Abstract
Objective:
Pediatric studies for new biological agents are mandated by recent legislation, necessitating careful thought to evaluation of emerging multiple sclerosis (MS) therapies in children with MS. Challenges include a small patient population, the lack of prior randomized clinical trials, and ethical concerns. The goal of this meeting was to assess areas of consensus regarding clinical trial design and outcome measures among academic experts involved in pediatric MS care and research.
Methods:
The Steering Committee of the International Pediatric MS Study Group identified key focus areas for discussion. A total of 69 meeting attendees were assembled, including 35 academic experts. Regulatory and pharmaceutical representatives also attended, and provided input, which informed academic expert consensus decisions.
Results:
The academic experts agreed that clinical trials were necessary in pediatric MS to obtain pharmacokinetic, safety and efficacy data, and regulatory approval allowing for greater medication access. The academic experts agreed that relapse was an appropriate primary outcome measure for phase III pediatric trials. An international standardized cognitive battery was identified. The pros and cons of various trial designs were discussed. Guidelines surrounding MRI studies, pharmacokinetics, pharmacodynamics, and registries were developed. The academic experts agreed that given the limited subject pool, a stepwise approach to the launch of clinical trials for the most promising medications is necessary in order to ensure study completion. Alternative approaches could result in unethical exposure of patients to trial conditions without gaining knowledge.
Conclusion:
Consensus points for conduct of clinical trials in the rare disease pediatric MS were identified amongst a panel of academic experts, informed by regulatory and industry stakeholders.
There are limited studies informing the use of disease-modifying treatments in children with multiple sclerosis (MS).1 No therapies are approved for pediatric MS by the US Food and Drug Administration (FDA), and only limited interferon use in adolescents with MS has been approved by the European Medicines Agency (EMA).1 As in adults, close to 40% of pediatric patients with MS discontinue treatment due to intolerance, toxicity, persisting relapses, or nonadherence, supporting the need for new therapeutic options in children.2 Several new molecules have shown efficacy in phase III trials and could be available in the near future for adults with MS.
Recent legislation in the United States and Europe now mandates pediatric studies for new medicinal products. In Europe, a pediatric investigation plan (PIP) must be submitted to the EMA. Similarly, the Pediatric Research Equity Act (PREA) in the United States requires pediatric studies for any new active molecule, new dosage form, or new route of administration. A full or partial waiver is possible if the treated condition does not occur in the pediatric population or if studies are not feasible or appropriate or safe for the age group. Additionally, the Best Pharmaceuticals Act for Children (BPCA) in the United States provides for voluntary pediatric drug assessments via written requests issued by the FDA, with the incentive of eligibility of an additional 6 months of market exclusivity.
The International Pediatric MS Study Group (IPMSSG) is a voluntary group of over 150 academic physicians and researchers treating or studying MS in children convened under the auspices of the MS International Federation (MSIF). Details regarding its governance and structure are available on its Web site (www.ipmssg.org). The IPMSSG recently published a consensus statement endorsed by 50 of its members summarizing its views on evaluation of new and emerging therapeutics for MS in children.1
Several challenges were identified in this initial consensus statement, which included the limited number of patients available for studies, the specifics of clinical trial design, and the implementation of planned studies. In order to address these issues, it was deemed necessary to hold a face-to-face meeting of key stakeholders including physicians treating children with MS, MS societies, and regulatory agency and pharmaceutical company representatives.
METHODS
Meeting participants.
A meeting was held on January 19–20, 2012, in Washington, DC, sponsored by the US National MS Society (NMSS) and the MSIF. There were 69 meeting attendees including 25 IPMSSG members, of which 9 were from the IPMSSG Steering Committee, as well as 10 invited guests from the academic medical community with expertise in fields including biostatistical analysis, clinical trial design, MS therapeutics, MRI analysis in MS, and neuropsychology, a member of the Children's Oncology Group (COG), and a member of the pediatric rheumatology network. The academic experts (AEs) were identified by the IPMSSG Steering Committee for expertise in the listed fields. The list was ratified by the IPMSSG Secretariat. Representatives from the EMA, FDA, and Health Canada were in attendance. Representatives from each of the pharmaceutical companies with drugs that had completed phase II clinical trials as of August 2011 attended. Staff leadership from the NMSS, MSIF, MS Society of Canada, and the Italian MS Society participated.
Meeting agenda.
Prior to the meeting, 7 workgroups of academic experts prepared summaries on the following: clinical outcome measures, clinical trial design, MRI outcome measures, cognitive outcome measures, pharmacokinetics, pharmacodynamics (safety and biological mechanisms), and long-term registries. A premeeting report was sent to participants 2 weeks before the meeting summarizing key items to be addressed and short summaries from each workgroup. On the first meeting day, IPMSSG members gave presentations reviewing the published IPMSSG therapeutics consensus statement,1 current knowledge about the epidemiology and disease course of pediatric MS, and use of first- and second-line treatments in pediatric MS. The current chair of the COG network reviewed this organization's experience. Then, members from each workgroup presented summaries. Representatives from the pharmaceutical companies and regulatory agencies were also invited to present their views. The second day was dedicated to discussion of the points raised on the first day as well as identification of consensus points for the meeting report.
Meeting report logistics.
All attendees were invited to participate in discussions. The report summarizes major areas of discussion, factual points, and areas of consensus achieved among the academic participants, henceforth termed “expert academic consensus.” The consensus points listed reflect only the opinions of the academic participants and do not include those of the regulatory agencies or pharmaceutical companies that participated in the meeting. The representatives of the EMA, FDA, and Health Canada did not participate in any way in discussions regarding the number and sequence of proposed trials and were not present during these discussions.
