Abstract
Exposure to infectious microbes is a likely confounder after a nuclear terrorism event. In combination with radiation, morbidity and mortality from an infection may increase significantly. Pulmonary damage after low-dose low-LET irradiation is characterized by an initial diffuse alveolar inflammation. By contrast, inhaled fungal spores produce localized damage around pulmonary bronchioles. In the present study, we assessed lung injury in C57BL/6 mice after combined exposures to whole-body X radiation and inhaled fungal spores. Either animals were exposed to Aspergillus spores and immediately irradiated with 2 Gy, or the inoculation and irradiation were separated by 8 weeks. Pulmonary injury was assessed at 24 and 48 h and 1, 2, 4, 8, and 24 weeks later using standard H&E-stained sections and compared with sham-treated age-matched controls. Immunohistochemistry for invasive inflammatory cells (macrophages, neutrophils and B and T lymphocytes) was performed. A semi-quantitative assessment of pulmonary injury was made using three distinct parameters: local infiltration of inflammatory cells, diffuse inflammation, and thickening and distortion of alveolar architecture. Radiation-induced changes in lung architecture were most evident during the first 2 weeks postexposure. Fungal changes were seen over the first 4 weeks. Simultaneous combined exposures significantly increased the duration of acute pulmonary damage up to 24 weeks (P < 0.01). In contrast, administration of the fungus 8 weeks after irradiation did not produce enhanced levels of acute pulmonary damage. These data imply that the inhalation of fungal spores at the time of a radiation exposure alters the susceptibility of the lungs to radiation-induced injury.
INTRODUCTION
Pulmonary fungal infections from airborne Aspergillus fumigatus spores are a problem for immunosuppressed patients (1). However, individuals with normal immune systems rarely develop pulmonary aspergillosis, even when environmentally exposed to high concentrations of conidia, due to resistance resulting from lifelong exposure (2, 3). Studies modeling pulmonary injury after exposure to Aspergillus spores have demonstrated that the extent of fungal infection is related to the severity of immune modulation (4, 5). We hypothesize that exposure to Aspergillus spores in combination with other pulmonary damage caused by a secondary insult will likely change the tolerance of the lungs to injury and modulate the immune response.
The respiratory system is continually exposed to airborne contaminants. Ambient particulate matter present in inhaled air has the potential to cause acute and chronic pulmonary injury and is associated with respiratory diseases such as bronchilitis, pneumonia and asthma (6). The wide dispersal of particulate matter, organic irritants and other pollutants after the destruction of the World Trade Center in 2001 (7) produced extensive inhalation injuries and led to increased rates of new-onset and persistent respiratory health effects (8, 9). Longer-term health effects were also reported for people with pre-existing pulmonary diseases such as the asthmatic residents of Lower Manhattan (10).
Under current threat assessments, a nuclear or biological terrorist attack is considered probable. An urban area is considered the most likely target to achieve the maximal psychological impact (11–14). The inclusion of conventional explosives to ensure widespread dispersal of any harmful agents is also anticipated (15, 16). After a nuclear or radiological explosive event, pulmonary injury would occur either as a result of inhaled radioactive particles or from external contaminants (17). Moreover, the biological effects of radiation exposure are likely to be exacerbated by associated trauma injuries, toxic chemicals and possibly endemic opportunistic pathogens in the local environment. Infectious disease agents could also be dispersed intentionally to provide an additional biological threat.
A combined exposure to multiple cytotoxic modalities is likely to change tissue susceptibility to injury since radiation exposure impairs immune response. For example, an increase in mortality has been reported when radiation exposure occurs in combination with thermal burns (18, 19). The mechanism underlying the synergism is unknown, but an increased susceptibility to infections has been proposed (20–22). Increased mortality associated with a secondary bacterial exposure has also been reported after exposure to low radiation doses (<1 Gy); the susceptibility to infection was dependent on exposure time (23–25). Synergistic effects between viral infection and radiation have been observed (26), but currently no data exist for combined exposure to radiation and common fungal species.
Accidental radiation exposure produces radiation pneumonitis and respiratory dysfunctions (27, 28). The impact of these radiation-induced lung injuries on the susceptibility to opportunistic infections from airborne endemic biological agents is unknown, but it is likely to have an adverse synergistic effect. Aspergillus fumigatus is a ubiquitous soil-dwelling fungus. Despite life-long exposure and low morbidity in immune-competent individuals, infections are common after large-scale environmental disruption that causes the airborne distribution of the spores. For example, elevated Aspergillus infection rates have been reported at construction sites and during hospital renovations (2, 29–32). An explosive device causing comparable environmental disruption has the same potential to increase the risk of Aspergillus infection to victims and first responders. Radiation injury that compromised lung function could reduce the clearance of Aspergillus conidia, exacerbating the risk of invasive pulmonary aspergillosis (33).
We hypothesize that radiation damage in combination with a coincident exposure to fungal spores would alter or prolong pulmonary injury in excess of that seen after radiation exposure alone, leading to additional pulmonary health risks. To the best of our knowledge, no studies on combined risk have been performed previously; the purpose of this study is to define how each of these hazards changes the susceptibility to the other and whether there is an alteration in health outcome.
MATERIALS AND METHODS
Animals
Female C57BL/6 mice, 6–8 weeks of age and weighing approximately 20 g, were obtained from Charles River Laboratories (Wilmington, MA) and acclimated for 1 week prior to treatment. Animals were housed in standard rodent plastic cages with integrated filter tops to limit their chances of contracting pulmonary infections from external sources. Water and standard rodent chow were given ad libitum. Treatment groups were (1) control group (vehicle inoculation and sham irradiation); (2) 2 Gy whole-body irradiation; (3) intranasal inoculation with A. fumigatus spores; or (4) 2 Gy immediately followed by inoculation with A. fumigatus. There were five animals per treatment group and time. The experimental protocol was approved by the Institute Animal Care and Use Committee of William Beaumont Hospital.
