Abstract
Objectives:
Our objectives were to 1) determine whether first-trimester use of gabapentin is associated with an increased risk for major malformations; 2) examine rates of spontaneous abortions, therapeutic abortions, stillbirths, mean birth weight and gestational age at delivery; and 3) examine rates of poor neonatal adaptation syndrome following late pregnancy exposure.
Methods:
The study design was prospective. Women were included who initially contacted the services between 5 and 8 weeks with a comparison group of women exposed to nonteratogens, collected in a similar manner.
Results:
We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. Among infants who were exposed to gabapentin up until delivery, 23 of 61 (38%) were admitted to either the neonatal intensive care unit or special care nursery for observation and/or treatment, vs 6 of 201 (2.9%) live births in the comparison group (p < 0.001). There were 2 cases of possible poor neonatal adaptation syndrome in neonates exposed to gabapentin close to delivery, compared with none in the comparison group, although it must be noted that these infants were concomitantly exposed to other psychotropic drugs. Among the women who took gabapentin, the major indications were pain (n = 90; 43%) and epilepsy (n = 71; 34%); the remainder were for other indications, mostly psychiatric.
Conclusion:
Our results suggest that although this sample size is not large enough to make any definitive conclusions, and there was no comparator group treated with other antiepileptic drugs, gabapentin use in pregnancy does not appear to increase the risk for major malformations. This finding and the increased risk for low birth weight and preterm birth require further investigation.
Gabapentin (Neurontin; Pfizer Canada Inc., Kirkland, QC) is an antiepileptic drug designed to treat partial seizures and is a γ-aminobutyric acid analog that differs both structurally and pharmacologically from other classes of antiepileptic drugs.1
The drug was approved by the US Food and Drug Administration for use in epilepsy in 1993 and subsequently for neuropathic pain in 2002.2 However, despite the increasing number of patients receiving gabapentin, there is only limited information regarding the safety of this medication when used during pregnancy.
A study from the European Gabapentin Registry included prospective and retrospective data with a total of 51 outcomes and 44 live births of women with epilepsy and other disorders exposed to gabapentin during pregnancy. The researchers reported 2 major malformations in infants exposed to gabapentin in the first trimester of pregnancy.3 In another group of 7 women with hyperemesis gravidarum, 2 congenital defects were reported.4 A cohort study in Denmark reported on 59 fetuses exposed to gabapentin during pregnancy, and documented 1 major malformation and 6 spontaneous abortions.5 Recently, the North American antiepileptic drug pregnancy registry reported on 145 fetuses (monotherapy), and documented 1 major malformation with no information on other outcomes.6
Considering the increasing number of pregnant women who may be taking gabapentin for other conditions during pregnancy, such as restless legs syndrome,7 and paucity of information regarding the safety of this medication in pregnancy, more information regarding fetal safety is required.
The objectives of our study were 3-fold: 1) to determine whether gabapentin exposure during pregnancy increases the rate of major malformations above the baseline population rate of 1% to 3%; 2) to examine the rates of stillbirths, spontaneous abortions, therapeutic abortions, gestational age at birth, and mean birth weight in exposed infants; and 3) to determine whether neonates experienced poor neonatal adaptation syndrome, which includes symptoms such as jitteriness, tachycardia, hypothermia, vomiting, hypoglycemia, irritability, hypertonia, eating/sleeping difficulties, convulsion, and respiratory stress.
METHODS
Data for this research were obtained from teratogen information services (TISs) as well as a pharmacovigilance center in several countries, which included Toronto (Canada), London (Canada), Lyon (France), Newcastle-Upon-Tyne (England), Florence (Italy), and Seoul (Korea). TISs provide evidence-based information regarding the safety and/or risks associated with exposure to drugs for pregnant and lactating women and their health care providers. French data were collected by one of the European TIS members from several French pharmacovigilance centers that use procedures similar to those of TISs, although requests are received mostly from physicians. The United Kingdom TIS does not currently routinely collect data from women, as it is their health provider who makes the initial inquiry. However, the same data are collected in the same manner in all centers, be it by a physician or an information specialist at a TIS. The services are run by various sources, such as universities, hospitals, and other academic centers.