IPMSSG survey.
The IPMSSG Steering Committee administered a survey to its members 1 month after the meeting, which requested numbers of patients seen in the past 5 years, incidence of new patients seen in the past year, and opinions regarding points raised at the meeting. These responses are provided in Results.
RESULTS
The meeting focused on immunomodulatory therapies, since these are currently the most widely studied in MS. However, it was recognized that neuroprotective, remyelinating, or restorative therapies are forthcoming and require further discussion for consideration in the pediatric population.
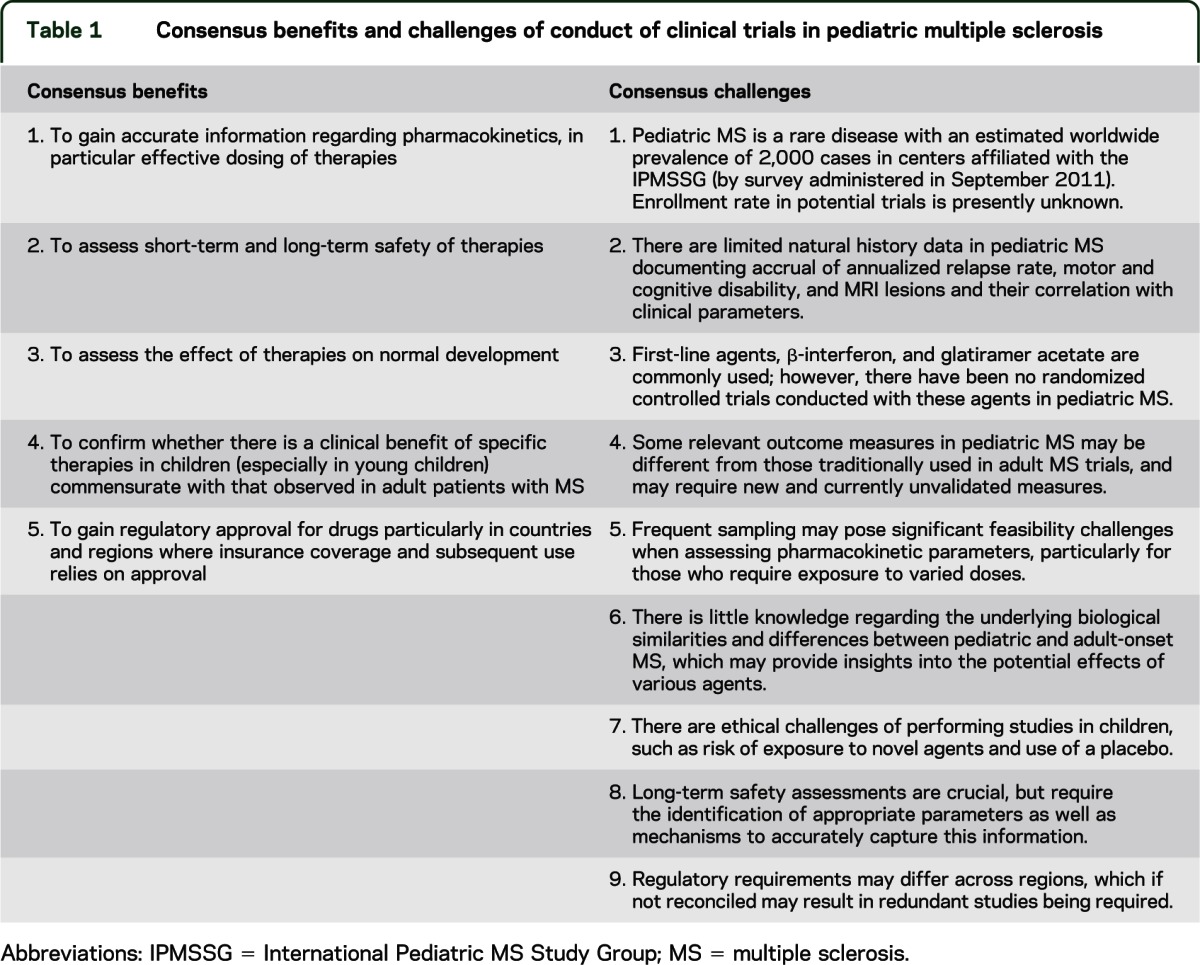
There was consensus among the expert academic meeting attendees that the conduct of clinical trials of appropriate and safe therapies was important in the pediatric MS population for reasons summarized in table 1. The group identified several major challenges in the pediatric MS study design also summarized in table 1.
Table 1.
Consensus benefits and challenges of conduct of clinical trials in pediatric multiple sclerosis
Precedents in other pediatric disorders.
COG has had a pivotal role in transforming childhood cancer from a virtually incurable disease 50 years ago to a current combined 5-year survival rate of 80%. Development of COG Network for clinical-translational trials, including a Group Operation Center, Statistics and Data Center supported by the National Cancer Institute and over 200 member institutions, allowed the development of clinical trial protocols for childhood cancers including many rare disorders. Similar models have been employed for other rare pediatric disorders including cystic fibrosis and rheumatologic conditions in children.
AE consensus agreed implementation of COG model would significantly benefit clinical trials in pediatric MS. This model would include the identification of clinical trial sites, development of a centralized institutional review board, and development of formal advisory panels to regulatory agencies and pharmaceutical companies regarding the appropriateness, feasibility, optimal design, and implementation of clinical trials in pediatric MS.
