Abstract
Objective:
To evaluate the utility of rare cell capture technology (RCCT) in the diagnosis of leptomeningeal metastasis (LM) from solid tumors through identification of circulating tumor cells (CTCs) in the CSF.
Methods:
In this pilot study, CSF samples from 60 patients were analyzed. The main patient cohort consisted of 51 patients with solid tumors undergoing lumbar puncture for clinical suspicion of LM. Those patients underwent initial MRI evaluation and had CSF analyzed through conventional cytology and for the presence of CTCs using RCCT, based on immunomagnetic platform enrichment utilizing anti–epithelial cell adhesion molecule antibody-covered magnetic nanoparticles. An additional 9 patients with CSF pleocytosis but without solid tumors were separately analyzed to ensure accurate differentiation between CTCs and leukocytes.
Results:
Among the 51 patients with solid tumors, 15 patients fulfilled criteria for LM. CSF CTCs were found in 16 patients (median 20.7 CTCs/mL, range 0.13 to >150), achieving a sensitivity of 100% as compared with 66.7% for conventional cytology and 73.3% for MRI. One patient had a false-positive CSF CTC result (specificity = 97.2%); however, that patient eventually met LM criteria 6 months after the tap. CSF CTCs were not found in any of the additional 9 patients with CSF pleocytosis.
Conclusion:
RCCT is an accurate, novel method for the detection of LM in solid tumors, potentially providing earlier diagnostic confirmation and sparing patients from repeat lumbar punctures.
Leptomeningeal metastasis (LM) is a devastating complication of cancer, and is often considered in the differential diagnosis when patients with cancer present with new neurologic symptoms.1 However, confirming the diagnosis of LM can be difficult, particularly at early stages. The diagnosis is based on CSF cytologic analysis and/or MRI findings. Brain and spine MRIs have been increasingly preferred for the initial evaluation of LM because of their noninvasive nature and convenience to patients. However, MRI findings can be equivocal, and unequivocal findings may only appear in late-stage disease (figure 1). CSF cytopathologic analysis provides diagnostic confirmation of LM, but is associated with a relatively low sensitivity (approximately 50% on the first lumbar puncture) and is highly examiner-dependent; repeat lumbar punctures are often required, which may increase sensitivity up to 90% with 3 samples.2,3
Figure 1. Examples of MRI findings.
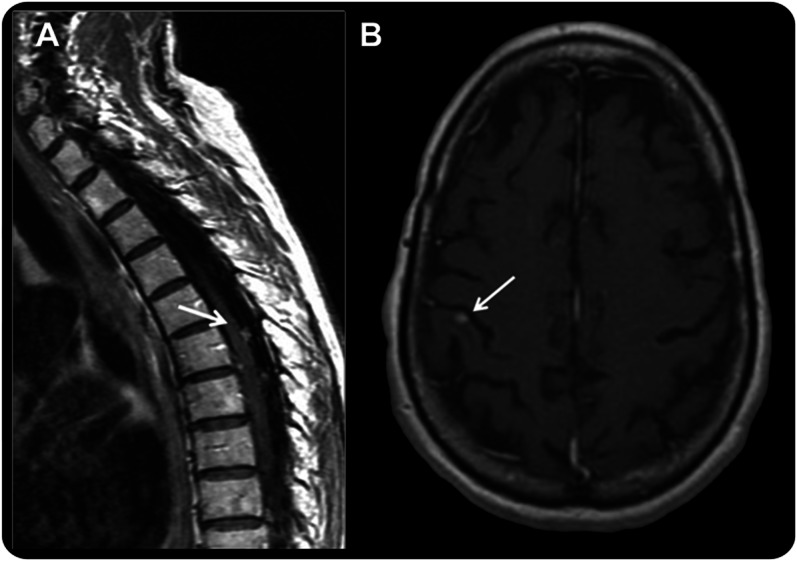
(A) Sagittal T1 postgadolinium image of the spine showing typical enhancing subarachnoid nodules considered in this study as unequivocal for the diagnosis of leptomeningeal metastasis (LM). (B) Axial T1 postgadolinium image of the brain in a patient with non–small cell lung cancer who developed progressive, mild gait ataxia and was found to have superficial, small, contrast-enhancing lesions (arrow). Given the superficial location of the lesions, the MRI was considered suspicious but not unequivocal for LM because the lesion seems intraparenchymal, posing a diagnostic problem. The CSF analysis showed 1 white cell/mm3 and negative cytology, but CSF circulating tumor cell (CTC) analysis was positive (0.13 CTC/mL). The lumbar puncture was repeated 3 weeks later, and both conventional cytology and CSF CTCs were positive, confirming the diagnosis of LM, as anticipated by the CSF CTC results.