Aspergillus Inoculation
Aspergillus fumigatus, genome reference strain Af293, was obtained from Dr. Gregory S. May of the M.D. Anderson Cancer Center. The strain was originally isolated clinically from a patient who eventually succumbed to invasive aspergillosis. This strain has previously been shown to produce invasive pulmonary aspergillosis in mice, with a reported LD90 of 5 × 106 in immunosuppressed CD1 mice. A. fumigatus conidia were grown on Sabouraud 2% glucose agar in 50-ml agar flasks at 37°C for 12–14 days. On average, 2.25 × 1010 spores were produced per 50-ml plate. Spores were harvested into PBS with 0.05% Tween, adjusted to an inoculation concentration of 5 × 107 spores in 30 μl, and mice were subsequently inoculated via manual intranasal delivery. For the inoculation, mice were lightly anesthetized with 0.5% isoflurane, and an extended pipette tip containing the spores was placed just in the nasal cavity and the spore-containing fluid was slowly released as micro-droplets into the nostril. Sham-treated animals received vehicle alone.
Radiation Schedule
Animals were whole-body irradiated at room temperature using a 160 kVp Faxitron X-ray machine (0.5 mm copper and aluminum filters) at a dose rate of 0.69 Gy/min. A dose of 2 Gy was delivered. The mice were not anesthetized and remained unrestrained but confined in a plexiglass irradiation device to maintain posture and dose homogeneity within the radiation beam (10 cm in diameter). X-ray dosimetry was performed in collaboration with Dr. Elwood Armour at Johns Hopkins University (Baltimore, MD). A mouse phantom was used along with EBT-GAFChromic film for calibration. This technique was devised by Dr. Armour and provides a better dosimetry analysis for the experimental setup than can be achieved by an ionization chamber. To obtain maximum depth penetration, the Faxitron beam was filtered and used with a long source-to-skin distance. Copper filtration was adopted and supplied by Faxitron. After irradiation, the mice were returned to standard filter-top caging. Sham-treated animals were subject to all of the above procedures but the X-ray machine was not switched on.
Tissue Isolation
Mice were killed humanely by i.p. injection of 0.1 ml sodium pentobarbital (1:9 mixture of Nembutal® and heparin at a dose of 50 mg/kg) at 24 and 48 h and 1, 2, 4, 8 and 24 weeks post-treatment. The chest cavity was surgically opened with a midline incision and blood was harvested by cardiac puncture into a syringe containing 50 μl of heparin. The lungs were clamped to separate the right and left lungs. Intratracheal lung inflation by perfusion with 1 ml neutral-buffered formalin was performed on the left lung, which was then removed from the animal and preserved in 10% formalin overnight at 4°C. The right lung was removed from the thoracic cavity, crushed between two clean glass slides, and immersed in 5 ml PBS to release the spores. Dilutions of the lung solution were plated onto 2% Sabouraud glucose agar containing a penicillin/streptomycin solution to obtain Aspergillus colony-forming units per gram of lung tissue.
Histology
Formalin-fixed lungs were embedded in paraffin in the same orientation into individual tissue cassettes and cut on a microtome. Whole lung tissue sections, 5 μm thick, were mounted onto slides and subsequently deparaffinized and rehydrated through xylene and graded alcohols. Sections were stained with hematoxylin and eosin using an automated slide stainer (Thermo Scientific Microm HMS 740 Robotic Routine Stainer), covered with cover slips, and systematically analyzed in a blinded fashion. PAS and Grocott’s methenamine silver stain were used to detect the presence of fungi.
Evaluation of Tissue Damage
A semi-quantitative assessment of pulmonary injury was made by scanning the entire lung section at low (2×, 4×) and medium (10×) magnification. Damage was estimated according to three distinct parameters: local infiltration of inflammatory cells, diffuse inflammation, and thickening and distortion of tissue, which considered alveoli and bronchioles separately (34). Inflammation considered both perivascular and peribronchiolar inflammation such as pneumonia, exudative bronchiolitis with destruction of bronchi and alveolae, hemorrhagic necrosis, and the presence of resident and recruited inflammatory cells. Thickening and distortion of tissue accounted for progressive alveolar septal thickening, intrastitial and intra-alveolar edema, hyperplasia of type II pneumocytes, and invading inflammatory cells. Each of these individual parameters were evaluated on a 4-point scale and then combined to derive a final score that estimated the overall extent of gross tissue change across the entire lung section compared with sham-treated control animals. PAS- and silver-stained sections were analyzed to identify the presence of fungus within the lung tissue; each slide was given a score of 0, 1, 2 or 3 according the extent of staining.
Quantitative Evaluation of Tissue Damage
Damage to pulmonary tissue was further analyzed and quantified by measuring the thickness of alveolar walls and the amount of alveolar space within the tissue. Average alveolar wall thickness for each slide was measured from 10 randomly selected histological fields where 10 wall measurements were made per field at 40× objective power. Selected walls were measured in micrometers using image analysis software. Treatments were blinded to the scorer, and on completion the data were decoded to determine the mean (±SEM) for five animals per group. Alveolar wall space across entire lung sections was calculated from a mathematical algorithm applied to digital images of the sections at medium objective power, as described previously (34). Briefly, the images were segmented using pixel color as a discriminator to differentiate between alveolar wall and space, creating a binary image where 0 represents an alveolar wall pixel and 1 represents an alveolar space pixel. A numerical score, determined as the area ratio of alveolar wall as a product of alveolar space, was generated for each lung section. This score was used in addition to the average measured wall thickness to broadly quantify the extent of inflammation within the lung tissue, because inflammation leads to progressive thickening of the alveolar walls and a subsequent reduction in alveolar space. Likewise, infiltration of inflammatory cells within the alveolar space itself results in further space reduction. All image analysis was done using Nikon NIS-Elements image analysis software.
