Abstract
Objectives:
To determine the characteristics of adult-onset autoimmune chorea, and compare paraneoplastic and idiopathic subgroups.
Methods:
Thirty-six adults with autoimmune chorea were identified at Mayo Clinic (Rochester, MN) from 1997 to 2012. Medical record and laboratory data were recorded. Nonparaneoplastic (n = 22) and paraneoplastic cases (n = 14) were compared.
Results:
Women accounted for 21 patients (58%). Median age at symptom onset was 67 years (range 18–87 years). We estimated the incidence for Olmsted County was 1.5 per million person-years. Symptom onset was subacute in all. Chorea was focal (20 patients) or generalized (16 patients). Although chorea predominated, other neurologic disorders frequently coexisted (29 patients); abnormal eye movements were uncommon (4 patients). No patient had NMDA receptor antibody or any immunoglobulin (Ig)G yielding a detectable immunofluorescence binding pattern restricted to basal ganglia. Two had synaptic IgG antibodies novel to the context of chorea (GAD65, 1; CASPR2, 1). In the paraneoplastic group, 14 patients had evidence of cancer. Of 13 with a histopathologically confirmed neoplasm, small-cell carcinoma and adenocarcinoma were most common; 6 patients had a cancer-predictive paraneoplastic autoantibody, with CRMP-5–IgG and ANNA-1 being most common. In the idiopathic group, 19 of the 22 patients had a coexisting autoimmune disorder (most frequently systemic lupus erythematosus and antiphospholipid syndrome); autoantibodies were detected in 21 patients, most frequently lupus and phospholipid specificities (19 patients). The paraneoplastic group was older (p = 0.001), more frequently male (p = 0.006), had more frequent weight loss (p = 0.02), and frequently had peripheral neuropathy (p = 0.008).
Conclusions:
Autoimmune chorea is a rare disorder with rapid onset. Male sex, older age, severe chorea, coexisting peripheral neuropathy, and weight loss increase the likelihood of cancer.
Huntington disease is usually the foremost consideration for a neurologist evaluating adult-onset chorea. An autoimmune etiology is sometimes overlooked: paraneoplastic, parainfectious, or idiopathic. Prototypic disorders include CRMP-5 (collapsin response-mediator protein 5)-immunoglobulin (Ig)G–associated paraneoplastic chorea1 (usually related to small-cell carcinoma) and idiopathic phospholipid antibody-associated chorea.2 Sydenham chorea, a parainfectious immune-mediated disorder, lacks a proven autoantigen and is rare in later adulthood.3 There is a paucity of published data addressing clinical or serologic characteristics that aid the distinction of adult-onset autoimmune choreas, guide treatment, or inform outcomes. This report describes our 16-year experience with adult-onset autoimmune chorea, and compares paraneoplastic and idiopathic autoimmune forms.
METHODS
Patients.
The study used Mayo Clinic's computerized central diagnostic index, and was approved by the Mayo Clinic Institutional Review Board (IRB 08-6647). We reviewed 2,634 medical records of patients seen from January 1, 1997 to June 1, 2012, with 1 or more hyperkinetic movement disorder diagnoses (chorea, dystonia, dyskinesia, hemiballismus, myoclonus tremor) for whom the medical consultation notes contained the search terms antibody; immune; autoimmune; paraneoplastic; prednisone; steroid; intravenous immune globulin; IVIg; plasma exchange; plasmapheresis; Sydenham; and gravidarum. From these 460 medical records, we identified 36 patients who had a chorea-predominant neurologic presentation and suspicion for an autoimmune cause. These 36 patients were in either 1) the paraneoplastic group (n = 14) with chorea onset within 2 years of an active cancer diagnosis or accompanied by an autoantibody in serum or CSF with high positive predictive value for cancer, such as antineuronal nuclear antibodies type 1 (ANNA-1) or CRMP-5–IgG; or 2) the idiopathic group (n = 22) with clinical, serologic, or CSF findings (elevations in ≥1 of the following: CSF protein [>50 mg/dL], white cell count, IgG index, IgG synthesis rate, or oligoclonal bands) compatible with autoimmunity, but cancer evidence and risk factors lacking, and neurologic improvement after a trial of immunotherapy, or spontaneously. Video material documenting choreiform movements was available for 4 patients (videos 1–4 on the Neurology® Web site at www.neurology.org.).
We did not identify any patient with an isolated parainfectious etiology arising in adulthood; 3 patients had onset of Sydenham chorea in childhood and symptoms persisted after age 18 years. Chorea gravidarum was diagnosed in 3 patients but these patients were excluded from the study: 2 did not have an autoimmune cause identified; 1 had worsening of unresolved childhood-onset Sydenham chorea during pregnancy.
Laboratory methods.
All assay methods have been described previously.4–8 Serum and CSF were tested by standardized indirect immunofluorescence assay on a composite substrate of mouse cerebellum, midbrain, basal ganglia, thalamus, cerebral cortex, hippocampus, stomach, and kidney to detect IgG autoantibodies binding selectively to neuronal and glial nuclei (ANNA-1 [anti-Hu], type 2 [anti-Ri], and type 3; antiglial/neuronal nuclear antibody, type 1 [AGNA or SOX-1 antibody]), neuronal cytoplasm (Purkinje cell antibodies [types 1 {anti-Yo}, 2, and Tr], CRMP-5–IgG, and amphiphysin-IgG), or to hippocampal and basal ganglionic synapses.
IgG targeting specific neurotransmitter receptors in hippocampal synapses (NMDA [GluN1], AMPA [α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid] [GluA1 and GluA2], and γ-aminobutyric acid [GABA]B) were sought by indirect immunofluorescence on HEK293 cells transfected with appropriate cDNAs (Euroimmun, Lübeck, Germany).
