Abstract
Objective:
To explore the potential contribution of genetic variation in voltage-gated chloride channels to epilepsy, we analyzed CLCN family (CLCN1-7) gene variant profiles in individuals with complex idiopathic epilepsy syndromes and determined the expression of these channels in human and murine brain.
Methods:
We used parallel exomic sequencing of 237 ion channel subunit genes to screen individuals with a clinical diagnosis of idiopathic epilepsy and evaluate the distribution of missense variants in CLCN genes in cases and controls. We examined regional expression of CLCN1 in human and mouse brain using reverse transcriptase PCR, in situ hybridization, and Western immunoblotting.
Results:
We found that in 152 individuals with sporadic epilepsy of unknown origin, 96.7% had at least one missense variant in the CLCN genes compared with 28.2% of 139 controls. Nonsynonymous single nucleotide polymorphisms in the “skeletal” chloride channel gene CLCN1 and in CLCN2, a putative human epilepsy gene, were detected in threefold excess in cases relative to controls. Among these, we report a novel de novo CLCN1 truncation mutation in a patient with pharmacoresistant generalized seizures and a dystonic writer's cramp without evidence of variants in other channel genes linked to epilepsy. Molecular localization revealed the unexpectedly widespread presence of CLCN1 mRNA transcripts and the ClC-1 subunit protein in human and murine brain, previously believed absent in neurons.
Conclusions:
Our findings support a possible comorbid contribution of the “skeletal” chloride channel ClC-1 to the regulation of brain excitability and the need for further elucidation of the roles of CLCN genes in neuronal network excitability disorders.
CLCN1 was the first voltage-gated ion channel gene shown to cause muscle excitability disease, and ClC-1 chloride channel mutations remain the most frequent cause of inherited dominant (Thomsen) and recessive (Becker) nondystrophic myotonia.1–3 This disease link, coupled with robust expression in skeletal myocytes4 and lack thereof in brain tissue,5 resulted in ClC-1 being coined “the skeletal muscle chloride channel.” Voltage-dependent chloride currents resembling heterologously expressed ClC-1 and ClC-2 have been described in neurons,6,7 but their molecular composition and contribution to network excitability remain unclear. Although CLCN2 mutations have been reported in patients with epilepsy,8–10 the evidence for a functional role has been challenged,11 and seizures were not detected in Clcn2-deficient mice.12 Interestingly, Clcn3 colocalizes with the GABA vesicular transporter VGAT in hippocampal pyramidal neurons where it regulates loading of GABAergic synaptic vesicles.13 Deletion of Clcn3 in mice reduces the quantal size of inhibitory neurotransmission, causes seizures, and produces a pattern of early hippocampal cell loss similar to that of temporal lobe epilepsy.14,15
We hypothesized that if CLCN1 is expressed in human brain, then loss-of-function mutations that reduce chloride conductance and cause hyperexcitability in skeletal muscle could contribute to enhanced network excitability and increased susceptibility to seizures or other neurologic phenotypes. Using a combination of molecular genetic, biochemical, anatomical, and neurophysiologic techniques, we determined that CLCN1 is a likely candidate gene in a proband with idiopathic epilepsy (IE) and mild myotonic motor features, a novel neurologic phenotype that supports a functional role of CLCN chloride channels in epilepsy.
METHODS
Standard protocol approvals, registrations, and patient consents.
The Baylor College of Medicine (BCM) Institutional Review Board approved this study. Written informed consent for this research was obtained from all study participants from BCM and Meyer University Hospital of Florence.
Study population structure.
As described previously,16 we evaluated self-reported white Caucasian and white Hispanic adults with clinically confirmed generalized or localization-related epilepsy of unknown origin and the absence of identifiable familial, laboratory, or imaging risk factors for seizures, along with neurologically unaffected control individuals of equivalent ages examined at BCM affiliated hospitals. See e-Methods on the Neurology® Web site at www.neurology.org for inclusion criteria, table e-1 for cohort details, and table e-2 for additional information on diagnosis and clinical phenotype.
Channotype analysis of exomic sequencing data.
Using our Sanger exomic sequencing data,16 we performed channotype analysis and compiled personal structural variant profiles for all individuals in the sporadic IE and neurologically unaffected control cohorts. Genetic profiles were linked to a clinical phenotype database for genotype–phenotype studies.
Pedigree analysis.