Immunohistochemistry
Lung tissue was assessed for B and T lymphocytes, neutrophils and macrophage activity. Paraffin-embedded sections were deparaffinized in xylene and rehydrated through graded ethanols. For antigen retrieval, slides were heated for 20 min in 3-in-1 target retrieval solution, pH 9 (Dako, S2375). Immunohistochemical staining was performed automatically using a Dako AutostainerPlus. Briefly, sections were incubated with 3% H2O2 for 10 min to inactivate endogenous peroxidases, rinsed (Dako wash buffer), then blocked for 20 min with a rodent block (Rodent Block M, Biocare Medical no. RBM961H). Slides were then incubated with the following primary antibodies: CD3 (Abcam, no. ab5690) for T cells was applied for 30 min, CD79a (Abcam, no. 5691) for B cells for 30 min, activated macrophage marker CD68 (AbD Serotec, no. MCA1957GA) for 30 min, and neutrophil marker (AbD Serotec, no. MCA771G) for 40 min. After incubation, slides were rinsed in Dako wash buffer followed by addition of Envision flex HRP (Dako no. SM802) for 15 min. Finally, sections were reacted with substrate and chromagen (DAB) and counterstained with hematoxylin.
Data Analysis
Biological significance was determined using a non-parametric Mann-Whitney test. P values less than 0.05 were considered significant. Alveolar septal thickness was evaluated by Student’s t test assuming unequal variances, with P values less than 0.05 considered significant. The measured alveolar thickness and manual assessment of diffuse pulmonary damage (4-point scale) were compared by Spearman’s ρ correlation coefficient. ρ values <0.05 were considered significant.
RESULTS
Clearance of Fungal Spores
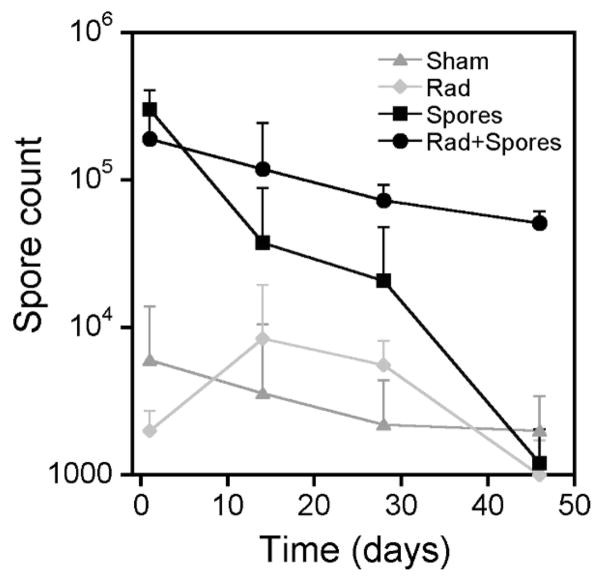
Initial experiments using a range of spore concentrations (5 × 106 to 5 × 108) were conducted to determine the maximum number of fungal spores that could be safely delivered; 5 × 107 spores in a 30 μl volume was found to be the optimum viscosity for intranasal inoculation and produced no adverse breathing complications after delivery. Using this regimen, no treatment-related morbidity was observed and animals remained asymptomatic with no physical signs of pulmonary distress and exhibited no behavioral or weight changes (data not shown). To assess the retention of the fungal spores after inoculation, an individual lung from each animal was crushed, and fixed dilutions of pulmonary suspension were plated on 2% Sabouraud glucose agar to obtain a count of Aspergillus colony-forming units per gram of tissue. Plotting the number of fungal colonies obtained as a function of time after inoculation indicated the rate of spore clearance (Fig. 1). A small number of spores were detected in the lungs of a few control (sham) and radiation-only animals (range 2 × 103–6 × 103) that were probably from routine environmental exposures. By contrast, mean (±SD) spore counts of 1.9 × 105 (±1.2 × 105) to 3 × 105 (±1 × 105) per gram of tissue were obtained 24 h after fungus inoculation for the fungus-exposed animals. No statistically significant difference in spore count was evident between the irradiated and nonirradiated animals at 24 h. However, 4 weeks after inoculation more spores were retained in the lungs of irradiated animals than in unirradiated animals (P < 0.01), and by 8 weeks spores were detected only in the lungs of animals given the combined treatment. These data demonstrate a slower pulmonary clearance of spores after radiation exposure. None of the groups retained spores at 24 weeks.
FIG. 1.
Spore count per gram of harvested lung tissue (mean ± SD) as a function of time after intranasal inoculation for animals given vehicle only (Sham), 2 Gy whole-body X irradiation (Rad), inoculated with 5 × 107 spores (Spores), and simultaneous combined radiation and fungal exposure (Rad + Spores).