Antibodies reactive with neural cation channel complexes (neuronal voltage-gated calcium channels [VGCCs, P/Q type and N type], voltage-gated potassium channel [VGKC] complexes, nicotinic acetylcholine receptors [AChR] of skeletal muscle type [α1 subunit], and neuronal ganglionic type [α3 subunit]) and glutamic acid decarboxylase 65-kDa isoform (GAD65) were detected by radioimmunoprecipitation assay. Skeletal muscle striational antibodies were detected by ELISA. CRMP-5–IgG and amphiphysin-IgG were additionally sought by recombinant Western blot assays. Sera yielding positive results for VGKC complex–IgG were tested further for IgG reactive with leucine-rich glioma-inactivated 1 (LGI1) protein or contactin-associated protein-2 (CASPR2) by a cell-based immunofluorescence assay (Euroimmun).
Statistical methods.
We compared data for paraneoplastic and idiopathic groups regarding demographic and clinical findings, treatment, and outcome (physician-reported) using Fisher exact test and Wilcoxon rank sum test where appropriate.
RESULTS
Twenty-one of the 36 patients were women (58%), and median age at onset was 67 years (range 18–87 years). All were evaluated by a Mayo Clinic staff neurologist at a median of 5 months after symptom onset (range 0.25–120 months). Onset was subacute in 34 patients and was not documented in 2 patients. Three patients were from Olmsted County, MN, giving an estimated incidence of 1.5 per million person-years (using an average annual population for the county of 132,905 [1997–2011], and assuming that all patients with autoimmune chorea were seen at Mayo Clinic).
Review of the records confirmed that the adventitious movements were indeed choreiform (semidirected, nonstereotyped, flowing from one muscle to the next; videos 1–4). Chorea was generalized in 16 patients (44%) and focal in 20 patients (56%), but became generalized in another 12 patients. Focal distributions included hemichorea in 9 patients, limbs in 8 patients (1 limb, 6 patients; both arms, 1 patient; both legs, 1 patient), and face and/or tongue in 3 patients. Abnormal saccadic eye movements were recorded in 4 patients (11%, all in the paraneoplastic group). In 3 patients (16, 19, and 27), the onset of chorea was within 3 months of initiating hormonal medication (estrogenic, 2; progesterone alone, 1). Chorea did not stop on discontinuing the medication.
No family history of chorea was reported, but 13 of 33 (40%) reported a family history of autoimmune disease. The following neurodegenerative etiologies were considered but excluded by blood tests: Huntington disease, 10 patients; dentatorubropallidoluysian atrophy, 3 patients; and neuroacanthocytosis, 2 patients. A smoking history was recorded in 23 patients (64%; 86% of paraneoplastic cases). Head MRI did not aid the diagnosis in any case. Evidence of remote basal ganglionic lacunar infarcts in the context of more widespread ischemic changes was observed in 4 patients. One or more neural antibodies were detected in 15 patients (42%). Immunofluorescence testing did not reveal any serum IgG binding selectively to basal ganglia. Follow-up information (median 12 months; range 2–228 months) was available for 33 patients (96%).
Paraneoplastic group.
The median age at symptom onset was 72 years (range 59–87 years), and 10 of the 14 patients (71%) were men (table e-1). Six reported a focal symptom onset; the remainder had generalized onset. By physician rating (reported for 11 patients), chorea was moderate or severe in 9 and mild in 2. Ten patients (71%) reported recent weight loss exceeding 4 kg (median 11; range 4–22 kg). Chorea was accompanied by additional neurologic symptoms/disorders in 12 patients. Peripheral neuropathy was most common (6, painful in 3). One or more coexisting autoimmune disorders were noted in 7 patients: hypothyroidism, 4; celiac disease, 3; diabetes mellitus, 1; and rheumatoid arthritis, 1.
At least 1 cancer was diagnosed within 2 years of chorea onset (13 of 14 patients); 6 patients had 1 or more paraneoplastic antibodies highly predictive of cancer (including 1 patient in whom cancer was not found) (table 1). Multiple neoplasms were documented in 5 patients: 3 had multiple coexisting cancers; 2 had an additional remote history of cancer. The 19 cancers identified were (table 1): small-cell lung carcinoma, 4; non–small-cell lung carcinoma, 3; hematologic, 3 (Hodgkin lymphoma, non-Hodgkin lymphoma, and chronic myeloid leukemia); colorectal adenocarcinoma, 2; prostate adenocarcinoma, 2; tonsillar squamous-cell carcinoma, 1; bladder carcinoma, 1; breast adenocarcinoma, 1; pancreatic adenocarcinoma, 1; and gastric adenocarcinoma, 1. Patient 4 (a smoker) developed chorea 178 months after squamous-cell tonsillar carcinoma was diagnosed; detection of CRMP-5–IgG at chorea presentation predicted small-cell carcinoma, but none was found.
Table 1.
Serum autoantibodies, CSF and oncologic findings, and treatment and outcome data for the paraneoplastic group
Neural autoantibodies with a high cancer-predictive value included CRMP-5–IgG, 5 patients; ANNA-1, 3; ANNA-2, 1; and amphiphysin-IgG, 1. Neural antibodies with lower cancer-predictive value included GAD65, 3 patients; VGCC (P/Q type), 2; striational, 2; and VGCC (N type), 1. No patient had detectable NMDA, AMPA, or GABAB receptor antibodies. Two patients had phospholipid antibodies, but neither had clinical manifestations of antiphospholipid syndrome (thrombotic event or miscarriage). One patient of 3 tested had an elevated antistreptolysin O (ASO) value; evidence of active streptococcal infection was lacking at chorea onset and sequential testing did not reveal an increase in titer.