Similarities and differences between pediatric and adult MS.
A key area of debate that emerged during the meeting was the extent of similarities and differences between pediatric and adult MS. This point is central in determining to what degree pediatric MS trial design may draw on a priori methodologies and results from adult MS clinical trials.
Studies following children within the first 2–5 years of disease demonstrated a higher frequency of clinical relapses3 (annualized relapse rate [ARR] 0.9–1.4) compared to adults, as opposed to those with longer follow-up times (ARR 0.5–0.8) (table e-1 on the Neurology® Web site at www.neurology.org). Compared to adult MS, children have an increased T2 lesion burden on MRI at disease onset4,5 and frequent cognitive deficits6,7 in contrast with slower disability progression measured by the Expanded Disability Status Scale (EDSS).8–11 This information, obtained through cohort-based studies, suggests higher levels of inflammation in children. The AEs agreed that there was limited information regarding the underlying immunopathobiology of pediatric MS and the effects of the developing immune, nervous, and endocrine systems, which may lead to differentially expressed disease outcomes.
The AEs agreed that potential differences between adult and pediatric MS suggest that results obtained in adults cannot be directly extrapolated to children, and reinforce the need for controlled trials in children ensuring that the sample size for the relevant age population is large enough to obtain robust data.
Incidence and prevalence of pediatric MS.
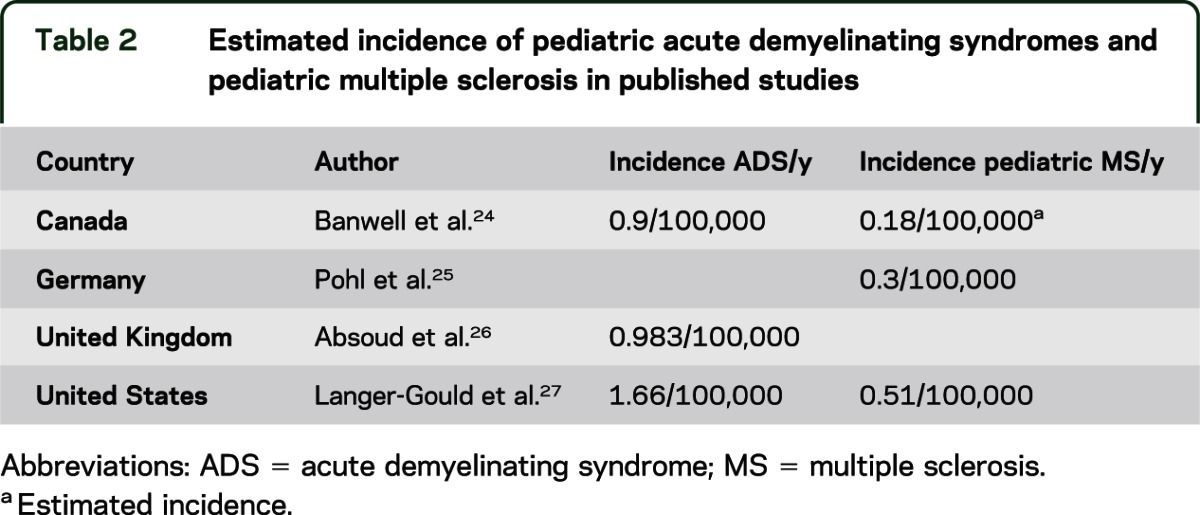
One of the major challenges in clinical trial planning is the low frequency of pediatric MS. The reported prevalence of pediatric MS differs among countries, with relatively frequent cases in Northern Europe, the United States, and Canada, and fewer cases reported from India, China, Japan, and Africa. Table 2 lists incidence studies of pediatric acute demyelinating syndrome (ADS) and MS. Results of a survey administered by the IPMSSG in September 2011 were presented. Among 50 responding clinicians, approximately 1,300 prevalent cases of pediatric MS were tabulated. It was estimated that there are likely at least 2,000 cases among IPMSSG members. Accurate assessments of the incidence and prevalence are required using standardized disease definitions.12
Table 2.
Estimated incidence of pediatric acute demyelinating syndromes and pediatric multiple sclerosis in published studies
Patient populations.
Current studies on relapse rates suggest higher rates early in the disease course; therefore, it may be important to restrict studies to patients within the first few years of disease in order to increase power. Twenty percent to 30% of patients with pediatric MS have onset before age 10. There was consensus among all except one AE attendee that children under the age of 10 should be included in therapeutic studies, particularly in pharmacokinetic (PK) and safety studies, since safe and effective dosing in young children is the most challenging to determine. Particular care should be taken in correctly diagnosing MS in young children, who may present differently compared to adolescents.13,14 Regulators expressed that in their view and given the change in the diagnostic criteria for adult MS,15 studies in clinically isolated syndromes (CIS) in adults were not necessary since these 2 entities overlapped significantly. However, the AEs expressed that these criteria have yet to be comprehensively validated in children and that consensus regarding the identification of children with CIS likely to have or develop MS is required, since many do not develop MS.
Clinical trial outcome measures.
Primary and secondary outcome measures have never been prospectively evaluated in therapeutic MS trials in children. Traditionally, relapse rate has been used as an outcome measure in adult MS studies. There was AE consensus that time to next relapse or annualized relapse rate should be used as a primary outcome measure in phase III trials. Time to next relapse after an initial attack of MS has been evaluated in a single report at a median of 1.1 year with 25% of the patients having a first relapse before 6 months.16 Time to further relapses is not established. EDSS, frequently used for secondary endpoints in adult studies, is typically low in children with MS,8 and is unlikely to change significantly in short-term trials. Novel scales and measures such as the 6-minute walk time are used in other diseases (i.e., neuromuscular disorders) but have to be validated in pediatric MS; thus no data allow current power calculations, and as such, these other outcome measures may not be considered as primary endpoints at present.