Lung Histology
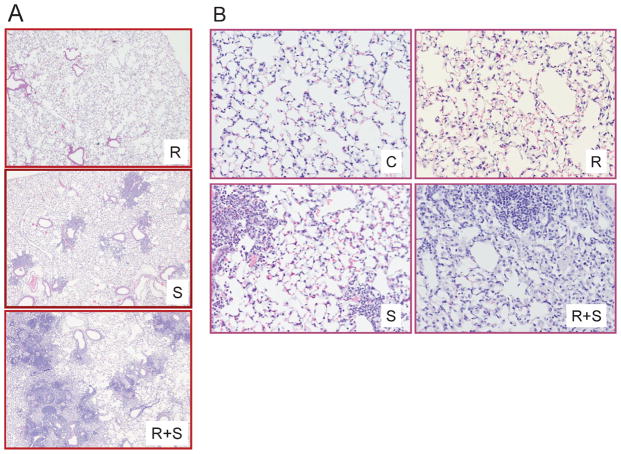
Since spore inoculation was anticipated to produce bronchial-related pulmonary damage involving an invasion of inflammatory cells, and the radiation treatment was expected to produce changes in alveolar architecture, the assessment of pulmonary injury from combined treatments (5 × 107 spores and 2 Gy) involved an assessment of both these parameters (Fig. 2). Control animals were inoculated with 30 μl vehicle (no spores) and sham-irradiated. In control animals, distinct alveolar sacs and single alveoli were evident, with visible pulmonary capillaries and vessels surrounding each alveolus (Fig. 2B). Adjacent alveoli were separated by a thin alveolar septum of flattened epithelial cells. Distinct type I and type II pneumocytes could be seen in the epithelium of the alveolar lining.
FIG. 2.
Panel A: H&E-stained lung sections showing representative changes in alveolar thickness seen after radiation only treatment (R), localized bronchial damage widely seen after fungal treatment only associated with the invasion of inflammatory cells (S), and alveolar thickening combined with localized damage when the dual treatments were given (R +S). Original magnification 200×. Panel B: Enlarged representative images 24 h post-treatment showing typical pulmonary architecture from untreated control animals (C), changes in alveolar thickening associated with exposure to 2 Gy whole-body X radiation (R), invasion of inflammatory cells around individual bronchioles that are typically seen after fungal inoculation only (S), and combined damage after dual treatment of radiation and fungal spores (R + S). Original magnification 20×.
In animals inoculated with 5 × 107 spores (sham-irradiated), the most evident alteration in lung tissue at 1–4 weeks postinoculation was an invasion of inflammatory cells localized around individual bronchioles (Fig 2A). Interstitial and intra-alveolar edema was also evident and contributed to the severity of the diffuse pattern of damage. Specific immunohistochemistry indicated an early invasion of neutrophils and lymphocytes followed by macrophages. By contrast, in irradiated animals (sham fungal inoculation), a diffuse subtle alveolar thickening was evident that was most discernible at 24 and 48 h postirradiation, with no distinct change in bronchiole structures. The mean alveolar wall thickness increased from 2.67 μM (±0.27) to 5.97 μM (±2.21) at 24 h after irradiation (P < 0.01). In contrast, animals inoculated with fungal spores (sham-irradiated) had a mean alveolar thickness of 3.26 μM (±0.49) that was not biologically different from controls.
Assessment of Damage from Combined Exposures
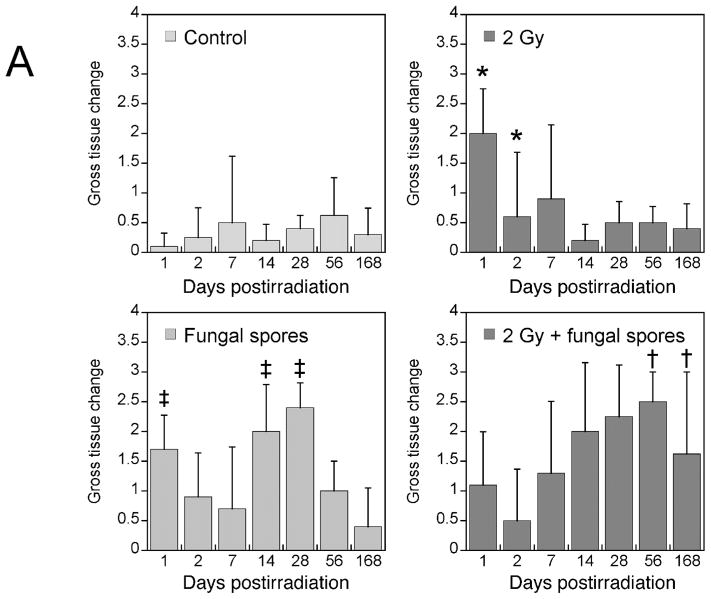
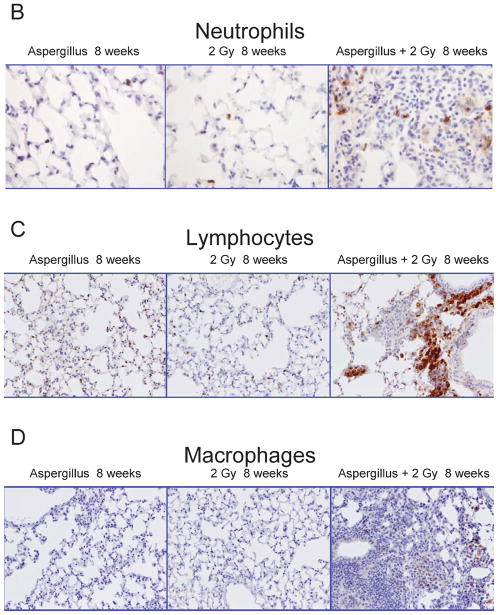
A semi-quantitative assessment of pulmonary injury was made by blinded observers using three distinct parameters: local infiltration of inflammatory cells, diffuse inflammation, and thickening and distortion of tissue (described in the Materials and Methods) to obtain a parameter called gross tissue change (Fig. 3A). This methodology was used to assess the distinct types of damage produced by the two insults. Similar gross tissue change scores were seen for the control animals over the 24-week period. Radiation-only treated animals (2 Gy; vehicle inoculation) showed a significant gross tissue change within the first week post-treatment (day 1, P = 0.021), after which scores were consistent with control values. Animals given only spores (5 × 107; sham radiation) exhibited gross tissue changes over the first 4 weeks with no damage by week 24; this was significantly different from controls at 24 h and 2–4 weeks (P < 0.01). Animals treated with both radiation and spores showed significantly (P = <0.01) more damage at 8 weeks and 24 weeks compared with radiation-only and spores-only animals. This response pattern reflected the pattern of neutrophil and lymphocyte invasion (Fig. 3B–D).