During the initial contact, which was early on in pregnancy, most frequently between 5 and 8 weeks of pregnancy, demographics, medical and obstetrical histories, as well as details of exposure and concurrent exposures were recorded on a standardized questionnaire. Shortly after birth to approximately 2 to 3 months after delivery at most services, researchers contacted women who had taken gabapentin and received oral and/or written consent to complete the follow-up pregnancy outcome questionnaire. Outcomes of interest included live birth, spontaneous or therapeutic abortion, ectopic pregnancy, stillbirth, presence or absence of major malformation, defined as structural anomalies in the offspring that have serious medical effects or require surgery (genetic and cytogenetic anomalies were excluded), birth weight, gestational age at delivery, and presence or absence of neonatal distress in the newborn period up to 2 weeks postnatal. At some of the programs, but not all, following completion of the questionnaire, a letter was sent to the infant's physician asking for verification of the information obtained from the mother regarding the baby's health.
Each woman was compared with another woman who contacted the same TIS or pharmacovigilance center with exposure to a nonteratogenic substance, for example, acetaminophen or antibiotics. They were matched for maternal age (±2 years), alcohol consumption, and smoking, as well as for gestational age at time of initial contact (±2 weeks). The latter is critical when calculating the incidence of spontaneous abortion, because the observed proportion of pregnancies ending in loss is highly dependent on the gestational age at which pregnancies are recognized and how the losses are identified.
Statistical analysis.
Maternal characteristics and pregnancy outcomes measured on a continuous scale were compared using unpaired Student t tests. Categorical variables were contrasted using χ2 tests. The p values ≤0.05 were considered statistically significant.