Chorea improved in 3 of 7 patients who received oncologic therapy and in 5 of 11 patients who received immunotherapy (table 1). Immunotherapy-related improvements were associated with corticosteroids alone (2 of 6 patients), plasma exchange (1 of 3 patients), cyclophosphamide (2 of 3 patients), steroids combined with IVIg (2 of 2 patients). Improvement was also variable with symptomatic therapies (6 of 12 patients, table 1). Four patients reported therapy side effects: worsening hyperkinetic movements, 1 (haloperidol), and worsening dysarthria, 1 (alprazolam).
Spontaneous improvement was reported in 8 patients, nonsustained in 7 patients, and complete remission in 1 (table 1). At last follow-up, 2 patients had died with metastatic carcinoma.
Idiopathic group.
The median age at symptom onset was 45 years (range 18–87 years), and 17 of the 22 patients (77%) were women (table e-2). A focal, segmental, or hemisymptom onset was reported in 14 patients (64%). By physician rating (reported for 21 patients), chorea was moderate or severe in 9 (42%) and mild in 12 (58%). Seven patients (32%) reported recent weight loss exceeding 4 kg (median 11; range 4–13 kg). Chorea was accompanied by additional neurologic symptoms/disorders in 14 patients (table e-2). Cognitive or behavioral symptoms were most common (9 patients) and were mild except for patient 15 who developed severe personality change (disinhibition and poor judgment). One patient had peripheral neuropathy. One or more coexisting autoimmune disorders were noted in 19 