Rare cell capture technology (RCCT) utilizing immunomagnetic platforms and antibody-covered ferroparticles has emerged as a new tool for capturing circulating tumor cells (CTCs) in the blood of patients with solid tumors. Analysis of peripheral blood CTCs has been explored as a prognostic marker of disease and response to anticancer treatments, particularly in prostate, colon, and breast cancers.4–6 Some studies have suggested that blood CTC enumeration may correlate with tumor burden and anticipates tumor progression. Moreover, blood CTCs have been used to characterize genetic and immunophenotypic changes over time, with the ultimate goal of guiding the management of targeted therapies.
Although several cell-surface antigens can be used to detect and isolate CTCs, the most frequently used marker is the epithelial cell adhesion molecule (EpCAM).7 EpCAM is a transmembrane glycoprotein found on the surface of epithelia, which is strongly expressed in various carcinomas, but that may also be found in other types of solid tumors. Anti-EpCAM–based RCCT (Veridex LLC, Warren, NJ) is an US Food and Drug Administration–approved methodology for capturing and enumerating blood CTCs in patients with solid tumors that is becoming widely available.8,9 We hypothesized that such methodology can be used to diagnose LM in solid tumors through the identification of CTCs in the CSF, and initiated a pilot study to evaluate the potential of this technology.
METHODS
In this study, we utilized RCCT for the evaluation of CSF samples from patients with solid tumors undergoing a lumbar puncture for a clinical suspicion of LM; results were compared with CSF standard cytopathologic analysis from that same sample and with initial MRI findings. Neuroimaging consisting of MRI of the brain or total spine (or both, as clinically indicated) was obtained in all patients. Patients that were receiving bevacizumab treatment were identified; bevacizumab is a vascular endothelial growth factor (VEGF) inhibitor that may reduce or eliminate contrast enhancement on MRI through a decrease in vascular permeability, potentially masking imaging findings of LM.10
After the MRI was obtained, patients underwent a lumbar puncture and standard CSF evaluation consisting of intracranial pressure measurement, CSF protein, glucose, white and red cell analysis, bacterial and fungal cultures, as well as conventional cytopathology analysis (cytocentrifuge). An additional CSF sample was obtained for evaluation of CSF CTCs (recommended amount: 7.5 mL).
A composite definition of LM was used as the gold standard for the purposes of evaluating the diagnostic performance of the first MRI, first conventional CSF cytology, and CTC enumeration on CSF obtained at the first lumbar puncture. Patients were considered to have a definitive diagnosis of LM if they had a positive CSF cytology or unequivocal MRI findings observed within 1 month of the initial evaluation; positive results found on repeat examinations obtained at the discretion of the treating physician within that timeframe were also used as confirmation of LM. The 1-month window was defined arbitrarily as the maximum timeframe during which definitive diagnosis of LM was considered relevant to the CSF CTC test. Unequivocal MRI findings were defined as leptomeningeal enhancement with subarachnoid nodules, enhancement in basal cisterns, or enhancement/clumping of nerve roots (figure 1A).11 Findings such as multiple superficial brain metastases, intraventricular masses, dural enhancement associated with epidural metastasis, or new hydrocephalus were considered suspicious but nondiagnostic (figure 1B). Patients were enrolled between November 2008 and February 2010. In a separate analysis, we also examined CSF samples from an additional 9 patients without solid tumors and increased CSF leukocytes (7 leptomeningeal lymphomatosis, 1 viral, and 1 fungal meningitis) to ensure that leukocytes were not misinterpreted as CSF CTCs. Because these 9 patients did not have solid tumors, they were not included in the calculation of sensitivity or specificity.
For the evaluation of CSF CTCs, the CellSearch CTC Kit (Veridex LLC, Warren, NJ) was used to enumerate CTCs of epithelial origin (CD45−, EpCAM+, and cytokeratins [CKs] 8, 18+, and/or 19+). The kit contains an EpCAM-bound antibody ferrofluid capture for immunomagnetic enrichment. After immunomagnetic capture, fluorescent reagents are added for identification and enumeration of CTCs. The fluorescent reagents include anti–CK-PE (phycoerythrin) specific for the intracellular protein CK, DAPI (4′,6-diamidino-2-phenylindole), which stains the cell nucleus, and anti–CD45-allophycocyanin specific for leukocytes. Thus, CTCs can be distinguished from contaminating leukocytes. The reagent/sample mixture is dispensed by the CellTracks AutoPrep System (Veridex) into a cartridge that is inserted into a MagNest cell presentation device. The strong magnetic field of the MagNest device attracts the magnetically labeled epithelial cells to the surface of the cartridge. The CellTracks Analyzer II automatically scans the entire surface of the cartridge, acquires images, and displays any event in which CK-PE and DAPI fluorescence are co-located. An event is classified as a tumor cell when its morphologic features are consistent with those of a tumor cell and it exhibits the phenotype EpCAM+, CK+, DAPI+, and CD45−.