MRI outcome measures.
Published MRI studies in pediatric MS have largely focused on defining the MRI features predictive of MS in children with CIS/ADS, or have analyzed associations with clinical outcomes.9,17
Two main issues were discussed:
1. The desirability of obtaining acceptance of MRI as a primary outcome. The AEs agreed that lesion accrual measured by MRI is an important outcome measure for clinical trials in children with MS. However, at present, regulatory opinion did not support MRI as a primary outcome measure in phase III studies in pediatric MS. Correlations between MRI features and longitudinal clinical outcomes may resolve this issue. Relationships between MRI measures and cognition have been demonstrated.18,19 Since accrual of physical disability is rarely observed in the pediatric age group, correlations between EDSS and MRI features may not be robust. Ongoing studies will clarify whether accrual of lesions in pediatric MS is an acceptable surrogate for clinical relapses in clinical trials. Using the number of cumulative new active lesions (CAL) in adult MS and assuming a similar behavior in pediatric MS, sample size for a phase II trial, which includes CAL at 4, 5, and 6 months as an endpoint, would require 50 patients in each group against placebo and 600 against active comparator. Consideration should be given to MRI as the primary endpoint in pediatric MS phase II trials and as a secondary endpoint in phase III trials, although further data are required on the rates of lesion accrual.
2. The best specific analysis suited for multicentric international trials. There was AE consensus that MRI analyses suited for clinical trials should rely on new lesion counts, rather than lesion volumes, to determine short-term disease activity. This is in part based on the occurrence of vanishing T2-bright lesions in very young patients.13 Core metrics should include new T2 counts at a minimum and gadolinium-enhancing lesion counts if possible. All clinical trials in pediatric MS should employ high-quality standardized MRI protocols and centralized analyses. The ability to use sedation for children requiring it was discussed, although strategies to avoid sedation have been developed in several centers and may be considered for trials.
Advanced MRI metrics, including whole brain or regional brain region volumes, magnetization transfer ratio, diffusion tensor imaging, and cortical lesions,5,18,20 were considered best suited as secondary or tertiary outcomes.
Cognitive outcomes.
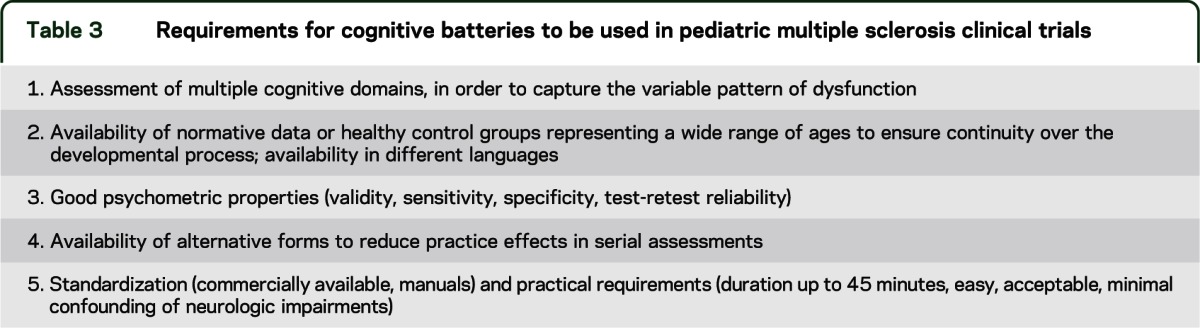
The consequences of MS in a developing brain and early cognitive dysfunction are major concerns that can be evaluated only in children and cannot be extrapolated from studies in adults.6,7,21,22 Cognitive function should be addressed in any therapeutic trial and requires the development of a core battery of neuropsychological tests applicable to international cross-sectional and longitudinal studies in pediatric MS. Challenges include differences in developmental trajectories and patterns of cognitive dysfunction in relation to patient age. Current research is limited by variable tools used by different research groups, preventing pooling of data across studies, and limited availability of validated assessment tools with satisfactory norms.6,21–23 Requirements for cognitive batteries to be used in pediatric MS clinical trials are listed in table 3.
Table 3.