Parental genomic DNA was extracted from blood (DNeasy Blood Kit, Qiagen, Hilden, Germany). PCR amplification of the mutation-containing exon 23 of the CLCN1 gene (NM_000083) was performed using primers targeting intron 22/23 and 3′ UTR yielding a 480-bp amplicon (table e-3). The 30 cycle PCR protocol had a 1-minute 60°C annealing step and 1-minute extension time. PCR products were size-resolved on a 2% agarose/Ethidium bromide gel, and gel extracted (Gel Extraction Kit, Qiagen). PCR products were sequenced in both directions (Genewiz, South Plainfield, NJ).
Human and mouse brain tissue.
De-identified human frozen postmortem brain tissue samples were obtained from the Department of Pathology (BCM) from previously healthy individuals who died accidently. Mice were killed by cervical dislocation, and whole brain, skeletal muscle, and heart were promptly removed under sterile, RNase-free conditions, dissected, and flash frozen.
Reverse transcriptase PCR to detect CLCN transcripts.
Tissues were homogenized with a Tissue Tearor. RNA was extracted using Trizol (Life Technologies, Philadelphia, PA) following the manufacturer's standard protocol. cDNA template was created using Phusion reverse transcriptase PCR (Finnzyme, New England Biosystems, Waltham, MA) according to the supplied protocol. PCR amplification of transcripts was performed (Hotstar, Qiagen) using primers spanning exon/exon boundaries (table e-3) with 35 (mouse) or 40 (human) cycles of a 1-minute 58°C annealing step and 1-minute second extension time amplification, respectively. PCR products were size-resolved and bands were excised and purified (Qiaquick Gel Extraction, Qiagen) for sequence confirmation (Genewiz).
In situ hybridization of brain Clcn1 mRNA.
An in situ hybridization (ISH) probe template targeting mouse Clcn1 exon 23 was produced by 2 rounds of PCR amplification (table e-3). Initial PCR templates were generated by PCR (Hotstar, Qiagen) of mouse brain cDNA (35 cycles: 1-minute 56°C annealing step and a 30-second extension time). The single amplicon was excised and purified (Qiagen Qiaquick Gel Extraction) for sequence confirmation and used in a 100-µL large-scale PCR reaction to produce >1 µg probe template. In vitro transcription reactions were performed on purified templates (QiaQuick PCR Purification, Qiagen) using the 10× DIG RNA Labeling Mix (Roche, Branchburg, NJ) to generate the antisense detection probe (SP6 promoter) or control sense probe (T7 promoter) ISH was performed by the IDDRC RNA In Situ Hybridization Core.17 Fresh-frozen wild-type mouse brains were sagittally sectioned at 25 µm, and incubated with labeled riboprobe for 5.5 hours at 63.5°C followed by a series of desalinizing washes. Colorimetric detection of probe binding used horseradish peroxidase (HRP)–conjugated anti-digoxigenin antibody followed by treatment with a neutravidin-alkaline phosphatase and nitroblue tetrazolum resulting in a blue precipitate. Slides were washed, postfixed with 4% paraformaldehyde, and coverslipped. Sections were scanned at 10× magnification in a series of overlapping tiles per section, then compiled into a single image. An automated detection algorithm18 colored cells with Clcn1 transcripts based on relative expression levels, while cells with no detectable precipitate remain uncolored.
Western blot detection of ClC-1 protein.
Tissues were homogenized (Tissue Tearor) in ice-cold RIPA buffer containing protease/phosphatase inhibitors (Santa Cruz Biotechnology, Santa Cruz, CA). Total lysate protein concentrations were determined (Protein Assay, Bio-Rad, Hercules, CA). Forty or 200 micrograms of each sample was separated on 8% Tris–HEPES sodium dodecyl sulfate polyacrylamide gel electrophoresis gels, analyzed by Western blot using rabbit polyclonal anti-ClC-1 antibody targeting the N-terminus (1:200 in vehicle, Millipore, Billerica, MA) and HRP-tagged goat antirabbit F(ab’)2 secondary antibody (1:500 dilution in vehicle, Molecular Probes, Eugene, OR), and detected with a chemiluminescent substrate (SuperSignal, Pierce Chemical, Rockford, IL).
RESULTS
CLCN gene variant enrichment in epilepsy.