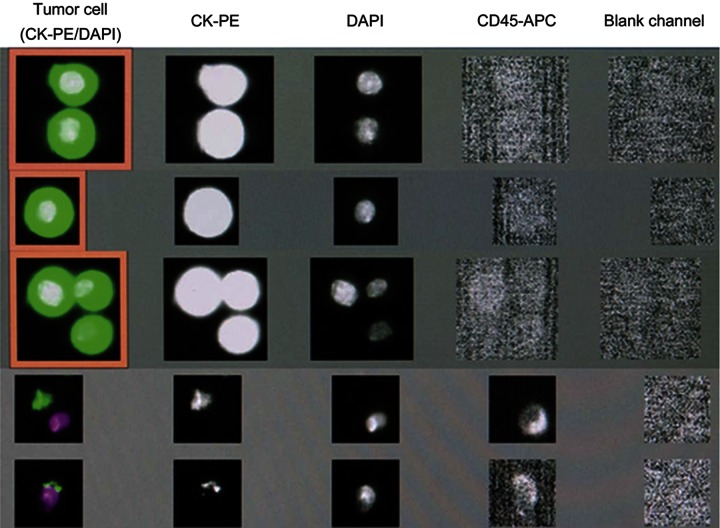
Enumeration of cells in the CSF samples was performed as follows. The samples were collected in the CellSave preservative tube in which they could be stored for up to 96 hours at room temperature before processing. No centrifugation was used. The CellTracks AutoPrep System software requires red blood colored specimens. To allow for analysis of a clear fluid sample such as the CSF, the control mode was used. A 3.5-mL sample was dispensed into a cartridge ready for analysis using the CellTracks Analyzer II. Once the sample was processed, the filled cartridge within the MagNest device was allowed to incubate in the dark for a minimum of 20 minutes and was scanned within 24 hours. As exemplified in figure 2, the images taken from the cartridge were reviewed and the observed objects were considered CTCs if they were CK-PE+, DAPI+ and CD45−. The results were reported as the number of CTCs/mL of CSF. CSF CTC analysis was considered to be positive if >0 EpCAM-expressing cells/mL were identified. The number of CTCs/mL was capped at 150 CTCs/mL. Patients with CTCs <5/mL had images and captured CTCs reviewed by a cytopathologist to confirm those corresponded to tumor cells and were comparable to the tumor cells visualized in the conventional cytology. Clinical follow-up information was obtained through chart review.
Figure 2. Screen view of CSF analysis utilizing the CellTracks Analyzer II system.
The first 3 rows represent examples of circulating tumor cells (CTCs). They are identified when all of the following criteria are met: the cell is positive for both the epithelial cell marker (CK-PE) and the nuclear dye (DAPI), negative for the leukocyte marker (CD45/APC), and negative in the blank channel. The cells depicted in the last 2 rows do not meet these criteria and therefore are not considered CTCs. CK-PE = cytokeratin-phycoerythrin (specific for intracellular cytokeratin); DAPI = 4′,6-diamidino-2-phenylindole (specific for cell nucleus); CD45-APC = allophycocyanin (specific for leukocytes).
Standard protocol approvals, registrations, and patient consents.
All patients authorized the use of their CSF specimens for this study and signed an Institutional Review Board–approved informed consent.
RESULTS
CSF samples from 60 patients were evaluated with RCCT. As described above, the main study cohort consisted of 51 patients with solid tumors with a clinical suspicion of LM; CSF samples from an additional 9 patients with elevated leukocytes but without solid tumors were analyzed as additional negative controls but were not included in the main cohort.
Among the 51 patients with solid tumors, the most common primary cancers were breast and non–small cell lung cancer (table 1). Concomitant chemotherapy at the time of study inclusion, defined as any anticancer agent utilized in the previous 6 weeks, consisted of bevacizumab-containing regimens in 11 patients, other chemotherapy regimens in 32, and no treatment in 8.
Table 1.