FIG. 3.
Panel A: Gross tissue change as a function of time after treatment in the four treatment groups (mean ± SD, n = 5). Significant damage is evident early after X irradiation (*P = 0.021) compared with control animals but diminishes with time post-treatment. Exposure to Aspergillus spores produced early and later lung injury that was significantly different from that in control animals (†P < 0.01) but dissipated by 24 weeks. Combined simultaneous exposures resulted in significantly prolonged and elevated damage beyond 24 weeks (‡P < 0.01) compared with controls, radiation only and fungal inoculation only.
Panels B–D: Images showing antibody-specific immunohistochemistry staining for neutrophils (panel B), lymphocytes (panel C) and macrophages (panel D) from representative animals exposed to 5 × 107 fungal spores, 2 Gy total-body X radiation or fungal spores and X radiation. Brown color indicates the presence of the three cell types. Original magnification 400×.
Temporal Response of Combined Exposures
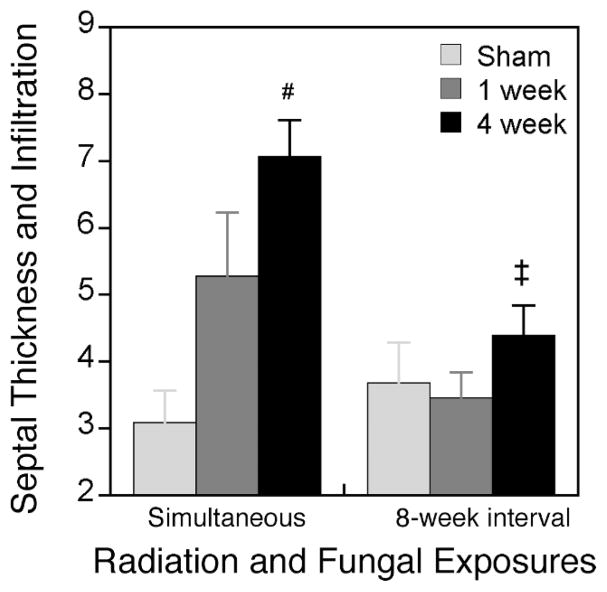
To assess the temporal response of combined exposures to radiation and fungal spores, animals were treated with 2 Gy whole-body X rays and then exposed to 5 × 107 fungal spores immediately or 8 weeks after the radiation treatment. Pulmonary damage was then assessed 1 and 4 weeks later (Fig. 4). After simultaneous exposure to radiation and spores, more pulmonary damage was seen at 4 weeks after treatment (7.07 ± 0.55) compared with 1 week (5.28 ± 0.95), which is consistent with the data in Fig. 3A. However, no difference in pulmonary damage was seen between the controls and 1- and 4-week assessments when the radiation and spores were separated by 8 weeks.
FIG. 4.
Septal thickness and inflammatory cell infiltration as a function of time between radiation and fungal exposures. Animals were exposed to radiation and fungal spores simultaneously or 8 weeks apart (8-week interval). Damage was then assessed at 1 and 4 weeks after the last treatment was given. For simultaneous exposures, significantly more damage was seen at 4 weeks than 1 week (#P < 0.05). Significantly less damage was seen at 4 weeks for the 8-week interval regimen compared with simultaneous exposures (‡P < 0.01).
DISCUSSION
An explosive radiological incident will release microscopic radioactive particles into the atmosphere that will be unavoidably inhaled into the lungs of the victims. This study was designed to determine if the pulmonary radiation damage will impair lung function, thereby increasing susceptibility to pulmonary infections from a microbial source. Aspergillus fumigatus spores were investigated because this is a naturally occurring fungus present in the environment and is known to produce infections after environmental disruption (2, 29, 30, 32). The C57BL/6 mouse was used as the model since previous studies had demonstrated that this strain was susceptible to Aspergillus infection (35) and that it exhibited transient changes in pulmonary architecture after acute radiation exposures (34). This study therefore reports the first murine model of combined lung injury after radiation and fungal spore exposure. The results demonstrate that low-dose irradiation prolongs fungal infection in lung and furthermore that pulmonary exposure to fungal spores can sensitize the lungs to the acute effects of injuries from low-dose radiation. Animals given a combined A. fumigatus exposure and irradiation had significantly increased pulmonary damage scores relative to control animals and mice given single treatments (Figs. 3 and 4).
The Aspergillus fumigatus inoculum and radiation dose used were selected because they produced minimal and transient pulmonary damage when given alone. Histopathological assessment of the fixed lung tissues indicated thickening of the alveoli septal wall after radiation alone; this was significantly increased and prolonged when the radiation was combined with fungal exposure (Fig. 3A). Moreover, exposure to fungal spores only was associated with an invasion of inflammatory cells, perivascular and peribronchial edema, and bronchiole cupping (Fig. 2). This type of pulmonary damage was also increased and prolonged when the combined exposures were given.