Standard protocol approvals, registrations, and patient consents.
Researchers, with the exception of the United Kingdom, contacted women who had taken gabapentin and obtained oral and/or written consent. This study was approved by the Research Ethics Board at The Hospital for Sick Children in Toronto, Canada, and at local research ethic boards at the other centers. In the United Kingdom, data collection is covered by Section 251 of the NHS Act, 2006.
RESULTS
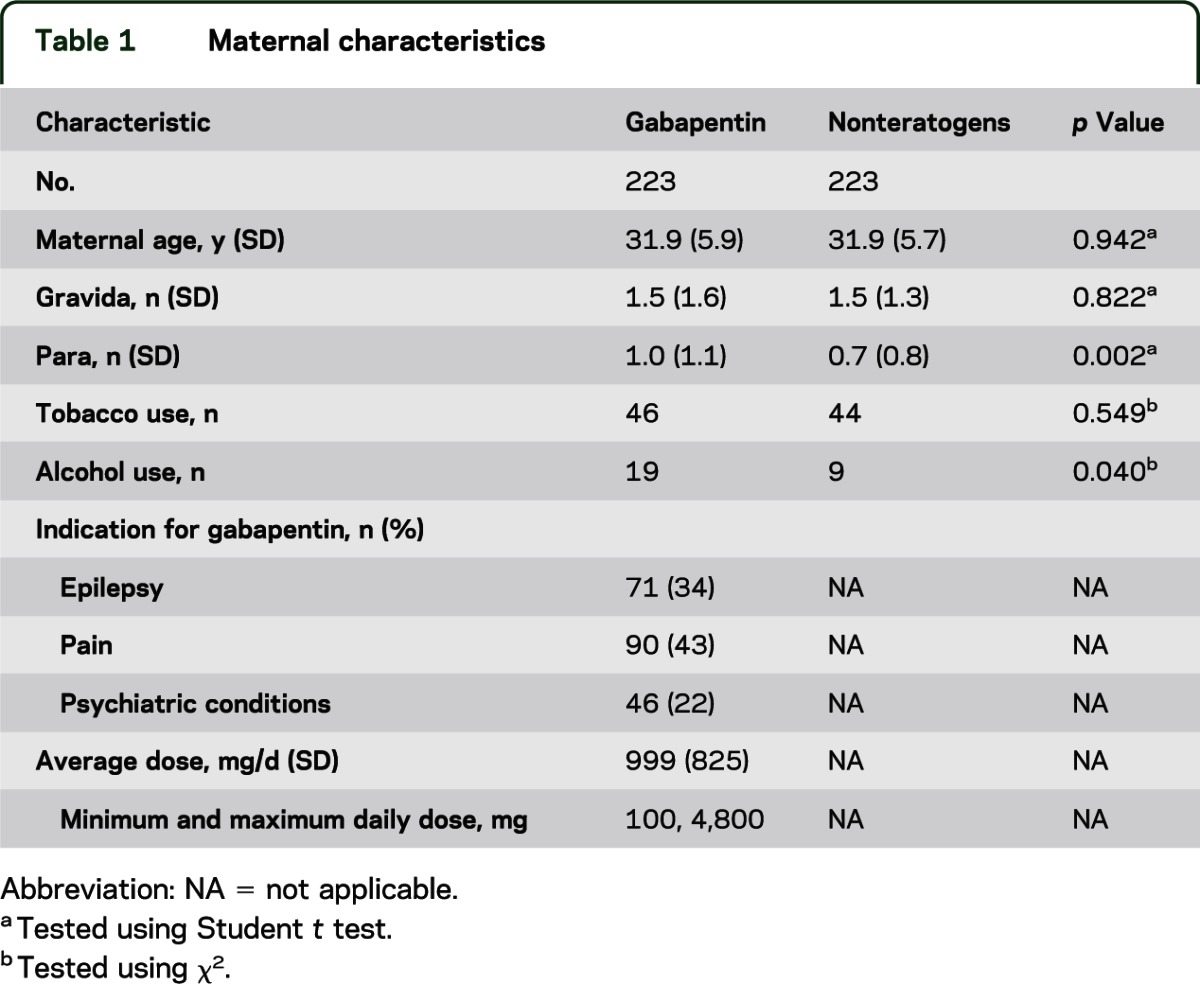
We completed data on the outcomes of 223 pregnancies exposed to gabapentin and compared them with 223 unexposed pregnancies. The maternal demographics were very similar on all characteristics, with the exception of the gabapentin group having significantly more women who consumed alcohol. However, the use of alcohol was minimal during pregnancy (mostly an occasional drink, or only prior to the woman finding out that she was pregnant) and was not associated with any adverse outcomes. Among the 223 women exposed to gabapentin, 207 (92.8%) reported the indication for use, and the major indication was pain (90, 43%); only 71 (34%) took it for the treatment of epilepsy. The psychiatric indications included 11% depression, 4% panic attacks/anxiety, 4% bipolar illness/psychosis, 2% obsessive-compulsive disorder, and 2% anorexia.
There were 182 women who reported dose and 173 who reported both dose and indication. Overall, the average dose was 1,000 mg (SD = 825 mg), with a range of 100 to 4,800 mg/d. The average dose among those taking it for epilepsy (n = 58) was 1,538 mg/d, 853 mg/d for pain (n = 73), and 538 mg/d for other indications (n = 42) (table 1).
Table 1.
Maternal characteristics
The pregnancy outcomes are presented in table 2. Among the mothers of children with major malformations, in 3 of 7, the indication was epilepsy (average dose = 1,300 mg/d in 2 women; 1 dose not reported), and 4 took it for pain (average dose = 1,500 mg/d).
Table 2.