Distribution of the different types of solid tumors enrolled in the main study cohort (n = 51)
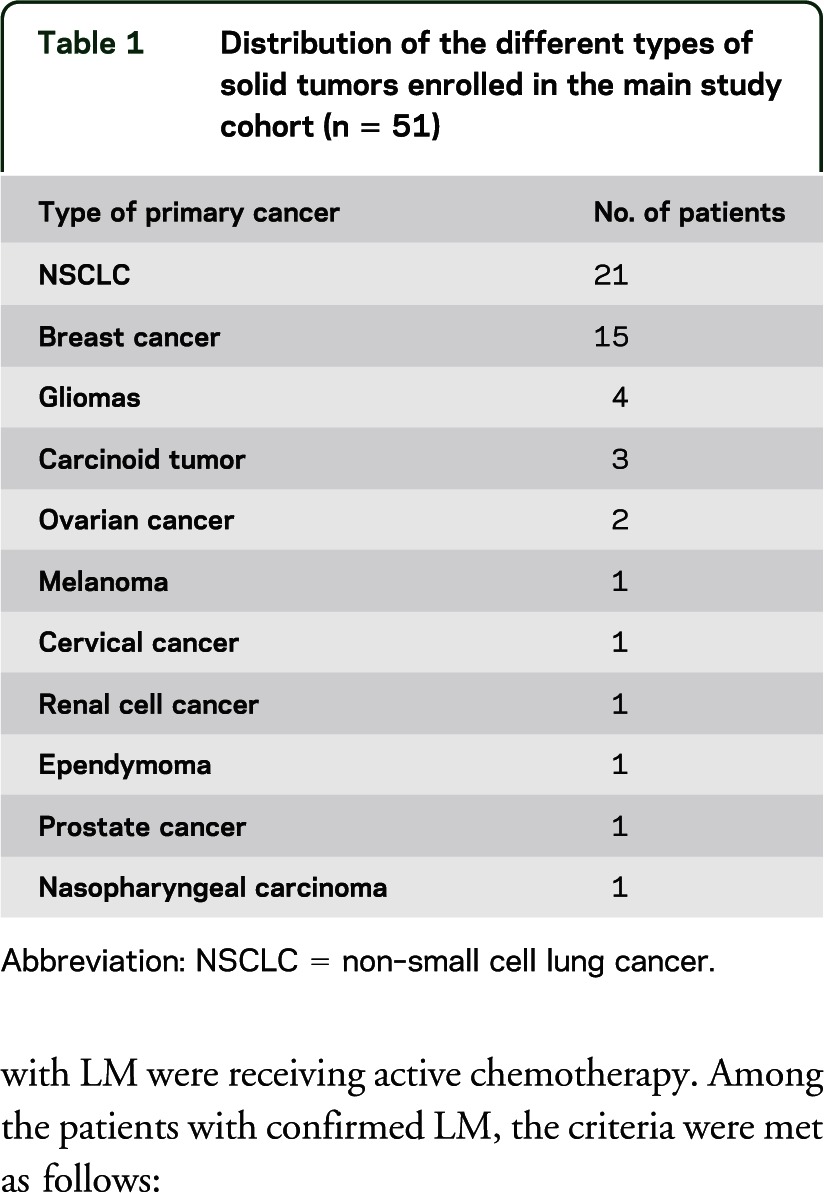
Among the 51 enrolled patients, 15 patients fulfilled criteria for LM, and LM was not present in the remaining 36 patients. Fourteen of these patients with LM were receiving active chemotherapy. Among the patients with confirmed LM, the criteria were met as follows:
At the time of initial lumbar puncture, conventional CSF cytology was positive for malignant cells in 10 patients and MRI demonstrated unequivocal findings of LM in 11; in 8 of these patients, both CSF cytology and MRI were positive for LM.
An additional 3 patients had positive CSF cytology on repeat lumbar puncture within 1 month of the initial procedure, 2 of whom had initial MRI findings suspicious but not unequivocal for LM. In these patients, both initial MRI and CSF cytology were nondiagnostic, and the diagnosis was only established with an additional lumbar puncture.
In the CSF analysis with RCCT at the time of the initial lumbar puncture, CTCs were absent in 35 patients and present in 16 patients (table 2), including all of the 15 patients who fulfilled LM criteria. The median number of CTCs detected was 20.27 CTCs/mL (range 0.13 to >150).
Table 2.