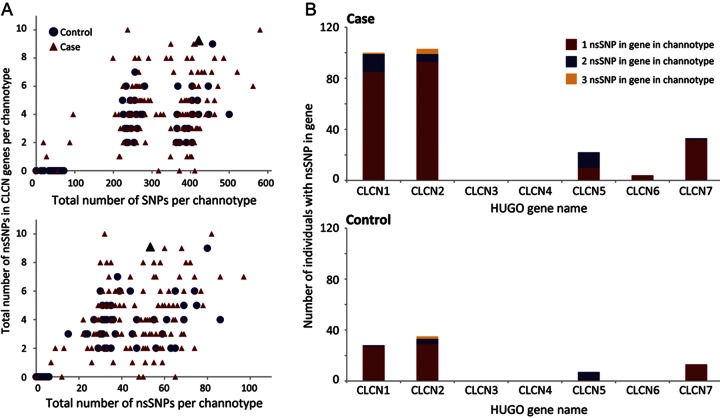
We newly evaluated the personal ion channel variant profiles in 152 individuals with sporadic IE and 139 neurologically asymptomatic controls.16 Among the 237 ion channel genes sequenced, we detected a relative enrichment in known and novel nonsynonymous single nucleotide polymorphisms (nsSNPs) in the voltage-gated chloride channel gene family (CLCN1-7). When examined cumulatively, 96.7% of individuals with IE had at least one missense variant in the CLCN genes compared with 28.2% of controls. The total number of CLCN missense variants observed in a personal profile (“channotype”) was independent of the number of nsSNPs identified in the individual regardless of disease status (figure 1A). While not detected in CLCN3 (NM_001243372) or CLCN4 (NM_001830), we identified missense variants in all other CLCN genes in individuals with IE (figure 1B). Notably, 3 times as many cases had nsSNPs in CLCN1 (NM_000083) and CLCN2 (NM_004366) vs controls. Of these nsSNPs, only 3 in CLCN1 (rs10282312, rs41276054, rs13438232) and 2 in CLCN2 (rs9820367, rs2228292) were previously reported in the database of single nucleotide polymorphisms (dbSNP). Interestingly, with the exception of a single CLCN2 variant (R644C) identified in a single control individual, the novel variants in these known channelopathy genes were found only in those with IE (9/9 CLCN1; 9/10 CLCN2). This trend was also observed in CLCN5-7 (accessions NM_000084, NM_001286, NM_001287, respectively), where 8/11 missense variants were novel to our study and 7/8 of these were identified solely in the IE population. We performed an in silico analysis of each singular nsSNP using SIFT19 and Polyphen220 algorithms to assess the projected functional impact of the amino acid substitution and found that 70.4% of patient genomes had one or more predicted deleterious mutations in the CLCN gene family as compared with 18.4% of controls. We also observed a greater frequency of compound (>1) missense variants within a single gene in the IE cohort where 7.9% of individuals with IE had 2 or more nsSNPs in the CLCN1 gene compared with <1% (1/139) in controls. This disparity in variant burden and enrichment of predicted damaging mutations in IE cases suggests that, if expressed within appropriate brain networks, CLCN genes could be considered as preeminent candidate genes for epilepsy.
Figure 1. Genetic variation in CLCN genes among individuals with sporadic idiopathic epilepsy compared with neurologically normal controls.
(A) Parallel exomic sequencing reveals that the total number of individuals with structural variants (nsSNPs) in CLCN genes is greater in an idiopathic epilepsy (IE) population compared with controls. Scattergrams of all individuals within each cohort show the total number of CLCN nsSNPs per individual plotted against number of single nucleotide polymorphisms (SNPs) (top panel) or nsSNPs contained in their channotype (bottom panel). The number of nsSNPs in the CLCN genes is independent of total SNP and nsSNP load in an individual channotype. The proband with the de novo truncation is identified as a black triangle. (B) Histograms show mutation burden of CLCN1–7. Individuals with IE have 3 times as many nsSNPs in CLCN1 and CLCN2, including a small number of individuals with 2 or more nonsynonymous variants within the same channel gene. No nsSNPs were identified in the CLCN3 or CLCN4 genes.
Channotype analysis of a proband with a novel de novo truncation in CLCN1.