patients (table 2): systemic lupus erythematosus (SLE), 8; antiphospholipid syndrome, 8 (6 had SLE, 5 had 1 or more episodes of arterial or venous thrombosis, and 3 had a history of 1 or more miscarriages); Sjögren syndrome, 4; rheumatoid arthritis, 3; autoimmune thrombocytopenic purpura, 3; autoimmune thyroid disease, 3; and diabetes mellitus, 2. Detected non-neural autoantibodies included the following: phospholipid antibody or lupus anticoagulant, 18; antinuclear antibody (ANA, 14; extractable nuclear antigen specificity, 7; double-stranded DNA specificity, 5); rheumatoid factor, 3; and thyroperoxidase antibody, 1. One or more neural autoantibodies were detected in 6 patients (table 2): GAD65, 3; N-type VGCC, 1; muscle AChR, 1; striational, 1; VGKC complex, 1. No patient had stiff-man syndrome, Lambert-Eaton syndrome, or myasthenia gravis. Further serologic evaluation of patient 15 revealed that the VGKC-complex antibody (0.20 nmol/L; normal ≤0.02 nmol/L) was CASPR2-IgG specific; LGI1-IgG was not detected.
Table 2.
Serum autoantibodies, CSF and autoimmune findings, treatment and data outcome for the idiopathic group
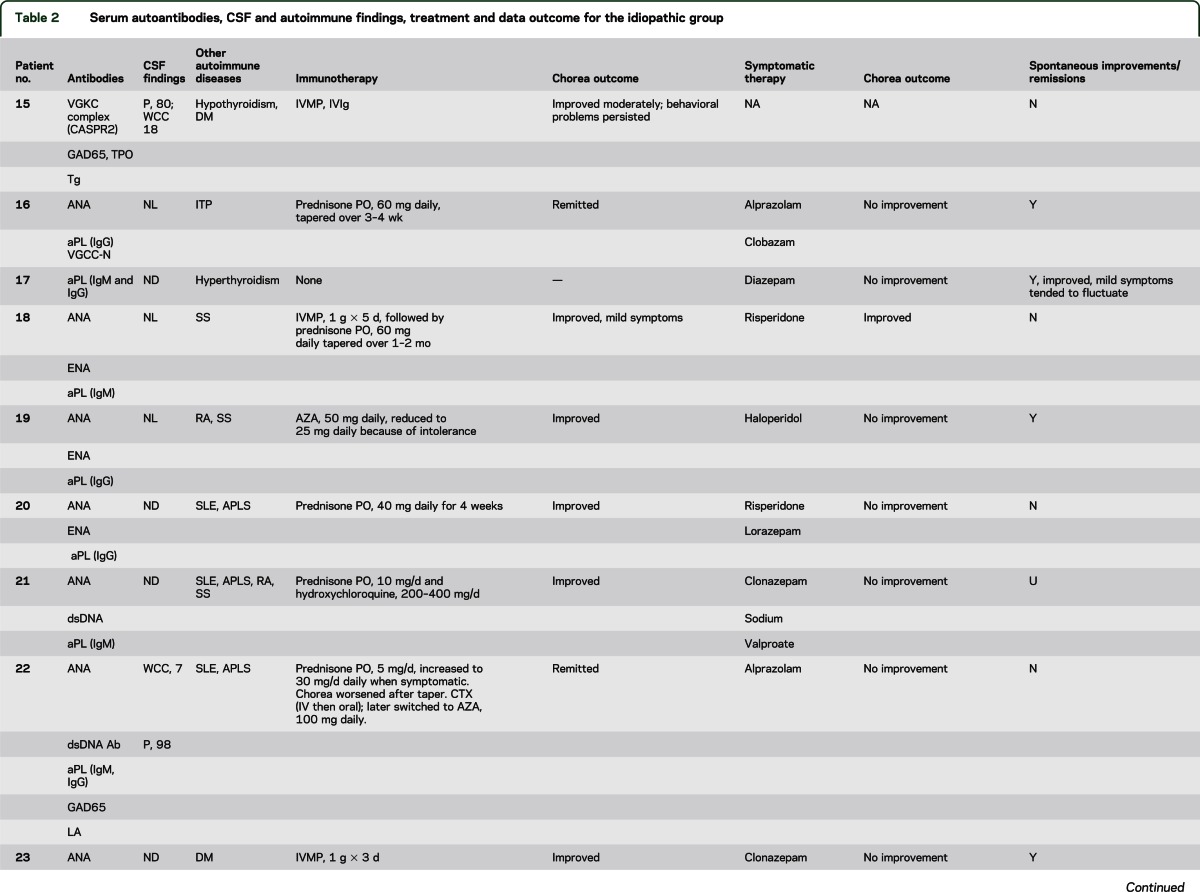
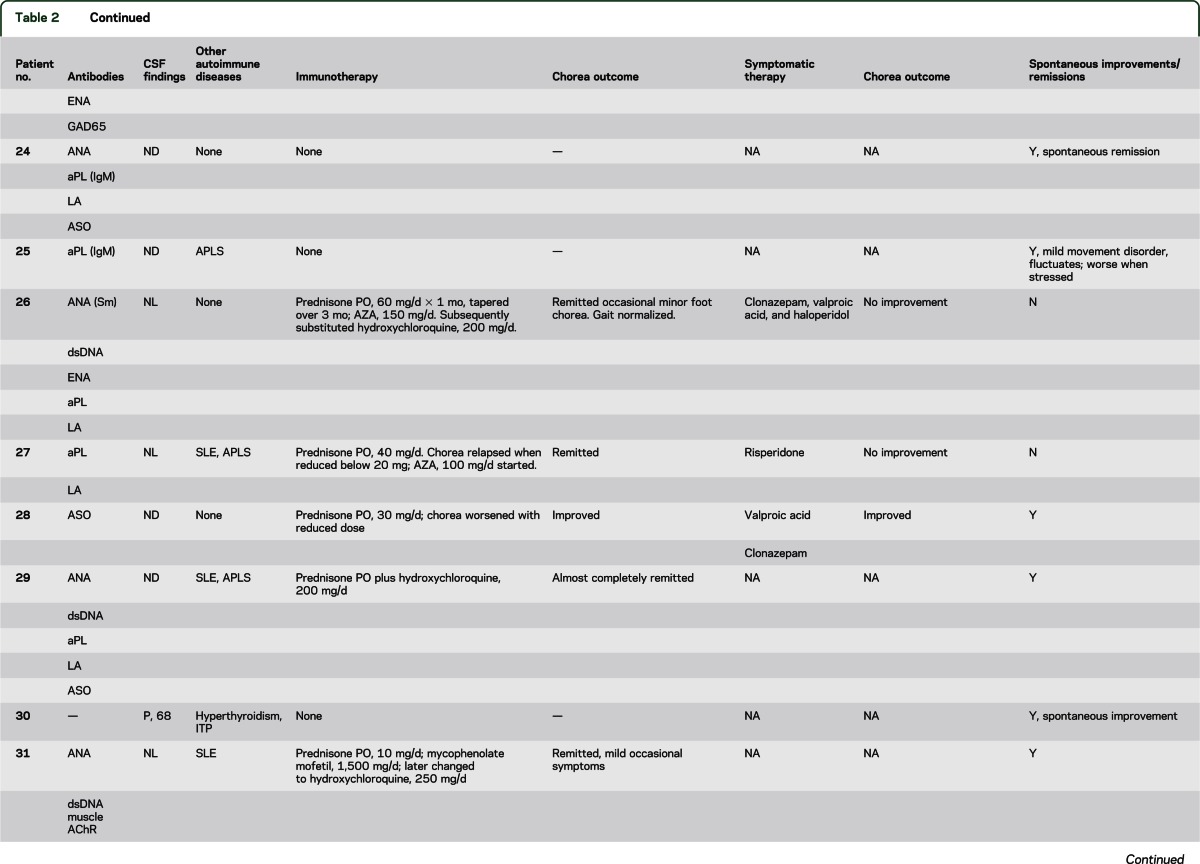
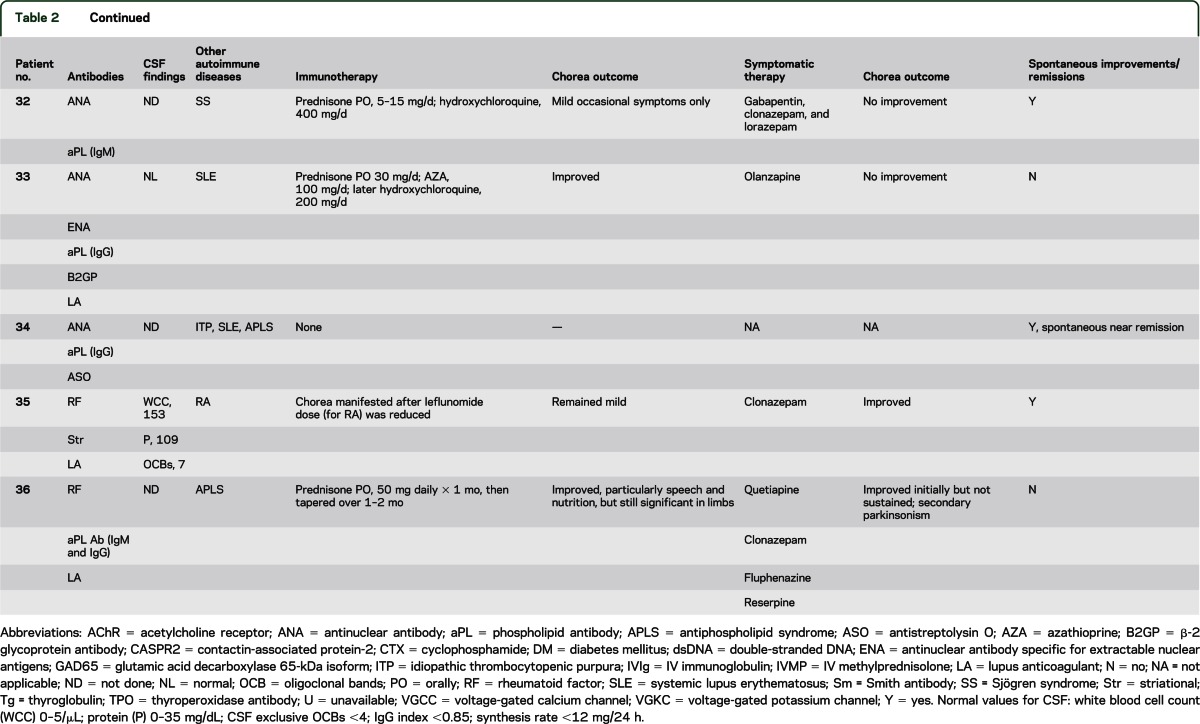
Four of 6 patients tested had an increased antistreptolysin O antibody value, but none had an increase in titer with sequential testing. There was no evidence of active streptococcal infection at chorea onset and no patient had a history of rheumatic fever or cardiac valvular disease.