CSF and MRI findings in patients with positive CSF CTCs
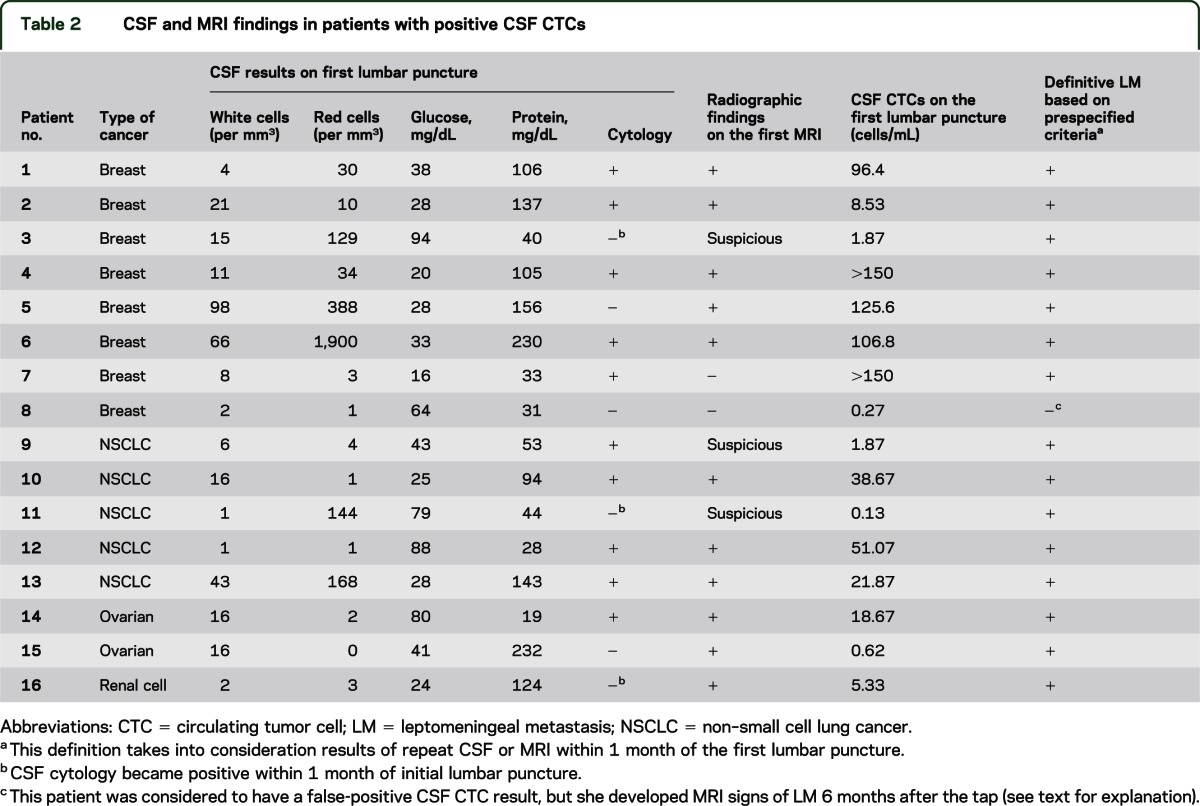
Table 3 summarizes the sensitivity and specificity of CSF CTCs as compared with conventional cytology and MRI. The sensitivity of the conventional cytology on the first lumbar puncture was 66.7% (95% confidence interval [CI], 38.3%–88.1%), the sensitivity of the first MRI was 73.3% (95% CI, 44.9%–92.2%), and the sensitivity of the CSF CTC analysis from the first lumbar puncture was 100% (95% CI, 78.1%–100%).
Table 3.
Results of initial conventional CSF cytology, MRI, and CSF CTC analyses
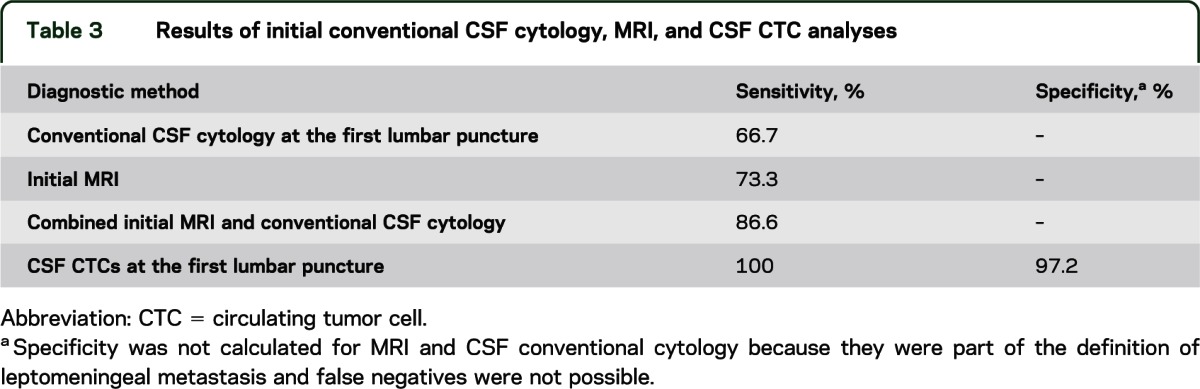
One patient with CSF CTCs did not meet criteria for LM. This patient had breast cancer with multiple superficial brain metastases and hydrocephalus and was found to have 0.27 CTCs/mL. The MRI was considered suspicious but nondiagnostic for LM, and the CSF cytology was negative. Therefore, the patient did not meet the prespecified criteria for LM and this was considered a false-positive CTC result for the purpose of this study. However, the patient was receiving paclitaxel treatment; bevacizumab was added immediately after the first lumbar puncture. Eventually, MRI evidence of LM developed 6 months after the initial tap. Considering this patient had a false-positive CSF CTC result, the specificity for the CSF CTCs on initial lumbar puncture was 97.2% (95% CI, 85.4%–99.9%). The positive predictive value of CSF CTCs was 93.8% (95% CI, 69.8%–99.8%) and the negative predictive value was 100% (95% CI, 90%–100%). Specificity and predictive values for the first MRI and conventional cytology at the first lumbar puncture were not calculated because they were both part of the gold standard definition, and false positives are therefore not possible.