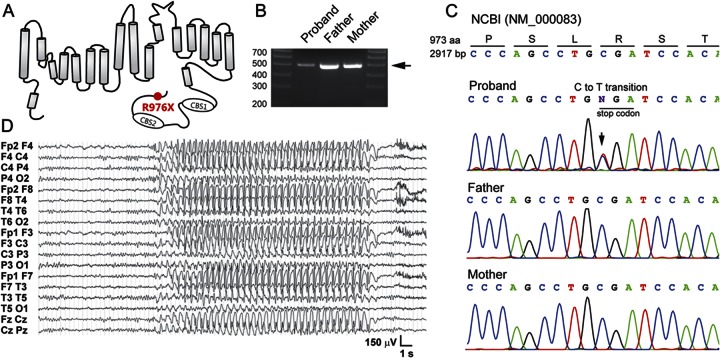
We identified a solitary novel nonsense mutation in the CLCN1 channel gene in one proband (1/291). This heterozygous nsSNP encodes a novel premature stop codon (R976X), resulting in truncation of the distal C-terminus of ClC-1 protein by 12 residues (figure 2A). This mutation has not been described in dbSNP or the 1000 Genomes Project. Since CLCN1 mutations produce hyperexcitable muscle phenotypes, we reasoned that, if de novo in the proband, the novel truncating mutation would identify CLCN1 as a priority candidate gene for epilepsy in this individual. A PCR fragment containing CLCN1 exon 23 was generated for both maternal and paternal alleles (figure 2B). Sequence analysis showed that the C to T transition was not present in either parent (figure 2C), confirming it as a spontaneous de novo mutation and therefore likely to contribute to the phenotype. The proband is also heterozygous for a known CLCN1 single nucleotide polymorphism (rs13438232, P727L)21 found in both patients and controls.16
Figure 2. Detection of a heterozygous de novo nonsense mutation in CLCN1 in a proband with childhood-onset idiopathic generalized epilepsy including cortical spike-wave absence seizures and a history of writer's cramp.
(A) Schematic diagram of a single α subunit of the ClC-1 channel protein showing the location of the novel C-terminal truncation mutation (R976X) identified in a single proband in the study cohort. (B) PCR amplification of the final coding exon (exon 23) of CLCN1 in the trio yielded a 550 bp product (arrow) used as a template in a Sanger sequencing reaction. (C) Sequence chromatograms for the trio compared to the NCBI reference gene (NM_000083) indicating the heterozygous base pair substitution encoding a premature stop codon in the proband. Neither parent has the mutation, indicating that it is de novo, resulting from a spontaneous C to T transition in the proband. (D) A multi-lead EEG recording from the proband during a typical absence seizure 20 seconds in duration exhibiting 3-Hz generalized spike-and-wave complexes.
We further examined the proband channotype and failed to find other compelling candidate variants among the 15 homozygous nsSNPs and 30 heterozygous nsSNPs in other ion channel genes. The only other novel variant identified in the proband was in CLCNKA (A287V), a subunit expressed primarily in kidney, which was found in both patient and control populations within our study.16 The remaining nsSNPs in the proband's channotype are considered common polymorphisms and have all been previously described in dbSNP. The only variant among known monogenic causes of epilepsy we recognized in our 237 ion channel screen of this individual was a single polymorphic nsSNP (rs891398) encoding a T125A missense variant in CHRNA2, a known gene for a nocturnal frontal lobe epilepsy syndrome.22 This variant is considered benign by both Polyphen2 and SIFT, which is consistent with its polymorphic prevalence in the human population, and is unlikely to be a cause of the generalized absence epilepsy phenotype in this proband.
Clinical history and comorbidities in the de novo R976X proband.
We explored the clinical history of the R976X proband and noted the neurologic phenotype of generalized pharmacoresistant epilepsy with mixed seizure types as well as mild myotonic features. The female proband is the only child of healthy, unrelated Italian parents. Following an unremarkable early development, a first seizure occurred during a febrile illness at 11 months of age. An EEG showed generalized spike-wave discharges, and valproate treatment was started. A second seizure appeared at 3 years, ethosuximide (ESM) was added, and the patient remained seizure-free. During a medication taper at age 14, generalized tonic-clonic seizures (GTC) appeared and persisted despite various drug combinations, including phenobarbital (PB) and lamotrigine (LTG). The emergence of clinical absence seizures was discovered at age 17. These were easily observed and recorded as typical absences, lasting about 10–12 seconds and accompanied by 3-Hz generalized spike-wave complexes (figure 2D), occurring several times daily. Background EEG activity remained normal. On occasion it was noticed that an absence seizure would evolve into a GTC seizure. Reintroduction of ESM and PB withdrawal were followed by full control of spontaneous absence seizures since age 21. The patient is right-handed. At age 9, a clinically significant dysfacility with handwriting raised a provisional diagnosis of myotonic writer's cramp.
EEGs obtained over the last several years continue to show interictal generalized spike-wave complexes without clinical seizures. Current treatment includes LTG, ESM, and levetiracetam. Attempts to withdraw ESM have been followed by reappearance of absence seizures. An MRI of the brain was normal. Cognitive testing with the Wechsler Adult Intelligence Scale and the Raven Progressive Matrices at 24 years of age demonstrated normal verbal scores and borderline performance scores. Handwriting that was once considered dysgraphic appeared normal. At age 26, her neurologic examination remains unremarkable, and electrophysiologic investigations including electrocorticography, blink reflex, motor and sensory nerve conduction studies, and needle EMG are all within normal limits with no evidence of myotonic discharge.