Requirements for cognitive batteries to be used in pediatric multiple sclerosis clinical trials
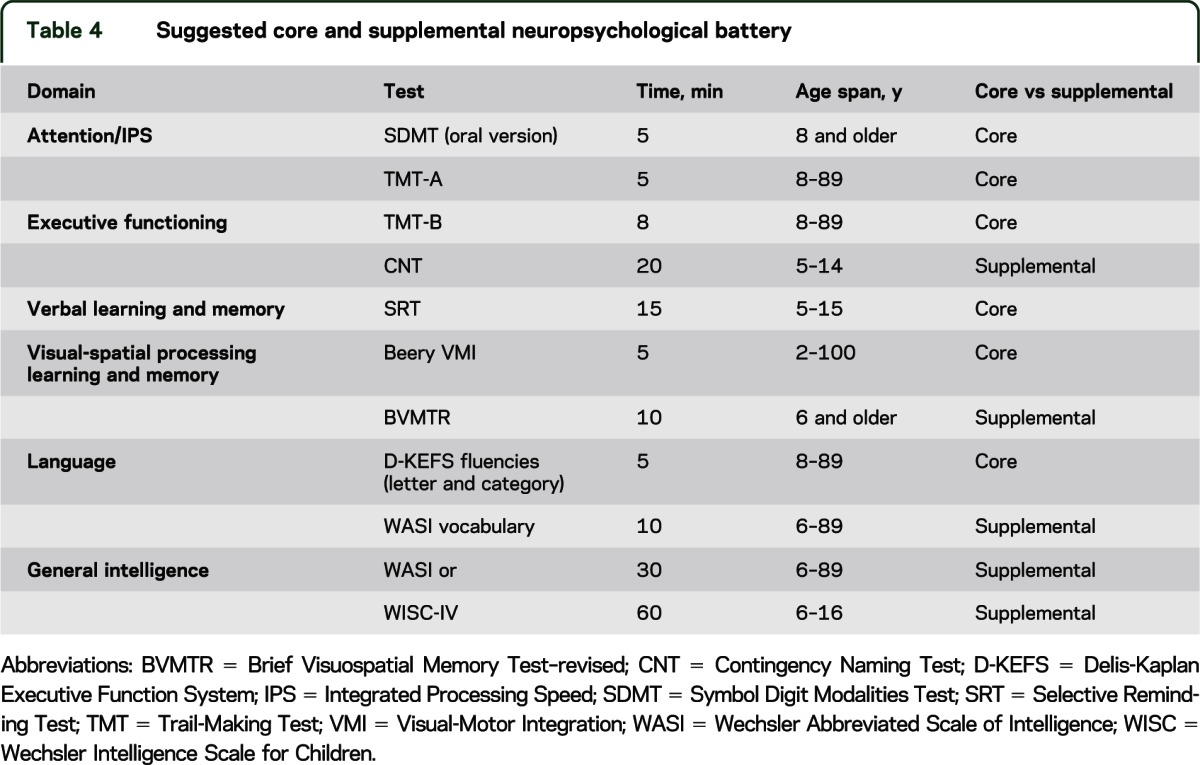
The AE-recommended core battery of 45 minutes duration and supplemental battery are detailed in table 4 with 1-year retest intervals on the basis of research in adults. Measures of fatigue, depression, and quality of life were also recommended in addition to cognitive assessment, although current information on the characteristics of different available assessment tools is insufficient to allow any specific recommendations.
Table 4.
Suggested core and supplemental neuropsychological battery
Clinical trial design.
AEs agreed that trials with a primary clinical endpoint, such as time to a new event or ARR, would translate more easily to clinical practice than phase II trials with MRI endpoints. MRI endpoints should, nevertheless, be included in pediatric MS trials as detailed in the MRI section. Clinical trials may take place in a phased approach with initial PK and dose-finding studies potentially combined in phase II studies and followed by definitive phase III studies.
Whether randomized controlled trials should use placebo or an active comparator was debated, since both strategies have advantages and challenges, summarized in table 5. In theory, placebo-controlled trials are ideal in the current environment as there are no approved treatments for pediatric MS, and placebo-controlled trials typically allow for smaller sample sizes than superiority trials (table e-2). However, this position may be difficult to accept because in some countries first-line disease-modifying therapies are commonly used off-label in pediatric MS.
Table 5.
Pros and cons of various clinical trial designs for pediatric multiple sclerosis
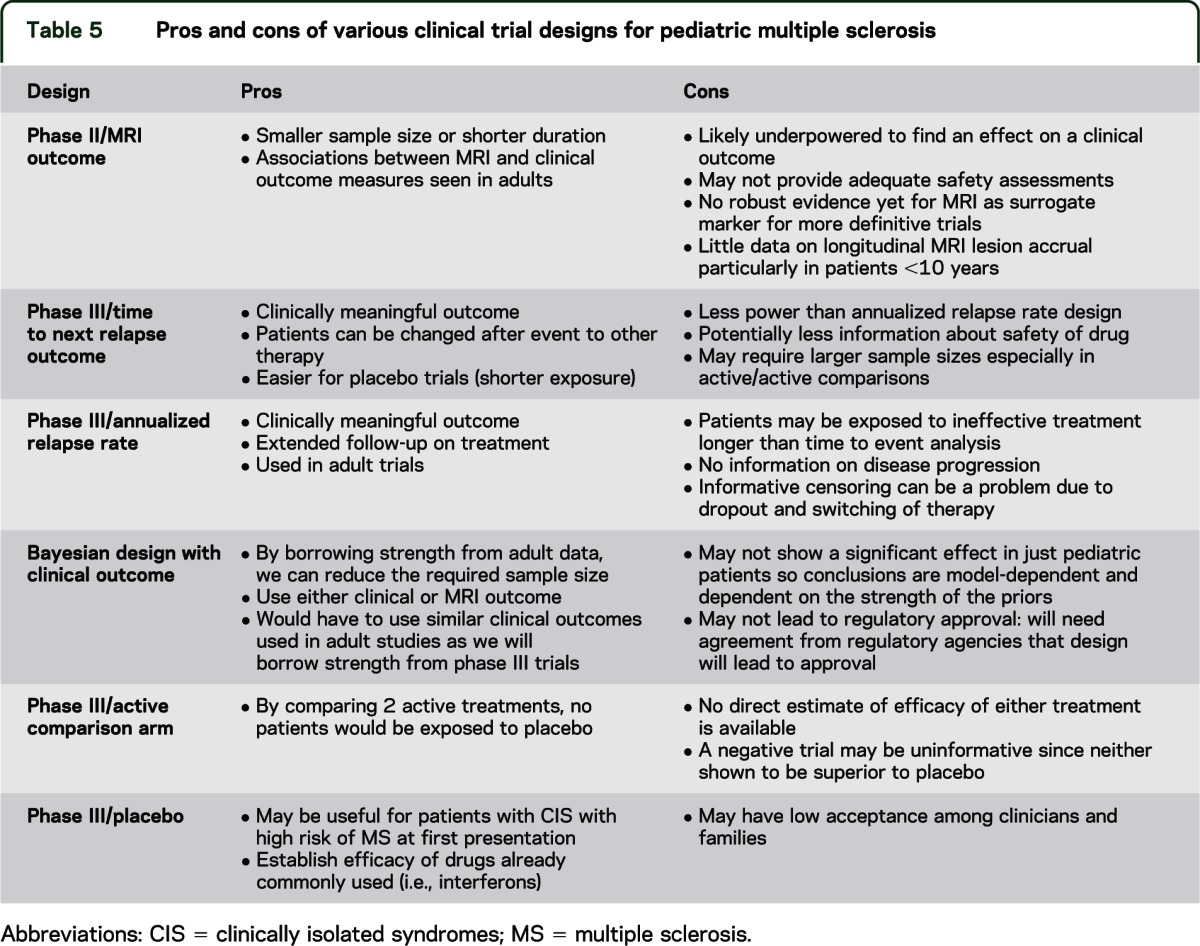
The main problems with superiority trials include the need for large sample sizes and the issue of choosing an active comparator as none is approved for pediatric MS. Moreover, comparison to “active comparator,” which itself has not been shown to be superior to placebo, may be uninformative. Use of Bayesian design incorporating a priori data from adult MS trials should lower the sample size required for trials, but would require the use of the same primary outcome measures and acceptance by regulatory agencies.
AE consensus surrounding clinical trial design was that additional data regarding available patient numbers, acceptance of placebo-controlled trials by treating neurologists and families, and detailed phase III data from adult studies of potential agents to be evaluated are required to advise on the specifics of studies. Finally, AE consensus was that a long-term registry was required to provide relevant data on long-term safety.
Pharmacokinetics.
To date, PK/pharmacodynamic studies have not been performed for any agent used in pediatric MS, and dosing recommendations are based purely on expert consensus. The workgroup discussed age-specific dosing requirements of medication, noting increased clearance of some drugs between 3 and 6 years of age and puberty. Lack of information regarding developmental profiles for hepatic and extrahepatic drug metabolizing enzymes may be an issue.
Feasibility regarding patient accrual and ethical concerns about exposures to unknown risks in children were discussed. Consequently, there was AE consensus about the need for predictive modeling, including 1) in silico techniques that provide simulations, or computer modeling of physically based PK/pharmacodynamic models, and 2) studies in juvenile animals to evaluate for safety. These methods may reduce the number of subjects required for PK studies.