One patient with glioblastoma, progressive gait ataxia, and hydrocephalus had MRI, CSF cytology, and CTCs that were negative for LM and was thus considered a true negative for the purpose of this study. However, this patient was being treated with bevacizumab and died 8 months later with no imaging evidence of LM, but autopsy showed brain and spinal cord LM.
Among the 9 additional patients with elevated CSF leukocytes and without solid tumors, no CSF CTCs were found.
DISCUSSION
In this study, we analyzed CSF samples from 60 patients utilizing immunomagnetic platform–based RCCT in order to establish the usefulness of this technology in the diagnosis of LM from solid tumors. We found that the detection of CSF CTCs was a robust diagnostic tool in a wide range of epithelial tumor types, outperforming standard diagnostic tools such as MRI and conventional CSF cytology analysis.
Despite advances in cancer management, the prognosis of LM from solid tumors has not changed in the past 30 years, with a median survival that remains 2 to 4 months.12–15 For poorly understood reasons, LM has been increasingly recognized in patients with solid tumors, not only in the setting of terminally ill patients, but also in patients with stable systemic disease and good performance status.16,17 Establishing the diagnosis of LM at earlier disease stages is crucial for the development of better therapies because treatments administered when a patient is already severely disabled are less likely to be effective or feasible.
Whereas the diagnosis of LM in hematologic malignancies has been facilitated by the use of CSF flow cytometry and PCR-based gene rearrangement studies, these techniques have not been useful in solid tumors. Alternatively, several studies have sought to identify protein-based CSF biomarkers able to accurately identify LM, particularly focusing on proteins involved in angiogenesis, tumor invasion, and cytokines.11,18,19 VEGF is the most studied biomarker for this indication.19–24 A recent study evaluating both CSF VEGF and stromal cell–derived factor-1 levels in 89 patients with breast cancer, lung cancer, and melanoma found high CSF VEGF levels in 67% to 75% of patients with positive CSF cytology and normal levels in 97% of patients with negative cytology; stromal cell–derived factor-1 levels were found to be less helpful.24 The sensitivity of CSF VEGF in various studies was 51% to 100%, with specificity up to 98%, although comparison with our results is difficult because most of those studies used cytology as a gold standard, itself a low-sensitivity examination. Several other proteins have also been investigated (table e-1 on the Neurology® Web site at www.neurology.org).2,11,19–25 However, as a whole, their development as CSF biomarkers has been difficult because each may be relevant to only specific tumor types, and CSF protein levels are influenced by serum protein levels. Serum proteins may penetrate the subarachnoid space at variable rates, particularly in the setting of breakdown of the blood-brain barrier. It must be noted that several neurologic diseases that are also part of the differential diagnosis in patients with cancer who present with neurologic symptoms, such as meningitis and inflammatory disorders, are associated with disruption of the blood-brain barrier, which may produce false-positive results. Therefore, correction formulas based on serum protein and albumin levels are required, seeking to differentiate local protein synthesis from passage through the blood-brain barrier, with variable results.19 Conversely, the CTC methodology is based on the direct identification of tumor cells, and therefore is not limited by such variations in the blood-brain barrier permeability. In the future, combining these different methodologies might be of interest, particularly in tumor-specific studies.
An inherent limitation of this and other studies seeking to establish diagnostic tools for LM is the lack of a gold standard, which ideally would correspond to autopsy examination in all patients performed close to the diagnostic procedure. Because this type of design is obviously not feasible, we chose to utilize the combination of both CSF cytology and MRI as the gold standard, as neither alone is sensitive enough to provide an accurate diagnosis. In a retrospective study evaluating a large series of patients with LM, MRI led to the diagnosis of LM in 53% of patients, and CSF cytology provided the diagnosis in 23%.13 In patients who underwent both MRI and CSF evaluation, discordance between the 2 techniques was frequent, with 37% of patients having a positive MRI and negative CSF, and 15% having a positive CSF and negative MRI, suggesting that both techniques are complementary. Other studies comparing CSF and MRI as diagnostic tools for LM have used various definitions of LM as a gold standard, and overall have found that MRI seems to perform better than CSF cytology in solid tumors, with a sensitivity ranging from 76% to 100%, vs 46% to 75% for CSF cytology.26–28 Although MRI is widely used in clinical practice, imaging findings are never truly unequivocal, given that other neurologic diseases may occasionally display similar findings, and mimic LM.