Chorea improvements with immunotherapy were attributed to corticosteroids alone in 14; corticosteroids and IVIg in 1, and azathioprine alone in 1 (table 2). Seven had complete or near-complete remission; 4 relapsed when the steroid dose was reduced. Steroid-sparing medications permitted maintenance of remission: hydroxychloroquine, 6; azathioprine, 5; cyclophosphamide, 1; and mycophenolate mofetil, 1. Spontaneous improvements or remission was reported for 13 patients; for 5 of these patients, immunotherapy was not deemed necessary. Symptomatic therapies were beneficial in 4 of 15 treated patients (table 2). Reported side effects included sedation, 2; parkinsonism, 1; bradyphrenia, 1; and exacerbation of autonomic symptoms, 1. At last follow-up, 3 patients had died: 1 of cardiac failure, 1 of cerebral venous sinus thrombosis, and 1 of unknown cause.
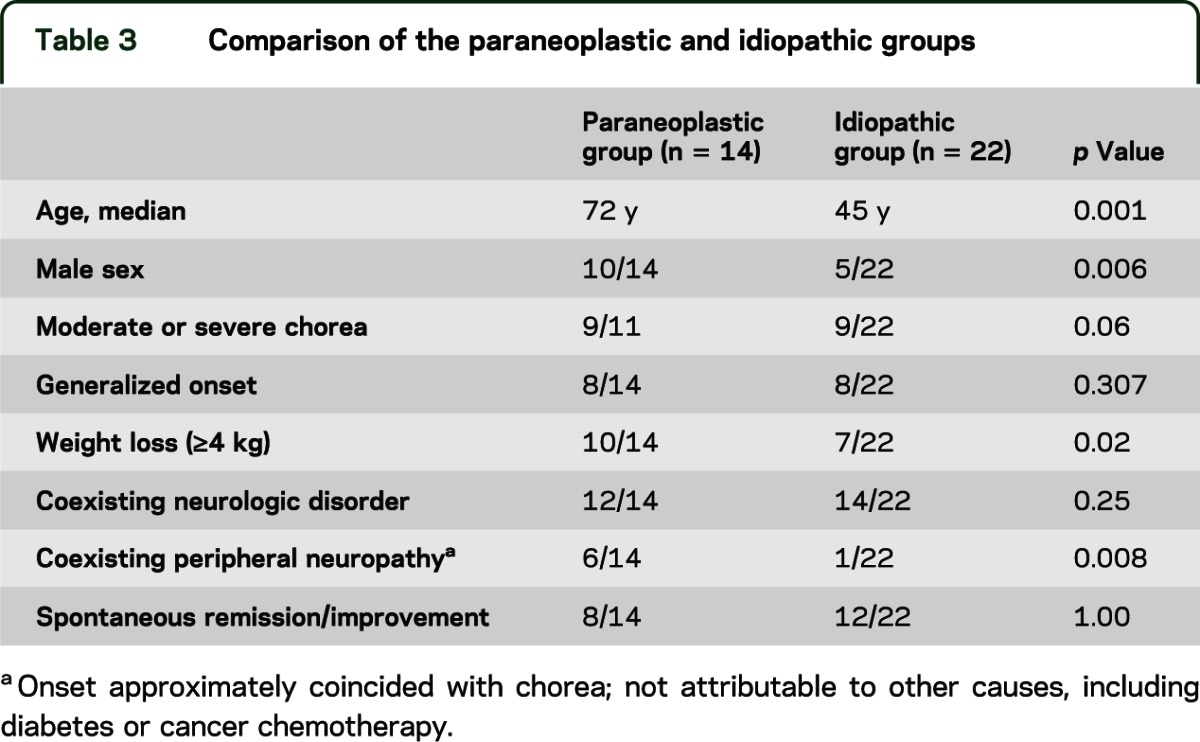
Comparison of paraneoplastic and idiopathic groups.
Patients in the paraneoplastic group were more likely to be older (p = 0.001) and male (p = 0.006). They were also more likely to have weight loss (p = 0.02) and coexisting peripheral neuropathy (0.008) (table 3).
Table 3.