Acute pulmonary inflammation in rodents has been extensively studied. The inflammation response is dependent on the generation of complement activation and the resulting production of cytokines and chemokines (36, 37). The results reported here indicate that exposure to low-dose radiation sensitizes the lungs to the damaging effects of fungal spore inhalation through inflammatory pathways as indicated by the invasion of neutrophils, macrophages and lymphocytes (Fig. 3).
The method of inoculation in the present study was direct intranasal delivery. While this method is less sophisticated than using an inhalation chamber (38, 39), it does provide a reproducible experimental model for an initial investigation to mimic a bioterrorism exposure and induce an immediate inflammatory response. Moreover, it allows sufficient spores to be delivered to induce pulmonary effects in immune competent animals so that effects with radiation could be examined. Intranasal delivery has been used previously in survival studies (40), although in combination with agar beads, allowing lower spore burdens to be delivered. Other models of pulmonary fungal infections have also used low fungal counts (4, 40–42), but these used immuno-compromised animals to encourage fungal infections.
It is well established that combined trauma can amplify the effects of radiation exposure (17, 43). Based on the present data using Aspergillus fumigatus, a combined fungal and radiation exposure exacerbates the damage response. The biodistribution patterns of other individual fungal species vary within the continental U.S. This is primarily due to differences in environmental conditions of temperature and humidity. For example, Coccidioides immitis, the etiologic agent responsible for coccidioidomycosis and the more well-known San Joaquin Valley Fever (which has already been classified as a bioterrorism agent), is distributed in the southwestern U.S. (44), while the fungal infection histoplasmosis caused by the dimorphic fungus Histoplasma capsulatum is prevalent in the Ohio River valley (45). The small size of the infective conidia of both Histoplasma (<5 nm) and Coccidioides (3–5 nm) allow infection via the pulmonary route and retention in the deep pulmonary spaces (46–48). Future studies should investigate the health risks from a combined exposure to radiation and fungal species other than Aspergillus sp.
Aspergillus fumigatus was chosen for this study because it is an environmentally ubiquitous microbe and because aspergillosis is medically relevant. Aspergillus exposure produces high morbidity and mortality of aspergillosis despite antifungal therapy (49). Although very expensive, antifungal prophylaxis is given for patients at high risk; such individuals are usually immune compromised or are working in areas of high fungal burden where spore dispersal is considered a problem, for example, during construction. Antifungal prophylaxis has been shown to be effective in a murine model of aspergillosis (50). In the current study we observed that low-burden pulmonary fungal exposure combined with low-dose irradiation only prolonged pulmonary damage and did not lead to any aspergillosis-related mortality. This was likely due to the low levels of damage produced, although we would predict that higher initial levels of lung damage would lead to clinical signs of aspergillosis. It is likely that the same would occur for a human population exposed to an explosive radiation terrorism event. Antifungal prophylaxis would not be warranted to prevent aspergillosis from environmental fungal spores. This conclusion may not hold if higher external radiation doses are received or damage occurs from inhaled isotopes of low or high LET. Further studies are needed using different ubiquitous fungal strains, other intentionally dispersed microbes, and combinations of inorganic particles and toxins in immune compromised animals to mimic human pulmonary diseases in the general population such as COPD. The age of the recipient animals should also be investigated since this is also known to influence radiation response.
In summary, the data presented in the current study indicate the combined exposure to Aspergillus spores and low-dose radiation produces more severe and prolonged lung damage than single exposure to either insult, although the longer-term effects of this elevated level of injury have yet to be determined.
Acknowledgments
This work was supported by Center for Biophysical Assessment and Risk Management Following Irradiation (CBARMFI, Rochester, NY), the William Beaumont Hospital Research Institute and Department of Radiation Oncology, William Beaumont Hospital. We thank Eric Hernady for guidance and helpful discussions regarding lung isolation. The authors wish to thank Dr. Mitual Amin for assistance with assessing lung histopathology, Barbara Pruetz in Beaumont BioBank for histopathological technical support and Kim Powell for growing the fungal spores.
References
- 1.Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol. 2001;66:257–262. doi: 10.1002/ajh.1054. [DOI] [PubMed] [Google Scholar]