Another limitation in our study is the fact that some of the included patients were receiving bevacizumab treatment. Because of the decrease in vascular permeability induced by VEGF blockade, bevacizumab may decrease or eliminate abnormal contrast enhancement on the MRI, masking signs of LM.10 Our patient with breast cancer who had a false-positive CSF CTC result was receiving bevacizumab and developed LM 6 months later as documented on a follow-up MRI; it is difficult to determine whether LM was already present at the time of the lumbar puncture, in which case this would have been a true positive and the specificity 100%. Likewise, the patient with glioblastoma on bevacizumab who was eventually found to have LM on autopsy 8 months after presentation of hydrocephalus may have constituted a false-negative result. In fact, we were unable to enroll patients with confirmed LM from nonepithelial tumors, given that they are relatively rare, accounting for only 7% of patients with solid-tumor LM.13 Therefore, our results largely apply to tumors of epithelial origin, which affect 93% of patients in whom this diagnosis is made. Larger confirmatory studies enriched for nonepithelial primary tumors and tumors known to have a low expression of EpCAM such as gliomas, sarcomas, melanomas, and non–small cell lung cancer are warranted; adding alternative antibodies7 and exploring other RCCTs would be warranted. Another limitation is that blood CTCs were not collected at the time of the lumbar puncture. However, it is highly unlikely that contamination from blood CTCs during the lumbar puncture would account for the CTCs identified in the CSF, given the extremely low concentrations of CTCs in the blood. Moreover, given the high discriminative power of RCCT in distinguishing blood from tumor cells, it is unlikely that blood contamination would result in false-positive CTC results, which is in line with the high specificity found in this study.
We describe a new method for the diagnosis of LM in epithelial solid tumors that seems to achieve improved sensitivity when compared with conventional CSF cytology and MRI, with the potential of confirming the diagnosis of LM at earlier disease stages, and sparing patients from the discomfort of repeat lumbar punctures. The methodology is relatively simple, examiner independent, previously validated in the blood, widely available, and does not require additional equipment or software; collected samples may be shipped at room temperature for analysis at referral centers. Future studies include validation of CSF CTCs in larger cohorts, exploring their use as markers of treatment response and failure in the management of LM,29 and molecular characterization of LM through analysis of tumor-enriched samples obtained with the use of RCCT.30
Supplementary Material
ACKNOWLEDGMENT
The authors are thankful to the Memorial Sloan-Kettering Cancer Center Medical Oncology Department attending physicians who referred patients to this study, Dr. Jerome Posner for advice on the manuscript, and Judith Lampron for editorial assistance (organizing figure files and manuscript grammar and spelling proofing).
GLOSSARY
- CI
confidence interval
- CK
cytokeratin
- CTC
circulating tumor cell
- DAPI
4′,6-diamidino-2-phenylindole
- EpCAM
epithelial cell adhesion molecule
- LM
leptomeningeal metastasis
- PE
phycoerythrin
- RCCT
rare cell capture technology
- VEGF
vascular endothelial growth factor
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Conception and design: Lakshmi Nayak, Antonio Omuro. Data collection and assembly: Lakshmi Nayak, Martin Fleisher, Rita Gonzalez-Espinoza, Oscar Lin, Chhui-Mei Liu, Antonio Omuro. Data analysis and interpretation: Lakshmi Nayak, Katherine Panageas, Anne Reiner, Antonio Omuro. Manuscript writing: Lakshmi Nayak, Antonio Omuro. Final approval of manuscript: Lakshmi Nayak, Martin Fleisher, Rita Gonzalez-Espinoza, Oscar Lin, MD, Katherine Panageas, Anne Reiner, Chhui-Mei Liu, Lisa M. DeAngelis, Antonio Omuro.