Comparison of the paraneoplastic and idiopathic groups
DISCUSSION
Although rare, autoimmune chorea is likely second only to Huntington disease as a cause of chorea in adults. Approximately 2 new patients were identified annually at our institution. We estimated the incidence in the local Olmsted County population to be 1.5 per million person-years, which is half the incidence reported for Huntington disease in the same population.9 The median onset age of autoimmune chorea was similar to that reported for Huntington disease,9 which was the initially considered diagnosis in several patients. Clinical factors that we find useful in distinguishing autoimmune chorea from a neurodegenerative disorder include a subacute symptom onset, fluctuating course with spontaneous improvements and remissions, frequent coexistence of neurologic disorders atypical for Huntington disease, and generally absence of eye-movement abnormalities (hypometric saccadic eye movements are characteristic of Huntington disease10). In our medical record search, the onset age of parainfectious (Sydenham) chorea was younger than 18 years, but symptoms sometimes persisted beyond childhood.
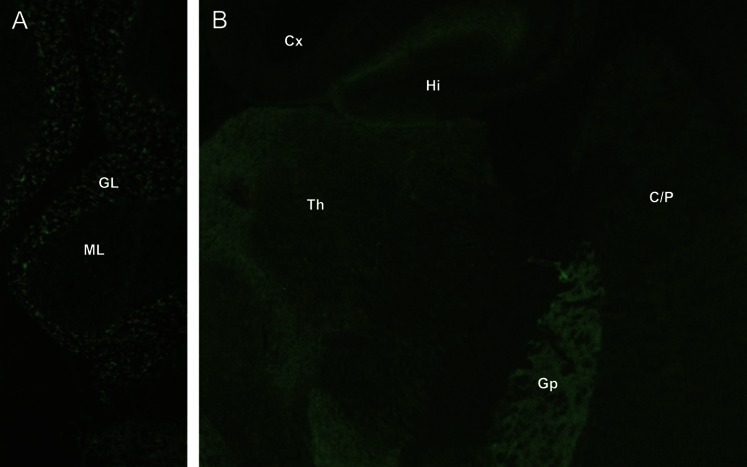
An autoimmune basis for chorea is also supported by clinical or serologic evidence of autoimmunity or cancer, inflammatory CSF, and immunotherapy responsiveness. Of note, we did not detect any basal ganglion–restricted autoantibodies using an optimized contemporary and clinically validated immunohistochemical assay for detecting synaptic antibodies. GAD65-IgG was detected in 1 patient using this methodology (figure; the radioimmunoprecipitation value was 760 nmol/L); GAD65-IgG was detected by radioimmunoprecipitation assay in 14% of patients overall (all other values were <0.10 nmol/L; the normal range is 0.00–0.02 nmol/L). The frequency of GAD65 autoantibody in healthy Olmsted County citizens older than 50 years is 8% (generally low values).11 The GAD65 enzyme is distributed throughout the brain, but is highly enriched in basal ganglia12 and is critical for GABAergic inhibitory neurotransmission in basal ganglia. Early pathologic signatures of Huntington disease are degenerative loss of striatal GABAergic neurons (and GAD65).13 Patients with neurologic manifestations of GAD65 autoimmunity (disorders characterized by loss of inhibitory neurotransmission, including stiff-man syndrome14 and autoimmune epilepsy15) typically have serum GAD65-IgG values >20 nmol/L. Our findings suggest that the GAD65 autoimmune neurologic spectrum may extend to chorea.16
Figure. GAD65-IgG detected by indirect immunofluorescence on mouse cerebellum.
(A) Cerebellum, (B) other brain regions (coronal section). Characteristic synaptic staining pattern is most prominent in the granular layer (GL) of cerebellum and globus pallidus (Gp). Less intensely stained regions include molecular layer of cerebellum (ML), thalamus (Th), caudate and putamen (C/P), hippocampus (Hi), and cerebral cortex (Cx). GAD65 = glutamic acid decarboxylase 65-kDa isoform; Ig = immunoglobulin.
Adult-onset autoimmune chorea falls into 2 distinct groups: paraneoplastic and idiopathic. The cancers we encountered most frequently were small-cell carcinoma and various adenocarcinomas. Other reported cancer associations include renal cell carcinoma17,18 and acute lymphoblastic leukemia.19 Our findings in patients with paraneoplastic chorea accord with the findings described by a European collaborative group in 13 patients, with respect to male predominance, late age at onset, subacuity, early generalization and severity, substantial weight loss, and poor treatment responses.17 We determined additionally that several of these characteristics pertain to paraneoplastic cases more than to idiopathic cases of chorea. We speculate that the greater weight loss in paraneoplastic cases reflects caloric expenditure from severe hyperkinetic movements,20 cancer-related cytokine effects,21 or both. The most striking factor distinguishing the 2 groups was the high frequency of accompanying peripheral neuropathy in the paraneoplastic group, which was not attributable to chemotherapy or diabetes. Although several clues emerging from this retrospective study of a rare disorder potentially aid the distinction of paraneoplastic from idiopathic autoimmune chorea, no attribute other than cancer and its predictive autoantibodies was absolutely restricted to one group. Suspicion for an autoimmune cause justifies comprehensive evaluation of serum and CSF for paraneoplastic antibodies, and an extended search for cancer. Detection of an autoantibody with high cancer predictive value (e.g., CRMP-5–IgG) may refine the cancer search to 1 or 2 cancer types (e.g., small-cell carcinoma, renal cell carcinoma, or thymoma).1 Distinctive basal ganglionic radiologic abnormalities were previously reported by our group in 5 of 15 patients with CRMP-5-IgG–associated chorea.1 Vigliani et al.17 reported similar radiologic findings in only 1 of 13 patients with paraneoplastic chorea. The lack of any radiologic abnormalities in the patients of this report may reflect the timing of radiologic testing in the course of the disease.