CLCN1 mRNA and protein expression in the brain.
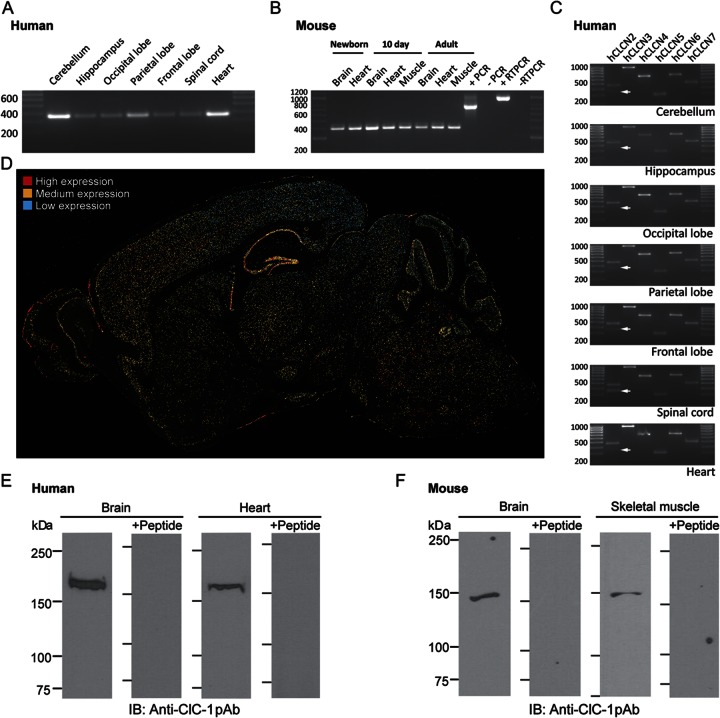
We hypothesized that if CLCN1 is indeed expressed in brain, then loss-of-function mutations that reduce chloride conductance could contribute to enhanced network excitability and increased susceptibility to seizures or other neurologic phenotypes. We used reverse transcriptase PCR to detect mRNA in human brain, and found that CLCN1 mRNA transcripts were expressed in all sampled regions, including cerebellum, hippocampus, spinal cord, occipital, parietal, and frontal lobes, and heart (figure 3A). A scan of human tissue revealed expression of the remaining voltage-gated chloride channel family members CLCN2–7 in the same regions (figure 3C). Interestingly, the primers targeting the N-terminus of CLCN2 detected a second 330-bp band in the spinal cord that was most visible in human hippocampus and neocortical regions (figure 3C) corresponding to a known CLCN2 isoform lacking the 132 bp encoded by exon 3.23 Similarly, we found Clcn1 mRNA in the brain and heart of developing and adult mice (figure 3B). Cellular localization of Clcn1 expression was performed by in situ hybridization in wild-type mouse brain. Neuronal expression level was highest in pyramidal and dentate granule cells of the hippocampal formation, cerebellar Purkinje cell layer, and in scattered brainstem nuclei. Medium levels of expression were evident in all laminae of frontal neocortex and thalamic relay nuclei. Lower levels were also diffusely present in other brain regions (figure 3D).
Figure 3. The ClC-1 “skeletal” chloride channel is expressed in human and murine nervous system.
(A) Reverse transcriptase PCR (RT-PCR) amplification using exon spanning primers detected the expected 330-bp product in all regions of the human brain as well as heart. (B) Similar amplification of a 400-bp product revealed that Clcn1 is expressed in the brain, heart, and skeletal muscle of the newborn, neonatal, and adult mouse. The positive and negative PCR controls targeted a 770-bp amplicon of mouse Tuba-1 (β-actin). (C) PCR amplification of cDNA from regionally dissected human pathology samples. All other members of the CLCN chloride channel family (CLCN2–7) were detected in all regions of the brain as well as in human heart. A known alternatively spliced isoform of CLCN2 lacking the 132 bp encoded by exon 3 was detected (arrow) in addition to the full-length N-terminal isoform. (D) In situ hybridization detection of Clcn1 transcripts in the mouse brain show distinct patterns of expression across brain regions with low levels of expression (blue) throughout. Expression level is highest (red cells) in the hippocampus where transcripts were detected in neurons of the pyramidal layer and dentate granule cell layer. Notable expression was also observed in the cerebellar Purkinje cell layer. Moderate expression (yellow) levels are observed in the frontal neocortex and thalamic relay nuclei. High-resolution image provided as figure e-1. (E) An immunoreactive band corresponding to the expected molecular weight of the ClC-1 α subunit protein was detected in protein lysate from human pathologic samples of human brain and heart using Western immunoblotting using a rabbit polyclonal ClC-1–specific antibody. Preincubation of the antibody with the peptide against which the antibody was raised abolishes detection of the bands in both brain and heart protein lysates. (F) An immunoreactive band corresponding to ClC-1 channel subunit protein was detected in 200 μg of murine brain lysate. The apparent molecular weight of this immunoreactive band in brain was the same as that detected in 40 μg of murine skeletal muscle lysate. Preincubation of the antibody with the peptide against which the antibody was raised abolishes detection of the bands in both brain and skeletal muscle protein lysates.