STUDY FUNDING
This study was funded by the Memorial Sloan-Kettering Cancer Center Department of Neurology Research and Development Fund.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Taillibert S, Laigle-Donadey F, Chodkiewicz C, Sanson M, Hoang-Xuan K, Delattre JY. Leptomeningeal metastases from solid malignancy: a review. J Neurooncol 2005;75:85–99 [DOI] [PubMed] [Google Scholar]
- 2.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 1982;49:759–772 [DOI] [PubMed] [Google Scholar]
- 3.Glass JP, Melamed M, Chernik NL, Posner JB. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology 1979;29:1369–1375 [DOI] [PubMed] [Google Scholar]
- 4.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897–6904 [DOI] [PubMed] [Google Scholar]
- 5.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol 2009;10:233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781–791 [DOI] [PubMed] [Google Scholar]
- 7.Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol 2004;35:122–128 [DOI] [PubMed] [Google Scholar]
- 8.Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 2007;13:7053–7058 [DOI] [PubMed] [Google Scholar]
- 9.Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 2007;13:920–928 [DOI] [PubMed] [Google Scholar]
- 10.Kleinschmidt-DeMasters BK, Damek DM. The imaging and neuropathological effects of bevacizumab (Avastin) in patients with leptomeningeal carcinomatosis. J Neurooncol 2010;96:375–384 [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain MC, Glantz M, Groves MD, Wilson WH. Diagnostic tools for neoplastic meningitis: detecting disease, identifying patient risk, and determining benefit of treatment. Semin Oncol 2009;36:S35–S45 [DOI] [PubMed] [Google Scholar]
- 12.Herrlinger U, Forschler H, Kuker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci 2004;223:167–178 [DOI] [PubMed] [Google Scholar]
- 13.Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology 2010;74:1449–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waki F, Ando M, Takashima A, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol 2009;93:205–212 [DOI] [PubMed] [Google Scholar]
- 15.Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol 2012;7:382–385 [DOI] [PubMed] [Google Scholar]
- 16.Omuro AM, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 2005;103:2344–2348 [DOI] [PubMed] [Google Scholar]
- 17.Enting RH. Leptomeningeal neoplasia: epidemiology, clinical presentation, CSF analysis and diagnostic imaging. Cancer Treat Res 2005;125:17–30 [DOI] [PubMed] [Google Scholar]
- 18.Bach F, Bjerregaard B, Soletormos G, Bach FW, Horn T. Diagnostic value of cerebrospinal fluid cytology in comparison with tumor marker activity in central nervous system metastases secondary to breast cancer. Cancer 1993;72:2376–2382 [DOI] [PubMed] [Google Scholar]
- 19.Corsini E, Bernardi G, Gaviani P, et al. Intrathecal synthesis of tumor markers is a highly sensitive test in the diagnosis of leptomeningeal metastasis from solid cancers. Clin Chem Lab Med 2009;47:874–879 [DOI] [PubMed] [Google Scholar]
- 20.Stockhammer G, Poewe W, Burgstaller S, et al. Vascular endothelial growth factor in CSF: a biological marker for carcinomatous meningitis. Neurology 2000;54:1670–1676 [DOI] [PubMed] [Google Scholar]
- 21.Herrlinger U, Wiendl H, Renninger M, Forschler H, Dichgans J, Weller M. Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: diagnostic and prognostic value. Br J Cancer 2004;91:219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reijneveld JC, Brandsma D, Boogerd W, et al. CSF levels of angiogenesis-related proteins in patients with leptomeningeal metastases. Neurology 2005;65:1120–1122 [DOI] [PubMed] [Google Scholar]
- 23.van de Langerijt B, Gijtenbeek JM, de Reus HP, et al. CSF levels of growth factors and plasminogen activators in leptomeningeal metastases. Neurology 2006;67:114–119 [DOI] [PubMed] [Google Scholar]
- 24.Groves MD, Hess KR, Puduvalli VK, et al. Biomarkers of disease: cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J Neurooncol 2009;94:229–234 [DOI] [PubMed] [Google Scholar]
- 25.Brandsma D, Voest EE, de Jager W, et al. CSF protein profiling using Multiplex Immuno-assay: a potential new diagnostic tool for leptomeningeal metastases. J Neurol 2006;253:1177–1184 [DOI] [PubMed] [Google Scholar]
- 26.Straathof CS, de Bruin HG, Dippel DW, Vecht CJ. The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol 1999;246:810–814 [DOI] [PubMed] [Google Scholar]
- 27.Zeiser R, Burger JA, Bley TA, Windfuhr-Blum M, Schulte-Monting J, Behringer DM. Clinical follow-up indicates differential accuracy of magnetic resonance imaging and immunocytology of the cerebral spinal fluid for the diagnosis of neoplastic meningitis: a single centre experience. Br J Haematol 2004;124:762–768 [DOI] [PubMed] [Google Scholar]
- 28.Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol 1995;38:51–57 [DOI] [PubMed] [Google Scholar]
- 29.Patel AS, Allen JE, Dicker DT, et al. Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget 2011;2:752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev 2010;20:96–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.