It was informative that NMDA receptor antibody was not detected in any patient. Orolingual facial dyskinesia is characteristic of NMDA receptor autoimmune encephalitis, which also is frequently paraneoplastic.22 Patients with that disorder usually have stereotypic, repetitive, dyskinetic movements in contrast to random choreiform movements. Furthermore, dyskinesias in NMDA receptor autoimmune encephalitis coincide with characteristic psychiatric symptomatology. This is usually followed by profound encephalopathy, seizures, hypoventilation, and coma.23
VGKC-complex antibody of CASPR2 specificity was detected in patient 15 who had immunotherapy-responsive idiopathic chorea and disinhibited behavior. A recent report described immunotherapy-responsive chorea as the presentation of 2 patients with VGKC-complex autoantibody of LGI1 specificity. In contrast to our patient, these patients progressed to limbic encephalitis within weeks.24
Universal corticosteroid responsiveness was a major criterion supporting an autoimmune basis for the idiopathic chorea cases in this study. SLE and antiphospholipid syndrome were common accompaniments. Estrogenic oral contraceptives and pregnancy (chorea gravidarum) are recognized risk factors for the occurrence of chorea in patients with SLE or antiphospholipid syndrome.2,25 Anticoagulation therapy is sometimes instituted because an ischemic etiology (secondary to a hypercoagulable state) is assumed.26 However, no patient in this study had radiologic evidence of subacute ischemia of the basal ganglia. We therefore inferred that chorea did not result from a hypercoagulable state and concluded that anticoagulation should be reserved for treating thrombosis, but not chorea.27 The infrequency of neural-specific antibody detection in patients with idiopathic autoimmune chorea (5 of 21 patients) leads us to conclude that pertinent autoantibody markers remain to be discovered.
Immunotherapy responsiveness was an inclusion criterion for idiopathic autoimmune chorea. Only 60% of the paraneoplastic chorea patients improved with immunotherapy. Therapies and doses varied, but there are some general inferences for clinical practice. Patients with idiopathic autoimmune chorea whose symptoms are sufficiently troublesome to merit immunotherapy could be treated with daily oral prednisone, or weekly IV methylprednisolone, with transition to a steroid-sparing agent such as azathioprine over 3 to 6 months. Coexistence of another autoimmune disease (such as SLE) would further justify initiating immunotherapy. Cancer treatment is paramount for patients with paraneoplastic chorea. Paraneoplastic movement disorders can occur rarely in the context of initiating chemotherapy.28 This phenomenon may have occurred in patient 13 who was receiving imatinib treatment for chronic lymphocytic leukemia when chorea began. It is conceivable that leukemoid cell apoptosis induced by imatinib may have initiated an onco-neural antigen-specific immune response.
Immunotherapy may involve sequential trials of methylprednisolone (1,000 mg daily for 3–5 days, followed by 1,000 mg weekly for 5 weeks), IVIg (0.4 g/kg daily for 3 days followed by once weekly for 5 weeks), and plasma exchange (5–7 exchanges administered every other day for 10–14 days). With relentlessly progressive neurologic impairment, IV pulse or oral daily cyclophosphamide therapy may be beneficial and does not seem to increase the risk of metastatic disease.29,30 Symptomatic therapies (fluphenazine, risperidone, and benzodiazepines) are sometimes helpful. One patient in this study received tetrabenazine without improvement. Another benefited from a pharmacologically similar approach, combined therapy with a dopamine-depleting drug, reserpine, and the dopamine D2 blocker, fluphenazine.
Autoimmunity should be considered as a potentially treatable cause of chorea. Comprehensive autoimmune serologic testing aids diagnosis, and an informative profile of neural autoantibodies may lead to successful treatment of an otherwise unsuspected cancer.
Supplementary Material
GLOSSARY
- AChR
acetylcholine receptor
- ANNA-1
antineuronal nuclear antibodies type 1
- CASPR2
contactin-associated protein-2
- GABA
γ-aminobutyric acid
- CRMP-5
collapsin response-mediator protein 5
- GAD65
glutamic acid decarboxylase 65-kDa isoform
- Ig
immunoglobulin
- LGI1
leucine-rich glioma-inactivated 1
- SLE
systemic lupus erythematosus
- VGCC
voltage-gated calcium channel
- VGKC
voltage-gated potassium channel
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. O’Toole: acquisition of data, analysis and interpretation of data, drafting the manuscript. Dr. Lennon: analysis and interpretation of data, revising the manuscript for content, contribution of vital reagents/tools. Dr. Ahlskog, Dr. Matsumoto, Dr. Pittock, Dr. Bower, Dr. Fealey, Dr. Lachance: acquisition of data, revising the manuscript for content. Dr. McKeon: study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis, drafting/revising the manuscript for content, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