Using Western immunoblot analysis with a specific antibody to ClC-1, we detected a single immunoreactive band with apparent molecular weight of ∼170 kDa in membrane fractions of human brain and heart tissue (figure 3E). Preincubation with the antigenic peptide abolished detection, confirming specific epitope interaction. We also detected a single immunoreactive band of ∼150 kDa in protein lysates of whole mouse brain and, as a positive control, in murine skeletal muscle (figure 3F). Peptide-preincubation abolished detection in both tissues. The difference in apparent molecular weight between murine and human ClC-1 subunits likely reflects species differences in post-translational modification, or presence of dissociation-resistant auxiliary subunits.
DISCUSSION
Directed by the results of a large-scale exome screen for ion channel gene variants in epilepsy, we show for the first time that the voltage-gated chloride channel protein ClC-1 is expressed in human and murine brain, and that de novo mutation of this gene makes it a strong candidate for contribution to human idiopathic generalized epilepsy with mild focal myotonic/dystonic features in our proband. We postulated that if CLCN1 were functionally expressed in brain, mutations could contribute to hyperexcitability by compromising the chloride channel's contribution to the total membrane conductance and resting membrane potential, a mechanism that is well-established in myocytes.1 Regional expression was identified in cortical, hippocampal, and thalamic regions where loss of chloride currents could contribute to the mixed seizure phenotypes observed in the patient. The presence of Clcn1 in other subcortical structures, including the basal ganglia, subthalamus, and cerebellar Purkinje cell layer, raises the possibility of motor outflow deficits potentially contributing to the proband's mild myotonic/dystonic motor features, and, intriguingly, to the dystonia phenotype of other CLCN1-linked individuals with what has previously been regarded as a purely muscular movement disorder.
The distal location of the proband's CLCN1 C-terminal truncation is relevant to ClC-1 function in the membrane, and nearby mutations in the 117 amino acids downstream of the second cystathionine β-synthase (CBS2) domain have been identified in myotonia patients with varying loss-of-function effects on expression levels, macroscopic conductance, and voltage dependence.24,25 A known distal C-terminal myotonia mutation at residue 946 in human ClC-1 reduces macroscopic conductance and subunit protein levels by half, and studies of sequential serial C-terminal truncations made further upstream result in variable current densities as critical residues in CBS2 are removed.26 Likewise, different ClC-1 C-terminal variants in human myotonia result in a wide spectrum of clinical severity with variable penetrance. For example, the distal C-terminal truncation variant R894X in ClC-1 causes recessive myotonia congenita in some families and dominant myotonia in others.25,27 Possible explanations for this spectrum include allelic variation within the remainder of the gene, reduced penetrance of dominant-negative mutations, incomplete dominance, and founder effect.27–29 Indeed, the dose dependency of ClC-1 mutations on clinical phenotype has been described, and accumulation of deleterious mutations or allelic variation that favors increased variant mRNA level correlates with a more severe pathology.27,30–32
Heteromeric subunit complexity may also account for the uncommon co-occurrence of clinical epilepsy in human myotonia. There is widespread overlap of CLCN1–3 regional expression in brain networks. Although skeletal muscle ClC-1 channels are homodimers, ClC-1/ClC-2 heterodimers with unique biophysical properties can be formed.33 While the existence of native ClC-1/ClC-2 heterodimers has yet to be explored in neurons, these chimeric channels could partially rescue function and, depending on the mutation and cell type, contribute to differences in phenotype severity. Indeed, coexpression of a mutant ClC-1 found in dominant human myotonia congenita (P480L) suppresses ClC-2 current in a dominant-negative fashion.33
Our study illustrates how genetic screening for de novo truncations in voltage-gated ion channels can uncover novel candidates for pathogenic phenotypes in disparate tissues, pointing to overlooked or sometimes occult comorbid neurologic syndromes. In addition, the possibility of a central origin of the dystonic features of CLCN1-linked clinical phenotypes may be interesting to re-evaluate given the extensive expression of the channel throughout central motor control networks. Individuals with IE display a wide range of clinical comorbidities, many of which are secondary to functionally deficient ion channels in tissues outside the CNS.34–36 Elucidating the molecular determinants of such comorbidities may enhance their clinical detection and provide a more efficient therapeutic target in the treatment of multiple disorders caused by a single ion channel gene defect. These results are the first to link a single de novo truncation CLCN1 mutation with absence epilepsy and to localize CLCN1 message and ClC-1 protein to relevant regions in the brain. Our findings identify the need for further exploration of the role of CLCN-family channels in neuronal excitability disorders and confirm the heuristic value of personalized genomic profiling in epilepsy.