Pregnancy outcomes
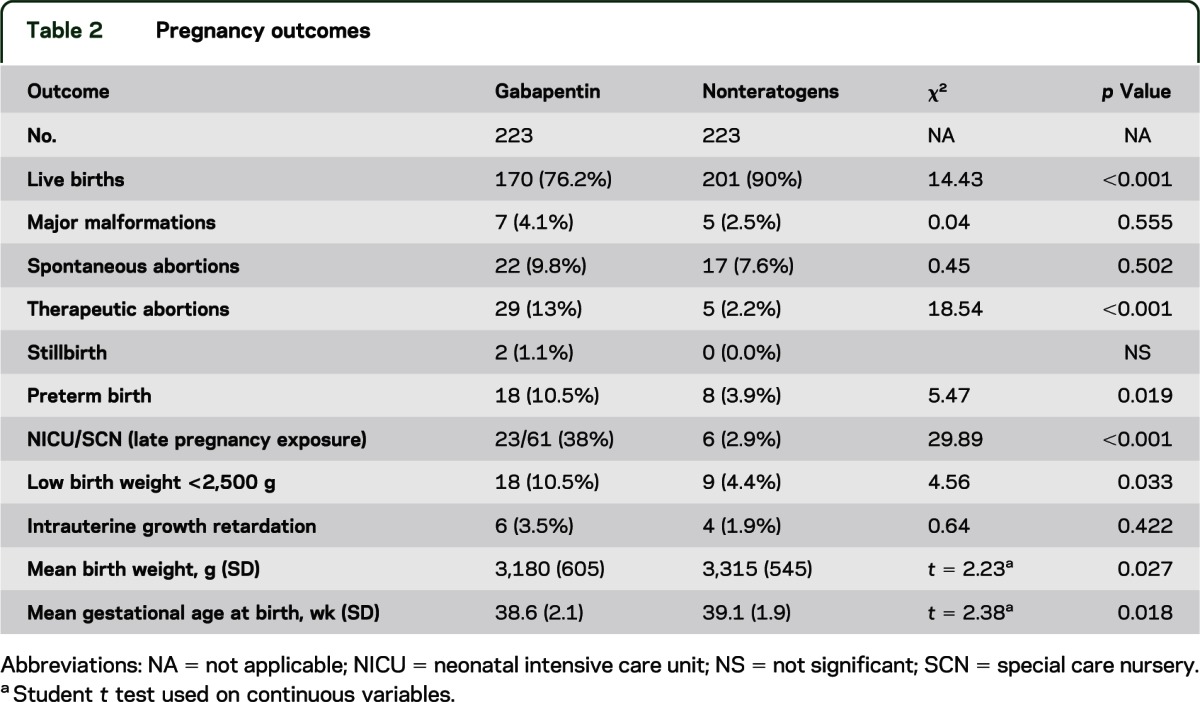
Rates of major malformations were similar in both groups, and in addition, none of the 36 women exposed only to gabapentin with no concomitant medications delivered a baby with a major malformation. The groups differed in rates of live births, therapeutic abortions, preterm births, low birth weight, and neonatal intensive care unit (NICU)/special care nursery (SCN). However, reported NICU/SCN admission rates included all exposed infants, regardless of time of exposure to gabapentin; consequently, some would have occurred long after maternal discontinuation of the drug. Of the 61 infants exposed up until delivery, 23 were admitted to either the NICU or SCN for observation and/or treatment vs 6 in the comparison group. The indications for admission included jaundice, low heart rate, hypotonia, hypoglycemia, respiratory distress, jitteriness, diarrhea, fever, and arrhythmia. All of these adverse events were self-limiting and resolved within a few days to a week. One infant was admitted for seizures in addition to respiratory distress syndrome, jaundice, seizures, and septicemia and was concomitantly exposed to trazodone, venlafaxine, eletriptan, and dimenhydrinate. Two of the neonates were described as having withdrawal symptoms. However, one of these infants was concomitantly exposed to vigabatrin, carbamazepine, and clobazam up until birth and the other was exposed to methadone throughout pregnancy. The symptoms were self-limiting and resolved within a few days to a week.