Pharmacodynamics.
The group agreed that all tolerability and safety issues identified in adult MS clinical trials and the ongoing postmarketing experience are relevant to pediatric MS. Additionally, there are particular concerns in pediatric populations with respect to immune maturity, immune repertoire, vaccine efficacy and safety, primary infection acquisition, and endocrine, musculoskeletal, and neurologic development as known side effects of drugs may be more pronounced or different in children. The group surmised that we should also learn from the experiences of other disciplines using immune-directed therapies in children (e.g., pediatric rheumatology, pediatric oncology).
Long-term drug safety registry.
The AE consensus was that a single long-term drug safety registry focused on serious adverse events, development, and fertility should be used by all countries for all children with MS who have received or are receiving treatments in the context of clinical trials or off-label use, since children may be enrolled in more than one study during their childhood years and, most certainly, during their lifetime. A single registry is simpler, increases sample size, and ensures standardization of data collection.
The registry can be either physician or patient (and parent) driven. Physician-driven registries can gather demographic and clinical information. Patient-driven databases can provide valuable information regarding quality of life as well as functional, emotional, and cognitive outcome. Data should be captured in a simple, user-friendly format.
An effective governance structure is important for maintaining a registry. Governance ensures the integrity of data collected, data analysis and quality control, and dissemination especially of serious adverse events.10
Timing and implementation of clinical trials.
At the time of the meeting, there were 8 approved PIPs posted on the EMA Web site. In addition, pediatric assessments under the PREA and written requests may be submitted under the BPCA act to the FDA. The AEs agreed that given the number of patients known to the IPMSSG who may be available for clinical trials, effective completion of only 1–2 studies with sample sizes of between 250 and 500 patients, each within a 2- to 3-year time period, is feasible. Such restrictions result in the need to prioritize trials for the most promising and safe therapies to evaluate. Development of methods to reduce sample sizes or trial duration, and still gain critical information to inform clinical practice and achieve regulatory approval, is crucial. Shorter, placebo-controlled trials may be acceptable to clinicians and families, provided that use of a placebo arm is coupled with stringent escape criteria such as offering standard of care treatment in the event of new disease activity. Use of MRI outcome measures may reduce the sample size required, since accrual of MRI lesions occurs more frequently than clinical relapses. Statistical modeling, including the use of Bayesian statistics to incorporate a priori data from adult trials or the identification and validation of more sensitive clinically relevant outcome measures, may reduce sample size. Trial designs should be explored in association with pharmaceutical companies and will have to satisfy regulatory requirements.