- 2.Arnow PM, Andersen RL, Mainous PD, Smith EJ. Pulmonary aspergillosis during hospital renovation. Am Rev Respir Dis. 1978;118:49–53. doi: 10.1164/arrd.1978.118.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Millner PD, Marsh PB, Snowden RB, Parr JF. Occurrence of Aspergillus fumigatus during composting of sewage sludge. Appl Environ Microbiol. 1977;34:765–772. doi: 10.1128/aem.34.6.765-772.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon DM, Polak A, Walsh TJ. Fungus dose-dependent primary pulmonary aspergillosis in immunosuppressed mice. Infect Immun. 1989;57:1452–1456. doi: 10.1128/iai.57.5.1452-1456.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JM, Tang CM, Van Noorden S, Holden DW. Virulence of Aspergillus fumigatus double mutants lacking restriction and an alkaline protease in a low-dose model of invasive pulmonary aspergillosis. Infect Immun. 1994;62:5247–5254. doi: 10.1128/iai.62.12.5247-5254.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dockery DW, Pope CA, III, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 7.Lioy PJ, Weisel CP, Millette JR, Eisenreich S, Vallero D, Offenberg J, Buckley B, Turpin B, Zhong M, Chen LC. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect. 2002;110:703–714. doi: 10.1289/ehp.02110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin S, Reibman J, Bowers JA, Hwang SA, Hoerning A, Gomez MI, Fitzgerald EF. Upper respiratory symptoms and other health effects among residents living near the World Trade Center site after September 11, 2001. Am J Epidemiol. 2005;162:499–507. doi: 10.1093/aje/kwi233. [DOI] [PubMed] [Google Scholar]
- 9.Reibman J, Lin S, Hwang SA, Gulati M, Bowers JA, Rogers L, Berger KI, Hoerning A, Gomez M, Fitzgerald EF. The World Trade Center residents’ respiratory health study: new-onset respiratory symptoms and pulmonary function. Environ Health Perspect. 2005;113:406–411. doi: 10.1289/ehp.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szema AM, Khedkar M, Maloney PF, Takach PA, Nickels MS, Patel H, Modugno F, Tso AY, Lin DH. Clinical deterioration in pediatric asthmatic patients after September 11, 2001. J Allergy Clin Immunol. 2004;113:420–426. doi: 10.1016/j.jaci.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Andersson KG, Mikkelsen T, Astrup P, Thykier-Nielsen S, Jacobsen LH, Schou-Jensen L, Hoe SC, Nielsen SP. Estimation of health hazards resulting from a radiological terrorist attack in a city. Radiat Prot Dosimetry. 2008;131:297–307. doi: 10.1093/rpd/ncn173. [DOI] [PubMed] [Google Scholar]
- 12.Coleman CN, Hrdina C, Bader JL, Norwood A, Hayhurst R, Forsha J, Yeskey K, Knebel A. Medical response to a radiologic/nuclear event: integrated plan from the Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services. Ann Emerg Med. 2009;53:213–222. doi: 10.1016/j.annemergmed.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Coleman CN, Parker GW. Radiation terrorism: what society needs from the radiobiology-radiation protection and radiation oncology communities. J Radiol Prot. 2009;29:A159–169. doi: 10.1088/0952-4746/29/2A/S11. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez AJ, Taylor Lauriston S. Lecture: Radiation protection in the aftermath of a terrorist attack involving exposure to ionizing radiation. Health Phys. 2005;89:418–446. doi: 10.1097/01.hp.0000179340.57348.26. [DOI] [PubMed] [Google Scholar]
- 15.Harper FT, Musolino SV, Wente WB. Realistic radiological dispersal device hazard boundaries and ramifications for early consequence management decisions. Health Phys. 2007;93:1–16. doi: 10.1097/01.HP.0000264935.29396.6f. [DOI] [PubMed] [Google Scholar]
- 16.Musolino SV, Harper FT. Emergency response guidance for the first 48 hours after the outdoor detonation of an explosive radiological dispersal device. Health Phys. 2006;90:377–385. doi: 10.1097/01.HP.0000196111.16261.ed. [DOI] [PubMed] [Google Scholar]
- 17.DiCarlo AL, Hatchett RJ, Kaminski JM, Ledney GD, Pellmar TC, Okunieff P, Ramakrishnan N. Medical countermeasures for radiation combined injury: radiation with burn, blast, trauma and/or sepsis. Report of an NIAID Workshop, March 26–27, 2007. Radiat Res. 2008;169:712–721. doi: 10.1667/RR1295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks JW, Evans EI, Ham WT, Jr, Reid JD. The influence of external body radiation on mortality from thermal burns. Ann Surg. 1952;136:533–545. doi: 10.1097/00000658-195209000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiang JG, Jiao W, Cary LH, Mog SR, Elliott TB, Pellmar TC, Ledney GD. Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat Res. 2010;173:319–332. doi: 10.1667/RR1892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishima S, Yukioka T, Matsuda H, Shimazaki S. Mild hypotension and body burns synergistically increase bacterial translocation in rats consistent with a “two-hit phenomenon”. J Burn Care Rehabil. 1997;18:22–26. doi: 10.1097/00004630-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Shires GT, Dineen P. Sepsis following burns, trauma, and intra-abdominal infections. Arch Intern Med. 1982;142:2012–2022. [PubMed] [Google Scholar]
- 22.Yan Y, Ran X, Wei S. Changes of immune functions after radiation, burns and combined radiation-burn injury in rats. Chin Med Sci J Chin Acad Med Sci. 1995;10:85–89. [PubMed] [Google Scholar]
- 23.Ledney GD, Elliott TB, Harding RA, Jackson WE, III, Inal CE, Landauer MR. WR-151327 increases resistance to Klebsiella pneumoniae infection in mixed-field- and gamma-photon-irradiated mice. Int J Radiat Biol. 2000;76:261–271. doi: 10.1080/095530000138916. [DOI] [PubMed] [Google Scholar]
- 24.Whitnall MH, Elliott TB, Harding RA, Inal CE, Landauer MR, Wilhelmsen CL, McKinney L, Miner VL, Jackson WER, Seed TM. Androstenediol stimulates myelopoiesis and enhances resistance to infection in gamma-irradiated mice. Int J Immunopharmacol. 2000;22:1–14. doi: 10.1016/s0192-0561(99)00059-4. [DOI] [PubMed] [Google Scholar]
- 25.Elliott TB, Brook I, Harding RA, Bouhaouala SS, Peacock SJ, Knudson GB. Bacillus anthracis infection in irradiated mice: susceptibility, protection, and therapy. Mil Med. 2002;167:103–104. [PubMed] [Google Scholar]
