Abstract
Objective:
To assess differences in gray matter (GM) atrophy between 2 Parkinson disease (PD) subtypes: the tremor dominant (TD) subtype and the postural instability gait difficulty (PIGD) subtype.
Methods:
Patients were classified as belonging to the predominately PIGD (n = 30) or predominately TD (n = 29) subtype. Voxel-based morphometry was used to compare GM in these 2 subtypes and to evaluate correlations between predefined regions of interest and the degree of symptoms. In the regions where GM atrophy was associated with symptoms, the relationship between GM volumes and functional connectivity was examined.
Results:
GM was reduced in the predominately PIGD group, compared with the predominately TD group, in areas that involve motor, cognitive, limbic, and associative functions (p < 0.05, false discovery rate corrected). Lower GM volumes in the pre–supplementary motor area (SMA) and in the primary motor area were associated with increased severity of PIGD symptoms (r = −0.42, p < 0.001; r = −0.38, p < 0.003, respectively). Higher GM volumes within the pre-SMA were associated with stronger functional connectivity between the pre-SMA and the putamen (r = 0.415, p < 0.025) in the patients with predominately PIGD.
Conclusions:
In patients with PD, PIGD symptoms are apparently associated with GM atrophy in motor-related regions and decreased functional connectivity. GM degeneration and a related decrease in spontaneous coactivation between cortical and subcortical motor-planning areas may partially account for the unique clinical characteristics of a subset of patients with PD.
Patients with Parkinson disease (PD) can be classified into the tremor dominant (TD) subtype or the postural instability gait difficulty (PIGD) subtype.1 These 2 subtypes differ in their motor and cognitive symptoms. In addition to the marked changes in gait, patients with PIGD have an increased risk for developing cognitive deterioration2,3 and dementia.4,5 The neural basis for these disparate manifestations of PD is lacking.
Gray matter (GM) atrophy may underlie the emergence of the 2 motor subtypes as it relates to both cognitive decline and gait changes. Such atrophy has been related to normal aging,6–8 mild cognitive impairment,9 Alzheimer disease,10 and lower gait speed.11 GM atrophy was increased in one study of patients with PD.12 Visual assessment of GM changes did not detect differences between the PD subtypes13; however, this methodologic approach may not have been sufficiently sensitive to detect subtle GM changes.
In the present study, 3 complementary MRI methods were applied to evaluate GM differences between the PD subtypes. We speculated that GM atrophy would be more extensive in the PIGD group because these patients usually have greater cognitive and motor impairments. First, a whole-brain comparison was applied using voxel-based morphometry (VBM). Second, the relationship between GM atrophy and clinical symptoms was evaluated in specific regions of interest (ROIs) that were found to be related to PD.12,14–16 Finally, in regions that showed such a relationship, the association between GM atrophy and functional connectivity within the motor network was examined.
METHODS
Study participants.
One hundred ten patients with idiopathic PD were recruited for this study. Further details of the methods used are described in appendix e-1 on the Neurology® Web site at www.neurology.org.
Standard protocol approval, registration, and patient consent.
Patients with idiopathic PD were recruited from the outpatient Movement Disorders Unit at the Tel-Aviv Sourasky Medical Center and from other affiliated clinics. All subjects provided informed written consent before participating in the study, as approved by the Human Research Ethics Committee of Tel Aviv Sourasky Medical Center.
Protocol outline.
Patients were studied on 2 separate occasions. The first visit included a neurologic and clinical examination; on a separate visit that took place within 2 weeks of the first visit, the participants underwent MRI testing in the “on” medication state. MRI testing took approximately 1.5 hours.
Clinical evaluation.