Birth weight and preterm birth.
There were 18 infants with a low birth weight of <2,500 g in the gabapentin group and 9 in the comparison group. Among those with a birth weight <2,500 g, the average gestational age at birth was 35 weeks (range 29–40) in the gabapentin group compared with 35.1 weeks (29–40) in the comparison group (p = 0.95).
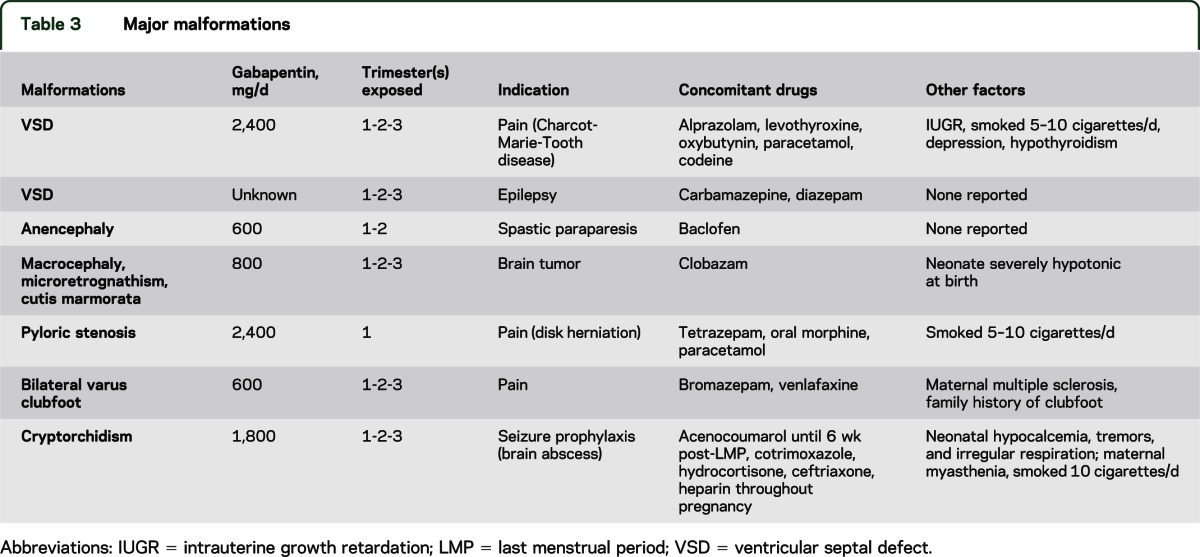
Table 3 lists the details of the malformations identified in 7 infants exposed to gabapentin, including doses taken, concomitant medications, and other factors possibly exerting an influence on outcomes. In the comparison unexposed group, 5 infants had malformations, which included 2 with ventricular septal defects, a dysplastic kidney (identified as requiring a transplant), bladder exstrophy, and bilateral hexadactyly plus bilateral colomba (in both optic nerves). Of the 223 women exposed to gabapentin, 142 were exposed in the first trimester only and had been taking it before becoming pregnant, 10 in the first and second trimesters only, 1 in the second trimester only, 1 in the third trimester only, 1 in the second and third trimesters only, and 59 before and throughout pregnancy. We did not have details of trimester exposure for the 9 remaining cases; however, none of these infants were noted as having a major malformation or any other adverse outcome.
Table 3.
Major malformations
DISCUSSION
To our knowledge, this is the largest prospective comparative study to date reporting on a number of pregnancy and neonatal outcomes after exposure to gabapentin during pregnancy. However, this is a small number when considering the possibility that there are probably thousands of women of childbearing age taking this drug worldwide.