The IPMSSG endorses a collaborative effort for pediatric MS clinical trials. Dilution of effort through an “open market” approach will lead to insufficient enrollment in multiple concurrent clinical trials and failure to bring appropriate therapies to the pediatric population in a timely fashion. This would raise ethical concerns as it could unnecessarily expose patients to trial conditions and possible side effects without the benefit of gained knowledge. An alternate approach endorsed by the AEs at the meeting was the prioritization of the most promising agents for pediatric MS and a timely stepwise approach to initiating and completing these clinical trials. The elected Steering Committee of the IPMSSG expressed a strong interest in continuing to provide input on these issues through the incorporation of opinion from the larger IPMSSG membership, AEs, and patient groups. In addition, they expressed interest in continuing to provide information on demographic and clinical features of pediatric MS and study feasibility to the regulatory agencies and pharmaceutical companies and in working closely with industry to identify clinical trial sites, recruit patients for appropriate studies, and advise on clinical trial design (table 6).
Table 6.
Potential roles of an international pediatric multiple sclerosis clinical trials network

Next steps.
The Steering Committee of the IPMSSG laid out what it considers the next steps in the process of initiating appropriate clinical trials in a timely fashion: 1) ascertainment of clinicians and potential clinical trial sites; 2) informed evaluation of available therapies for study; 3) detailed discussions with expert panels regarding tailored clinical trial designs; and 4) continued interaction with regulators and pharmaceutical industry on the above points.
DISCUSSION
Clinical trials in pediatric MS are an opportunity to gain well-defined PK, dosing, and efficacy data for appropriate therapies in this population and to allow children to benefit from the many advances in the MS field. The logistics of study design and clinical trial implementation require thoughtful and cooperative approaches from clinical, academic, and industry stakeholders. This meeting represents a first step in that process.
Supplementary Material
GLOSSARY
- ADS
acute demyelinating syndrome
- AE
academic expert
- ARR
annualized relapse rate
- BPCA
Best Pharmaceuticals Act for Children
- CAL
cumulative active lesion
- CIS
clinically isolated syndromes
- EDSS
Expanded Disability Status Scale
- EMA
European Medicines Agency
- FDA
US Food and Drug Administration
- IPMSSG
International Pediatric MS Study Group
- MS
multiple sclerosis
- MSIF
MS International Federation
- NMSS
National MS Society
- PIP
pediatric investigation plan
- PK
pharmacokinetic
- PREA
Pediatric Research Equity Act
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
T. Chitnis and Marc Tardieu contributed to design and drafting for intellectual content. Maria Pia Amato, Brenda Banwell, Amit Bar-Or, and Angelo Ghezzi contributed to design and drafting for intellectual content. Andrew Kornberg, Lauren Krupp, Daniela Pohl, Kevin Rostasy, and Silvia Tenembaum contributed to design and drafting for intellectual content. Emmanuelle Waubant and Evangeline Wassmer contributed to design and drafting for intellectual content.
STUDY FUNDING
The meeting was sponsored by the US National MS Society and the MS International Federation.
DISCLOSURE
T. Chitnis has acted as an advisor/consultant/advisory board member or speaker for Biogen-Idec, Merck-Serono, Novartis, Sanofi-Aventis, and Teva. She has received research support from Merck-Serono. M. Tardieu has acted as an advisor/consultant/advisory board member or speaker for Biogen Idec, Genzyme, and Novartis. M.P. Amato has acted as an advisor/consultant/advisory board member or speaker for Biogen Idec, Bayer Schering Pharma, Merck Serono, and Sanofi-Aventis. She has received research support from Biogen Idec, Bayer Schering Pharma, Merck Serono, Teva, and Sanofi-Aventis. B. Banwell has acted as an advisor/consultant/advisory board member or speaker for Biogen Idec, Merck Serono, Novartis, and Teva. A. Bar-Or has acted as an advisor/consultant/advisory board member or speaker for Amplimmune, Aventis, Bayhill Therapeutics, Biogen Idec, Berlex/Bayer, Diogenix, Eli Lilly, Genentech, Glycominds, GlaxoSmithKline, GJGGF, EMD Serono, Novartis, Ono Pharma, Sanofi-Aventis, Roche, Teva, and Wyeth. He has received research support from Ammplimmune, EMD Serono, Novartis, Roche, and Teva. A. Ghezzi acted as an advisor/consultant/advisory board member or speaker for Actelion, Allergan, Bayer-Schering, Biogen-Dompè, Merck-Serono, Novartis, Sanofi-Aventis, and Teva. A. Kornberg has acted as an advisor/consultant/advisory board member or speaker for Biogen Idec. L. Krupp acted as an advisor/consultant/advisory board member or speaker for Acorda, Axon Advisors Genentech, Bayer, Biogen Idec, EMD-Serono, Novartis, Pfizer, Sanofi-Aventis, and Teva. D. Pohl has acted as an advisor/consultant/advisory board member or speaker for or received honoraria from Bayer-Schering, Biogen-Idec, Merck-Serono, and Teva. K. Rostasy reports no disclosures. S. Tenembaum has acted as an advisor/consultant/advisory board member or speaker for Bayer Schering Pharma, Biogen Idec, Genzyme, Merck Serono, and Teva. E. Waubant has acted as an advisor/consultant/advisory board member or speaker for Actelion, Roche, Sanofi-Aventis, and Teva. She has received research support from Biogen Idec, Roche, and Sanofi-Aventis. E. Wassmer has acted as an advisor/consultant/advisory board member or speaker for Bayer, Biogen Idec, Genzyme, Merck Serono, Novartis, Shire, and UCB Pharmaceutical. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Chitnis T, Tenembaum S, Banwell B, et al. Consensus statement: evaluation of new and existing therapeutics for pediatric multiple sclerosis. Mult Scler 2012;18:116–127 [DOI] [PubMed] [Google Scholar]