- 26.Shoemaker MO, Tammariello R, Crise B, Bouhaouala SS, Knudson GB, Jackson WE, III, Ludwig GV, Smith JF. Combined effects of Venezuelan equine encephalitis IIIA virus and gamma irradiation in mice. Mil Med. 2001;166:88–89. [PubMed] [Google Scholar]
- 27.IAEA reports on Tokaimura accident. International Atomic Energy Agency. Health Phys. 2000;78:231. [PubMed] [Google Scholar]
- 28.Endo A, Yamaguchi Y. Analysis of dose distribution for heavily exposed workers in the first criticality accident in Japan. Radiat Res. 2003;159:535–542. doi: 10.1667/0033-7587(2003)159[0535:aoddfh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan A, Beck C, Buckley T, Geyh A, Bova G, Merz W, Perl TM. The ability of hospital ventilation systems to filter Aspergillus and other fungi following a building implosion. Infect Control Hosp Epidemiol. 2002;23:520–524. doi: 10.1086/502100. [DOI] [PubMed] [Google Scholar]
- 30.Sepkowitz KA, Farr BM. Three, if by air. Infect Control Hosp Epidemiol. 2003;24:470–471. doi: 10.1086/502244. [DOI] [PubMed] [Google Scholar]
- 31.Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect. 2006;63:246–254. doi: 10.1016/j.jhin.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Cooper EE, O’Reilly MA, Guest DI, Dharmage SC. Influence of building construction work on Aspergillus infection in a hospital setting. Infect Control Hosp Epidemiol. 2003;24:472–476. doi: 10.1086/502239. [DOI] [PubMed] [Google Scholar]
- 33.Tomee JF, van der Werf TS. Pulmonary aspergillosis. Neth J Med. 2001;59:244–258. doi: 10.1016/s0300-2977(01)00163-2. [DOI] [PubMed] [Google Scholar]
- 34.Downing L, Sawarynski KE, Li J, McGonagle M, Sims MD, Marples B. A simple quantitative method for assessing pulmonary damage after X irradiation. Radiat Res. 2010;173:536–544. doi: 10.1667/RR1712.1. [DOI] [PubMed] [Google Scholar]
- 35.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 36.Ward PA. Acute lung injury: how the lung inflammatory response works. Eur Respir J. 2003;44:22s–23s. doi: 10.1183/09031936.03.00000703a. [DOI] [PubMed] [Google Scholar]
- 37.Lentsch AB, Ward PA. Regulation of experimental lung inflammation. Respir Physiol. 2001;128:17–22. doi: 10.1016/s0034-5687(01)00260-2. [DOI] [PubMed] [Google Scholar]
- 38.Hoselton SA, Samarasinghe AE, Seydel JM, Schuh JM. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Med Mycol. 2010;48:1056–1065. doi: 10.3109/13693786.2010.485582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tolman JA, Wiederhold NP, McConville JT, Najvar LK, Bocanegra R, Peters JI, Coalson JJ, Graybill JR, Patterson TF, Williams RO., III Inhaled voriconazole for prevention of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2009;53:2613–2615. doi: 10.1128/AAC.01657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yonezawa M, Sugiyama H, Kizawa K, Hori R, Mitsuyama J, Araki H, Shimakura M, Minami S, Watanabe Y, Yamaguchi K. A new model of pulmonary superinfection with Aspergillus fumigatus and Pseudomonas aeruginosa in mice. J Infect Chemother. 2000;6:155–161. doi: 10.1007/s101560070015. [DOI] [PubMed] [Google Scholar]
- 41.Nawada R, Amitani R, Tanaka E, Niimi A, Suzuki K, Murayama T, Kuze F. Murine model of invasive pulmonary aspergillosis following an earlier stage, noninvasive Aspergillus infection. J Clin Microbiol. 1996;34:1433–1439. doi: 10.1128/jcm.34.6.1433-1439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheppard DC, Rieg G, Chiang LY, Filler SG, Edwards JE, Jr, Ibrahim AS. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2004;48:1908–1911. doi: 10.1128/AAC.48.5.1908-1911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiang JG, Jiao W, Cary LH, Mog SR, Elliott TB, Pellmar TC, Ledney GD. Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat Res. 2010;173:319–332. doi: 10.1667/RR1892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berman RJ, Friedman L, Pappagianis D, Smith CE. Survival of Coccidioides immitis under controlled conditions of temperature and humidity. Am J Public Health Nation’s Health. 1956;46:1317–1324. doi: 10.2105/ajph.46.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furcolow ML. Airborne histoplasmosis. Bacteriol Rev. 1961;25:301–309. doi: 10.1128/br.25.3.301-309.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crum N, Lamb C, Utz G, Amundson D, Wallace M. Coccidioidomycosis outbreak among United States Navy SEALs training in a Coccidioides immitis-endemic area—Coalinga, California. J Infect Dis. 2002;186:865–868. doi: 10.1086/342409. [DOI] [PubMed] [Google Scholar]
- 47.Vanselow NA, Davey WN, Bocobo FC. Acute pulmonary histoplasmosis in laboratory workers: report of 2 cases. J Lab Clin Med. 1962;59:236–243. [PubMed] [Google Scholar]
- 48.Werner SB, Pappagianis D, Heindl I, Mickel A. An epidemic of coccidioidomycosis among archeology students in northern California. N Engl J Med. 1972;286:507–512. doi: 10.1056/NEJM197203092861003. [DOI] [PubMed] [Google Scholar]
- 49.Denning DW, Marinus A, Cohen J, Spence D, Herbrecht R, Pagano L, Kibbler C, Kcrmery V, Offner F, Sylvester R. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. EORTC Invasive Fungal Infections Cooperative Group. J Infect. 1998;37:173–180. doi: 10.1016/s0163-4453(98)80173-4. [DOI] [PubMed] [Google Scholar]
- 50.Rieg G, Spellberg B, Schwartz J, Fu Y, Edwards JE, Jr, Sheppard DC, Ibrahim AS. Antifungal prophylaxis is effective against murine invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2006;50:2895–2896. doi: 10.1128/AAC.00299-06. [DOI] [PMC free article] [PubMed] [Google Scholar]