Supplementary Material
GLOSSARY
- BCM
Baylor College of Medicine
- dbSNP
database of single nucleotide polymorphism
- ESM
ethosuximide
- GTC
generalized tonic-clonic
- HRP
horseradish peroxidase
- IE
idiopathic epilepsy
- ISH
in situ hybridization
- LTG
lamotrigine
- nsSNP
nonsynonymous single nucleotide polymorphism
- PB
phenobarbital
Footnotes
Supplemental data at www.neurology.org
Editorial, page 1074
AUTHOR CONTRIBUTIONS
Tim T. Chen: manuscript preparation, study design, molecular/biochemical experiments. Tara L. Klassen: manuscript preparation, study design, molecular/biochemical experiments. Alica M. Goldman: patient recruitment and biochemical experiments. Carla Marini: patient recruitment and clinical phenotyping. Renzo Guerrini: patient recruitment and clinical phenotyping. Jeffrey L. Noebels: manuscript preparation, study design.
STUDY FUNDING
Supported by the National Institute of Neurological Disorders and Stroke NS 049130 and NS 29709 (J.L.N.), an Epilepsy Foundation Postdoctoral Fellowship (T.L.K.), the BCM IDDRC (5P30HD024064) and the Blue Bird Circle Foundation.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Matthews E, Fialho D, Tan S, et al. The non-dystrophic myotonias: molecular pathogenesis, diagnosis and treatment. Brain 2010;133:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George AL, Jr, Crackower MA, Abdalla JA, Hudson AJ, Ebers GC. Molecular basis of Thomsen's disease (autosomal dominant myotonia congenita). Nat Genet 1993;3:305–310 [DOI] [PubMed] [Google Scholar]
- 3.Koch MC, Steinmeyer K, Lorenz C, et al. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science 1992;257:797–800 [DOI] [PubMed] [Google Scholar]
- 4.Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature 1991;354:301–304 [DOI] [PubMed] [Google Scholar]
- 5.Steinmeyer K, Klocke R, Ortland C, et al. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature 1991;354:304–308 [DOI] [PubMed] [Google Scholar]
- 6.Madison DV, Malenka RC, Nicoll RA. Phorbol esters block a voltage-sensitive chloride current in hippocampal pyramidal cells. Nature 1986;321:695–697 [DOI] [PubMed] [Google Scholar]
- 7.Staley K. The role of an inwardly rectifying chloride conductance in postsynaptic inhibition. J Neurophysiol 1994;72:273–284 [DOI] [PubMed] [Google Scholar]
- 8.Reference was retracted in 2009. PMID: 19710717
- 9.Saint-Martin C, Gauvain G, Teodorescu G, et al. Two novel CLCN2 mutations accelerating chloride channel deactivation are associated with idiopathic generalized epilepsy. Hum Mutat 2009;30:397–405 [DOI] [PubMed] [Google Scholar]
- 10.Everett K, Chioza B, Aicardi J, et al. Linkage and mutational analysis of CLCN2 in childhood absence epilepsy. Epilepsy Res 2007;75:145–153 [DOI] [PubMed] [Google Scholar]
- 11.Niemeyer MI, Cid LP, Sepulveda FV, Blanz J, Auberson M, Jentsch TJ. No evidence for a role of CLCN2 variants in idiopathic generalized epilepsy. Nat Genet 2010;42:3. [DOI] [PubMed] [Google Scholar]
- 12.Blanz J, Schweizer M, Auberson M, et al. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J Neurosci 2007;27:6581–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stobrawa SM, Breiderhoff T, Takamori S, et al. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 2001;29:185–196 [DOI] [PubMed] [Google Scholar]
- 14.Riazanski V, Deriy LV, Shevchenko PD, Le B, Gomez EA, Nelson DJ. Presynaptic CLC-3 determines quantal size of inhibitory transmission in the hippocampus. Nat Neurosci 2011;14:487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickerson LW, Bonthius DJ, Schutte BC, et al. Altered GABAergic function accompanies hippocampal degeneration in mice lacking ClC-3 voltage-gated chloride channels. Brain Res 2002;958:227–250 [DOI] [PubMed] [Google Scholar]
- 16.Klassen T, Davis C, Goldman A, et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell 2011;145:1036–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaylaoglu MB, Titmus A, Visel A, Alvarez-Bolado G, Thaller C, Eichele G. Comprehensive expression atlas of fibroblast growth factors and their receptors generated by a novel robotic in situ hybridization platform. Dev Dyn 2005;234:371–386 [DOI] [PubMed] [Google Scholar]