There was no increased risk for major malformations, which is consistent with data from previous studies.3–6 There were 4 significant outcomes, one of which was live births, which correlated to the large number of therapeutic abortions in the gabapentin group compared with the comparison group. There were also higher rates of preterm births (p = 0.019) and low birth weight (p = 0.033) and this may have been attributable to a correlation between these 2 variables. An infant born weighing <2,500 g is considered low birth weight, and if the birth occurred before 37 weeks’ gestation, preterm birth. Both outcomes involve an increased risk of morbidity and mortality of the newborn. A low-birth-weight infant can be born full term, and a preterm infant may not necessarily be low birth weight. A measure used to combine these aspects is intrauterine growth retardation, known as “small for gestational age” and is a baby whose birth weight is below the 10th percentile, based on birth weight reference curves and stratified by infant sex and gestational age.8 There was no difference in the rates of small for gestational age in our cohort.
The other significant outcome was the number of admissions to the NICU/SCN, which included 23 of 61 (38%) neonates exposed to gabapentin in late pregnancy. However, not all of these neonates presented with symptoms, as some were admitted for observation, which is the policy for infants who have been exposed to psychotropic drugs throughout pregnancy in some institutions (anecdotal information). There were 2 cases in which it was noted that the infant had “withdrawal symptoms.” However, one of these infants was concomitantly exposed to vigabatrin, carbamazepine, and clobazam up until birth and the other one was concomitantly exposed to methadone throughout pregnancy. There are several case reports in the literature in adults suggesting that gabapentin withdrawal can occur at doses ranging from 400 to 8,000 mg/d. Patients experienced symptoms similar to those that develop with benzodiazepine withdrawal and were taking gabapentin for as little as 3 weeks to as long as 5 years.9 There is only 1 small study of 6 women who took gabapentin throughout pregnancy that reported neonatal outcomes. The authors suggested that there is probably an active transplacental transport of gabapentin, with accumulation in the fetus, which could be by the specific l-type amino acid transporter and is expressed in the placenta. The newborns appeared to have a slightly lower capacity to eliminate gabapentin than do adults. However, there were no reports of any adverse effects in these 6 neonates.10 In our study, the 2 infants were also exposed to other psychotropic drugs throughout pregnancy; consequently, it would be difficult to ascertain whether their symptoms were due to the gabapentin, the other drugs, or a combination of both.
As in all observational studies, there are strengths and limitations. The strengths of this type of study include a personal interview with the majority of the women, which involved detailed history-taking and included documentation of consumption of the drug during pregnancy. In addition, in many cases, details were verified with the child's physician. Using prospective comparative groups is considered Class II evidence because it allows comparisons between exposed and nonexposed groups. Because randomized controlled trials are unlikely to be conducted in pregnancy, this level of evidence is likely to be the highest available to physicians caring for women who require gabapentin pharmacotherapy during pregnancy. There were 2 main limitations that should be mentioned. First, we had a relatively small sample size, which has only 80% power to detect approximately a 3.5-fold increased risk for malformations above the baseline risk of 3%, with an α of 0.05. Typically, approximately 750 subjects in each group would be required to detect a 2-fold increase in major malformations and thousands would be required to detect rare malformations. The second limitation was that we did not have a comparative group of women who were treated for similar conditions with other medications (i.e., disease group) and therefore only had 1 comparison group of women who were unexposed to gabapentin.
Of interest was the low number of women (only one-third) taking gabapentin for epilepsy, and that number differed among countries. France had the largest number (61%), then the United Kingdom (28%), with the remaining countries ranging from 0% to 8%.
In our study, only 28% of the women continued taking gabapentin throughout pregnancy as two-thirds of the women (66%) discontinued in the first trimester, most following pregnancy confirmation between 6 and 8 weeks’ gestation. This number of women discontinuing their medication early in pregnancy has remained consistent for many years, despite reassuring results from many studies that have been published on pregnancy outcomes following exposure to various medications. This is most likely attributable to alarming information received from various sources. At Motherisk, we recently conducted a study in which we evaluated the impact of negative information from friends, family, health care providers, and the media on women who had taken an antidepressant during pregnancy. Most of the women reported that negative information they received from these sources affected their decision-making as to whether they continued taking their medication during pregnancy.11 Finally, there are no studies that have been conducted to examine possible long-term neurodevelopmental adverse effects of taking gabapentin in pregnancy and we did not attempt this in our study, as it requires more resources than we had available to us.
