15].
Electrical activation of pudendal afferents can evoke excitatory bladder responses in persons with SCI. It is not yet clear, however, whether IU stimulation will ameliorate or exacerbate detrusor sphincter dyssynergia.
V. Conclusion
In this study, we demonstrated evidence of two excitatory pudendal-to-bladder reflexes in persons with chronic SCI. The presence of these responses suggests the feasibility of using pudendal nerve stimulation as part of a neural prosthetic system aimed to restore bladder function. Further work is required, however, to determine whether the reflexes suppress or augment detrusor dyssynergia and to determine whether these reflexes can produce bladder emptying.
Acknowledgment
The authors thank Jean Maynor RN, Betty O'Neal NA, and John Beaudry PA.C for their assistance.
Financial support was provided by the Neilson Foundation and the National Institutes of Health (RO1 NS050514).
References
- 1.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol. 2006 Nov 15;577(Pt 1):115–26. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundin T, Carlsson CA, Kock NG. Detrusor inhibition induced from mechanical stimulation of the anal region and from electrical stimulation of pudendal nerve afferents. An experimental study in cats. Invest Urol. 1974 Mar;11(5):374–8. [PubMed] [Google Scholar]
- 3.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol. 2006 Jan;197(1):225–34. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Walter JS, Wheeler JS, Robinson CJ, Wurster RD. Inhibiting the hyperreflexic bladder with electrical stimulation in a spinal animal model. Neurourol Urodyn. 1993;12(3):241–52. doi: 10.1002/nau.1930120306. discussion 253. [DOI] [PubMed] [Google Scholar]
- 5.Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett. 1998 Mar 20;244(3):137–40. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- 6.Yoo PB, Grill WM. Minimally-invasive electrical stimulation of the pudendal nerve: A pre-clinical study for neural control of the lower urinary tract. Neurourol Urodyn. 2007;26(4):562–9. doi: 10.1002/nau.20376. [DOI] [PubMed] [Google Scholar]
- 7.Hansen J, Media S, Nohr M, Biering-Sorensen F, Sinkjaer T, Rijkhoff NJ. Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J Urol. 2005 Jun;173(6):2035–9. doi: 10.1097/01.ju.0000158160.11083.1b. [DOI] [PubMed] [Google Scholar]
- 8.Kirkham AP, Shah NC, Knight SL, Shah PJ, Craggs MD. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord. 2001 Aug;39(8):420–8. doi: 10.1038/sj.sc.3101177. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler JS, Jr., Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol. 1992 Jan;147(1):100–3. doi: 10.1016/s0022-5347(17)37145-8. [DOI] [PubMed] [Google Scholar]
- 10.Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett. 2004 Apr 22;360(1-2):9–12. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Yoo PB, Klein SM, Grafstein NH, Horvath EE, Amundsen CL, Webster GD, Grill WM. Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourol Urodyn. 2007;26(7):1020–3. doi: 10.1002/nau.20441. [DOI] [PubMed] [Google Scholar]
- 12.Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008 Jul;212(1):218–25. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo PB, Woock JP, Grill WM. Somatic innervation of the feline lower urinary tract. Brain Res. 2008 Dec 30;1246:80–7. doi: 10.1016/j.brainres.2008.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005 May;93(5):2688–97. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- 15.Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol Regul Integr Comp Physiol. 2008 Jun;294(6):R1880–9. doi: 10.1152/ajpregu.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]