- 18.Carson JP, Eichele G, Chiu W. A method for automated detection of gene expression required for the establishment of a digital transcriptome-wide gene expression atlas. J Microsc 2005;217:275–281 [DOI] [PubMed] [Google Scholar]
- 19.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res 2001;11:863–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods 2010;7:248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun C, Tranebjaerg L, Torbergsen T, Holmgren G, Van Ghelue M. Spectrum of CLCN1 mutations in patients with myotonia congenita in Northern Scandinavia. Eur J Hum Genet 2001;9:903–909 [DOI] [PubMed] [Google Scholar]
- 22.Aridon P, Marini C, Di Resta C, et al. Increased sensitivity of the neuronal nicotinic receptor alpha 2 subunit causes familial epilepsy with nocturnal wandering and ictal fear. Am J Hum Genet 2006;79:342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertelli M, Cecchin S, Lapucci C, et al. Quantification of chloride channel 2 (CLCN2) gene isoforms in normal versus lesion- and epilepsy-associated brain tissue. Biochim Biophys Acta 2007;1772:15–20 [DOI] [PubMed] [Google Scholar]
- 24.Hebeisen S, Fahlke C. Carboxy-terminal truncations modify the outer pore vestibule of muscle chloride channels. Biophys J 2005;89:1710–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer-Kleine C, Steinmeyer K, Ricker K, Jentsch TJ, Koch MC. Spectrum of mutations in the major human skeletal muscle chloride channel gene (CLCN1) leading to myotonia. Am J Hum Genet 1995;57:1325–1334 [PMC free article] [PubMed] [Google Scholar]
- 26.Macias MJ, Teijido O, Zifarelli G, et al. Myotonia-related mutations in the distal C-terminus of ClC-1 and ClC-0 chloride channels affect the structure of a poly-proline helix. Biochem J 2007;403:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duno M, Colding-Jorgensen E, Grunnet M, Jespersen T, Vissing J, Schwartz M. Difference in allelic expression of the CLCN1 gene and the possible influence on the myotonia congenita phenotype. Eur J Hum Genet 2004;12:738–743 [DOI] [PubMed] [Google Scholar]
- 28.Plassart-Schiess E, Gervais A, Eymard B, et al. Novel muscle chloride channel (CLCN1) mutations in myotonia congenita with various modes of inheritance including incomplete dominance and penetrance. Neurology 1998;50:1176–1179 [DOI] [PubMed] [Google Scholar]
- 29.Koty PP, Pegoraro E, Hobson G, et al. Myotonia and the muscle chloride channel: dominant mutations show variable penetrance and founder effect. Neurology 1996;47:963–968 [DOI] [PubMed] [Google Scholar]
- 30.Burgunder JM, Huifang S, Beguin P, et al. Novel chloride channel mutations leading to mild myotonia among Chinese. Neuromuscul Disord 2008;18:633–640 [DOI] [PubMed] [Google Scholar]
- 31.Shalata A, Furman H, Adir V, et al. Myotonia congenita in a large consanguineous Arab family: insight into the clinical spectrum of carriers and double heterozygotes of a novel mutation in the chloride channel CLCN1 gene. Muscle Nerve 2010;41:464–469 [DOI] [PubMed] [Google Scholar]
- 32.Bernard G, Poulin C, Puymirat J, Sternberg D, Shevell M. Dosage effect of a dominant CLCN1 mutation: a novel syndrome. J Child Neurol 2008;23:163–166 [DOI] [PubMed] [Google Scholar]
- 33.Lorenz C, Pusch M, Jentsch TJ. Heteromultimeric CLC chloride channels with novel properties. Proc Natl Acad Sci USA 1996;93:13362–13366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med 2009;1:2ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purtell K, Roepke TK, Abbott GW. Cardiac arrhythmia and thyroid dysfunction: a novel genetic link. Int J Biochem Cell Biol 2010;42:1767–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasuda K, Miyake K, Horikawa Y, et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet 2008;40:1092–1097 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.