Our results suggest that although this sample size is not large enough to make a definitive conclusion, gabapentin does not appear to increase the rate of major malformations above baseline. The other significant findings require further investigation before coming to any definitive conclusions. Infants exposed to gabapentin close to delivery, especially if concomitantly exposed to other psychotropics, should be monitored after birth for poor neonatal adaptation syndrome.
ACKNOWLEDGMENT
The participation of several French pharmacovigilance centers (in particular Dijon and Tours) is warmly acknowledged. Thanks to all of the women and/or their health care providers, without whom this study would not have been possible.
GLOSSARY
- NICU
neonatal intensive care unit
- SCN
special care nursery
- TIS
teratogen information service
AUTHOR CONTRIBUTIONS
Dr. Fujii, MD: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Mr. Goel, BSc: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Bernard, PhD, Dr. Pistelli, MD, Dr. Yates, MBCHB, Dr. Stephens, PhD, Dr. Han, MD, Dr. Matsui, MD: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Ms. Etwell, BSc: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Einarson, PhD, Dr. Koren, MD: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content. Ms. Einarson, RN: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
H. Fujii, A. Goel, N. Bernard, A. Pistelli, L.M. Yates, S. Stephens, J.Y. Han, D. Matsui, F. Etwell, T.R. Einarson, and G. Koren report no disclosures. A. Einarson for The Motherisk Program received an unrestricted educational grant to study the safety of Cymbalta (duloxetine) in pregnancy from Eli Lilly Inc., Canada. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Neurontin® Product Monograph Kirkland, QC: Pfizer Canada Inc.; October 25, 2011. [Google Scholar]
- 2.http://search.centerwatch.com/default.aspx?SearchQuery=gabapentin CenterWatch. Search results for gabapentin. Available at: Accessed December 15, 2012.
- 3.Montouris G. Gabapentin exposure in human pregnancy: results from the Gabapentin Pregnancy Registry. Epilepsy Behav 2003;4:310–317 [DOI] [PubMed] [Google Scholar]
- 4.Guttuso T, Jr, Robinson LK, Amankwah KS. Gabapentin use in hyperemesis gravidarum: a pilot study. Early Hum Dev 2010;86:65–66 [DOI] [PubMed] [Google Scholar]
- 5.Mølgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA 2011;305:1996–2002 [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Díaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78:1692–1699 [DOI] [PubMed] [Google Scholar]
- 7.Djokanovic N, Garcia-Bournissen F, Koren G. Medications for restless legs syndrome in pregnancy. J Obstet Gynaecol Can 2008;30:505–507 [DOI] [PubMed] [Google Scholar]
- 8.Wilcox AJ. On the importance—and the unimportance—of birthweight. Int J Epidemiol 2001;30:1233–1241 [DOI] [PubMed] [Google Scholar]
- 9.See S, Hendriks E, Hsiung L. Akathisia induced by gabapentin withdrawal. Ann Pharmacother 2011;45:e31. [DOI] [PubMed] [Google Scholar]
- 10.Ohman I, Vitols S, Tomson T. Pharmacokinetics of gabapentin during delivery, in the neonatal period, and lactation: does a fetal accumulation occur during pregnancy? Epilepsia 2005;46:1621–1624 [DOI] [PubMed] [Google Scholar]
- 11.Mulder E, Davis A, Gawley L, Bowen A, Einarson A. Negative impact of non-evidence-based information received by women taking antidepressants during pregnancy from health care providers and others. J Obstet Gynaecol Can 2012;34:66–71 [DOI] [PubMed] [Google Scholar]