O. O’Toole reports no disclosures. V. Lennon is a named inventor on a patent relating to AQP4 antibodies for diagnosis of NMO and receives royalties for this technology; is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and AQP4-IgG as a cancer marker; and receives research support from the NIH. J.E. Ahlskog and J. Matsumoto report no disclosures. S. Pittock is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and AQP4-IgG as a cancer marker and receives research support from Alexion Pharmaceuticals, Inc., the Guthy-Jackson Charitable Foundation, and the NIH. J. Bower, R. Fealey, and D. Lachance report no disclosures. A. McKeon receives research support from the Guthy-Jackson Charitable Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Vernino S, Tuite P, Adler CH, et al. Paraneoplastic chorea associated with CRMP-5 neuronal antibody and lung carcinoma. Ann Neurol 2002;51:625–630 [DOI] [PubMed] [Google Scholar]
- 2.Asherson RA, Harris NE, Gharavi AE, Hughes GR. Systemic lupus erythematosus, antiphospholipid antibodies, chorea, and oral contraceptives. Arthritis Rheum 1986;29:1535–1536 [DOI] [PubMed] [Google Scholar]
- 3.Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. JAMA 1992;268:2069–2073 [PubMed] [Google Scholar]
- 4.Lennon VA, Kryzer TJ, Griesmann GE, et al. Calcium-channel antibodies in the Lambert-Eaton syndrome and other paraneoplastic syndromes. N Engl J Med 1995;332:1467–1474 [DOI] [PubMed] [Google Scholar]
- 5.Thieben MJ, Lennon VA, Boeve BF, Aksamit AJ, Keegan M, Vernino S. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology 2004;62:1177–1182 [DOI] [PubMed] [Google Scholar]
- 6.McKeon A, Lennon VA, Lachance DH, Fealey RD, Pittock SJ. Ganglionic acetylcholine receptor autoantibody: oncological, neurological and serological accompaniments. Arch Neurol 2009;66:735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol 2001;49:146–154 [PubMed] [Google Scholar]
- 8.Pittock SJ, Lucchinetti CF, Parisi JE, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol 2005;58:96–107 [DOI] [PubMed] [Google Scholar]
- 9.Kokmen E, Ozekmekci FS, Beard CM, O'Brien PC, Kurland LT. Incidence and prevalence of Huntington's disease in Olmsted County, Minnesota (1950 through 1989). Arch Neurol 1994;51:696–698 [DOI] [PubMed] [Google Scholar]
- 10.Blekher T, Johnson SA, Marshall J, et al. Saccades in presymptomatic and early stages of Huntington disease. Neurology 2006;67:394–399 [DOI] [PubMed] [Google Scholar]
- 11.Walikonis JE, Lennon VA. Radioimmunoassay for glutamic acid decarboxylase (GAD65) autoantibodies as a diagnostic aid for stiff-man syndrome and a correlate of susceptibility to type 1 diabetes mellitus. Mayo Clin Proc 1998;73:1161–1166 [DOI] [PubMed] [Google Scholar]
- 12.Sheikh SN, Martin SB, Martin DL. Regional distribution and relative amounts of glutamate decarboxylase isoforms in rat and mouse brain. Neurochem Int 1999;35:73–80 [DOI] [PubMed] [Google Scholar]
- 13.Kumar P, Kalonia H, Kumar A. Huntington's disease: pathogenesis to animal models. Pharmacol Rep 2010;62:1–14 [DOI] [PubMed] [Google Scholar]
- 14.Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med 1990;322:1555–1560 [DOI] [PubMed] [Google Scholar]
- 15.Quek AM, Britton JW, McKeon A, et al. Autoimmune epilepsy: clinical characteristics and response to immunotherapy. Arch Neurol 2012;69:582–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittock SJ, Yoshikawa H, Ahlskog JE, et al. Glutamic acid decarboxylase autoimmunity with brainstem, extrapyramidal, and spinal cord dysfunction. Mayo Clin Proc 2006;81:1207–1214 [DOI] [PubMed] [Google Scholar]
- 17.Vigliani MC, Honnorat J, Antoine JC, et al. Chorea and related movement disorders of paraneoplastic origin: the PNS EuroNetwork experience. J Neurol 2011;258:2058–2068 [DOI] [PubMed] [Google Scholar]
- 18.Kujawa KA, Niemi VR, Tomasi MA, Mayer NW, Cochran E, Goetz CG. Ballistic-choreic movements as the presenting feature of renal cancer. Arch Neurol 2001;58:1133–1135 [DOI] [PubMed] [Google Scholar]
- 19.Schiff DE, Ortega JA. Chorea, eosinophilia, and lupus anticoagulant associated with acute lymphoblastic leukemia. Pediatr Neurol 1992;8:466–468 [DOI] [PubMed] [Google Scholar]
- 20.Pratley RE, Salbe AD, Ravussin E, Caviness JN. Higher sedentary energy expenditure in patients with Huntington's disease. Ann Neurol 2000;47:64–70 [PubMed] [Google Scholar]
- 21.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2002;2:862–871 [DOI] [PubMed] [Google Scholar]
- 22.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tofaris GK, Irani SR, Cheeran BJ, Baker IW, Cader ZM, Vincent A. Immunotherapy-responsive chorea as the presenting feature of LGI1-antibody encephalitis. Neurology 2012;79:195–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cervera R, Asherson RA, Font J, et al. Chorea in the antiphospholipid syndrome: clinical, radiologic, and immunologic characteristics of 50 patients from our clinics and the recent literature. Medicine 1997;76:203–212 [DOI] [PubMed] [Google Scholar]
- 26.Asherson RA, Derksen RH, Harris EN, et al. Chorea in systemic lupus erythematosus and “lupus-like” disease: association with antiphospholipid antibodies. Semin Arthritis Rheum 1987;16:253–259 [DOI] [PubMed] [Google Scholar]
- 27.Orzechowski NM, Wolanskyj AP, Ahlskog JE, Kumar N, Moder KG. Antiphospholipid antibody-associated chorea. J Rheumatol 2008;35:2165–2170 [DOI] [PubMed] [Google Scholar]
- 28.Samii A, Dahlen DD, Spence AM, Maronian NC, Kraus EE, Lennon VA. Paraneoplastic movement disorder in a patient with non-Hodgkin's lymphoma and CRMP-5 autoantibody. Mov Disord 2003;18:1556–1558 [DOI] [PubMed] [Google Scholar]
- 29.Colleoni M, Rocca A, Sandri MT, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol 2002;13:73–80 [DOI] [PubMed] [Google Scholar]
- 30.Vernino S, O'Neill BP, Marks RS, O'Fallon JR, Kimmel DW. Immunomodulatory treatment trial for paraneoplastic neurological disorders. Neuro Oncol 2004;6:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.