- 2.Yeh EA, Waubant E, Krupp LB, et al. Multiple sclerosis therapies in pediatric patients with refractory multiple sclerosis. Arch Neurol 2011;68:437–444 [DOI] [PubMed] [Google Scholar]
- 3.Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol 2009;66:54–59 [DOI] [PubMed] [Google Scholar]
- 4.Waubant E, Chabas D, Okuda DT, et al. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Arch Neurol 2009;66:967–971 [DOI] [PubMed] [Google Scholar]
- 5.Yeh EA, Weinstock-Guttman B, Ramanathan M, et al. Magnetic resonance imaging characteristics of children and adults with paediatric-onset multiple sclerosis. Brain 2009;132:3392–3400 [DOI] [PubMed] [Google Scholar]
- 6.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features of childhood and juvenile MS. Neurology 2008;70:1891–1897 [DOI] [PubMed] [Google Scholar]
- 7.MacAllister WS, Belman AL, Milazzo M, et al. Cognitive functioning in children and adolescents with multiple sclerosis. Neurology 2005;64:1422–1425 [DOI] [PubMed] [Google Scholar]
- 8.Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med 2007;356:2603–2613 [DOI] [PubMed] [Google Scholar]
- 9.Simone IL, Carrara D, Tortorella C, et al. Course and prognosis in early-onset MS: comparison with adult-onset forms. Neurology 2002;59:1922–1928 [DOI] [PubMed] [Google Scholar]
- 10.Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D. Early onset multiple sclerosis: a longitudinal study. Neurology 2002;59:1006–1010 [DOI] [PubMed] [Google Scholar]
- 11.Trojano M, Paolicelli D, Bellacosa A, Fuiani A, Cataldi S, Di Monte E. Atypical forms of multiple sclerosis or different phases of a same disease? Neurol Sci 2004;25(suppl 4):S323–S325 [DOI] [PubMed] [Google Scholar]
- 12.Krupp LB, Banwell B, Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007;68:S7–S12 [DOI] [PubMed] [Google Scholar]
- 13.Chabas D, Castillo-Trivino T, Mowry EM, Strober JB, Glenn OA, Waubant E. Vanishing MS T2-bright lesions before puberty: a distinct MRI phenotype? Neurology 2008;71:1090–1093 [DOI] [PubMed] [Google Scholar]
- 14.Chabas D, Ness J, Belman A, et al. Younger children with MS have a distinct CSF inflammatory profile at disease onset. Neurology 2010;74:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikaeloff Y, Caridade G, Tardieu M, Suissa S. Effectiveness of early beta interferon on the first attack after confirmed multiple sclerosis: a comparative cohort study. Eur J Paediatr Neurol 2008;12:205–209 [DOI] [PubMed] [Google Scholar]
- 17.Verhey LH, Branson HM, Shroff MM, et al. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: a prospective national cohort study. Lancet Neurol 2011;10:1065–1073 [DOI] [PubMed] [Google Scholar]
- 18.Till C, Ghassemi R, Aubert-Broche B, et al. MRI correlates of cognitive impairment in childhood-onset multiple sclerosis. Neuropsychology 2011;25:319–332 [DOI] [PubMed] [Google Scholar]
- 19.Tortorella P, Rocca MA, Mezzapesa DM, et al. MRI quantification of gray and white matter damage in patients with early-onset multiple sclerosis. J Neurol 2006;253:903–907 [DOI] [PubMed] [Google Scholar]
- 20.Absinta M, Rocca MA, Moiola L, et al. Cortical lesions in children with multiple sclerosis. Neurology 2011;76:910–913 [DOI] [PubMed] [Google Scholar]
- 21.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features in childhood and juvenile MS: two-year follow-up. Neurology 2010;75:1134–1140 [DOI] [PubMed] [Google Scholar]
- 22.MacAllister WS, Christodoulou C, Milazzo M, Krupp LB. Longitudinal neuropsychological assessment in pediatric multiple sclerosis. Dev Neuropsychol 2007;32:625–644 [DOI] [PubMed] [Google Scholar]
- 23.MacAllister WS, Boyd JR, Holland NJ, Milazzo MC, Krupp LB. The psychosocial consequences of pediatric multiple sclerosis. Neurology 2007;68:S66–S69 [DOI] [PubMed] [Google Scholar]
- 24.Banwell B, Kennedy J, Sadovnick D, et al. Incidence of acquired demyelination of the CNS in Canadian children. Neurology 2009;72:232–239 [DOI] [PubMed] [Google Scholar]
- 25.Pohl D, Hennemuth I, von Kries R, Hanefeld F. Paediatric multiple sclerosis and acute disseminated encephalomyelitis in Germany: results of a nationwide survey. Eur J Pediatr 2007;166:405–412 [DOI] [PubMed] [Google Scholar]
- 26.Absoud M, Lim MJ, Chong WK, et al. Paediatric acquired demyelinating syndromes: incidence, clinical and magnetic resonance imaging features. Mult Scler 2013;19:76–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langer-Gould A, Zhang JL, Chung J, Yeung Y, Waubant E, Yao J. Incidence of acquired CNS demyelinating syndromes in a multiethnic cohort of children. Neurology 2011;77:1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