Patients underwent a clinical assessment that included the Unified Parkinson's Disease Rating Scale (UPDRS). Symptoms were quantified using PIGD and TD scores. Balance, postural control, and gait speed were also evaluated. A computerized cognitive battery was used to compute an executive function index and a global cognitive score. For further details, see appendix e-1. A daily levodopa equivalent dosage was calculated for each patient, using a method previously described.17
All statistical analyses were 2-sided and conducted using the Statistical Package for Social Sciences (version 17 for Windows; SPSS Inc., Chicago, IL). Clinical and demographic differences between the 2 groups were evaluated using the Student t test for continuous variables, whereas χ2 tests were used for categorical variables.
Classification into PIGD and TD subtypes.
Of the 110 patients that were recruited for this study, MRI scans were not completed in 4 subjects (e.g., inability to lie down for an extended period of time). The remaining 106 patients were classified into PIGD or TD groups based on the ratio between the PIGD and tremor symptoms.1 Briefly, patients were classified as PIGD when the ratio of the mean tremor score divided by mean PIGD score was ≤1, whereas patients with a ratio of ≥1.5 were assigned to the TD group. When the ratio was >1 and <1.5, patients were classified as undetermined. After performing this ratio-based classification, 60 patients were classified as PIGD (mean age: 64.74 ± 8.03 years) and 37 patients were classified as TD (mean age: 65.98 ± 11.04 years). However, a large number of patients still showed mixed PD symptoms (see figure e-1). Because we aimed to identify the neural correlates that differentiate between the 2 subtypes, we applied a more strict exclusion criterion. Patients were excluded from the TD group if they had a PIGD score >3 or a tremor score <4. Patients were excluded from the PIGD group if their PIGD score was <4 or their tremor score was >3. Based on this classification into groups that best represented the 2 subtypes, with minimal overlap across symptom classes, 30 patients of predominately PIGD (p-PIGD) subtype (12 females, mean age: 64.95 ± 7.71 years) and 29 patients of predominately TD (p-TD) subtype (6 females, mean age: 64.64 ± 11.6 years) were identified.
MRI acquisition.
All of the MRIs were acquired on a 3.0-tesla (GE) scanner using an 8-channel head coil. For further details, see appendix e-1.
VBM data analysis.
For each group, the percentage of voxels classified as GM was computed. In addition, a whole-brain GM volumes comparison between the p-PIGD and p-TD subtypes was applied (see appendix e-1). The false discovery rate (FDR) (p < 0.05) correction for multiple comparisons was used.18 This whole-brain approach aimed to provide a comprehensive assessment of brain differences in GM between the groups. To understand the relationships between atrophy of specific brain areas and performance, correlations between GM volumes in predefined ROIs and clinical measurements were assessed as well. Correlations were evaluated using bivariate 2-tailed Spearman coefficient for discrete variables and Pearson correlation coefficient for continuous variables. The α error was set at 0.05. The Bonferroni correction for multiple comparisons was applied. Thus, only a p value (0.05/14) = 0.003 was considered significant.19
Regions of interest.
GM volumes were quantified in 14 ROIs, which were defined using the Wake Forest University PickAtlas.20 Cortical motor areas included the primary motor area, postcentral gyri, supplementary motor area (SMA), pre-SMA, and the cerebellum. Subcortical motor areas included the thalamus, caudate nucleus, putamen, and globus pallidus (internal and external). For each patient, the ROIs contralateral to the more affected side, defined by the side with a higher score on the UPDRS, were examined in relation to performance-based tests of symptoms. To define the left inferior frontal gyrus (IFG), which was shown to have robust GM atrophy in patients with PD compared with healthy controls, we selected a sphere of 8 mm around the following Montreal Neurological Institute coordinates: [−42, 20, −12], as published in a recent meta-analysis study.12
Resting-state functional connectivity analysis.
Functional connectivity detects very low frequency (<0.08 Hz) fluctuations in magnetic resonance signals.21 By identification of temporally correlated brain regions, i.e., functional networks, this analysis enables characterization of interregional neural interactions from spontaneous neuronal activity during rest.22 We investigated the level of connectivity with the motor network in regions that we found in the VBM analysis to be correlated with the PIGD score or with the tremor score (see also appendix e-1).
RESULTS
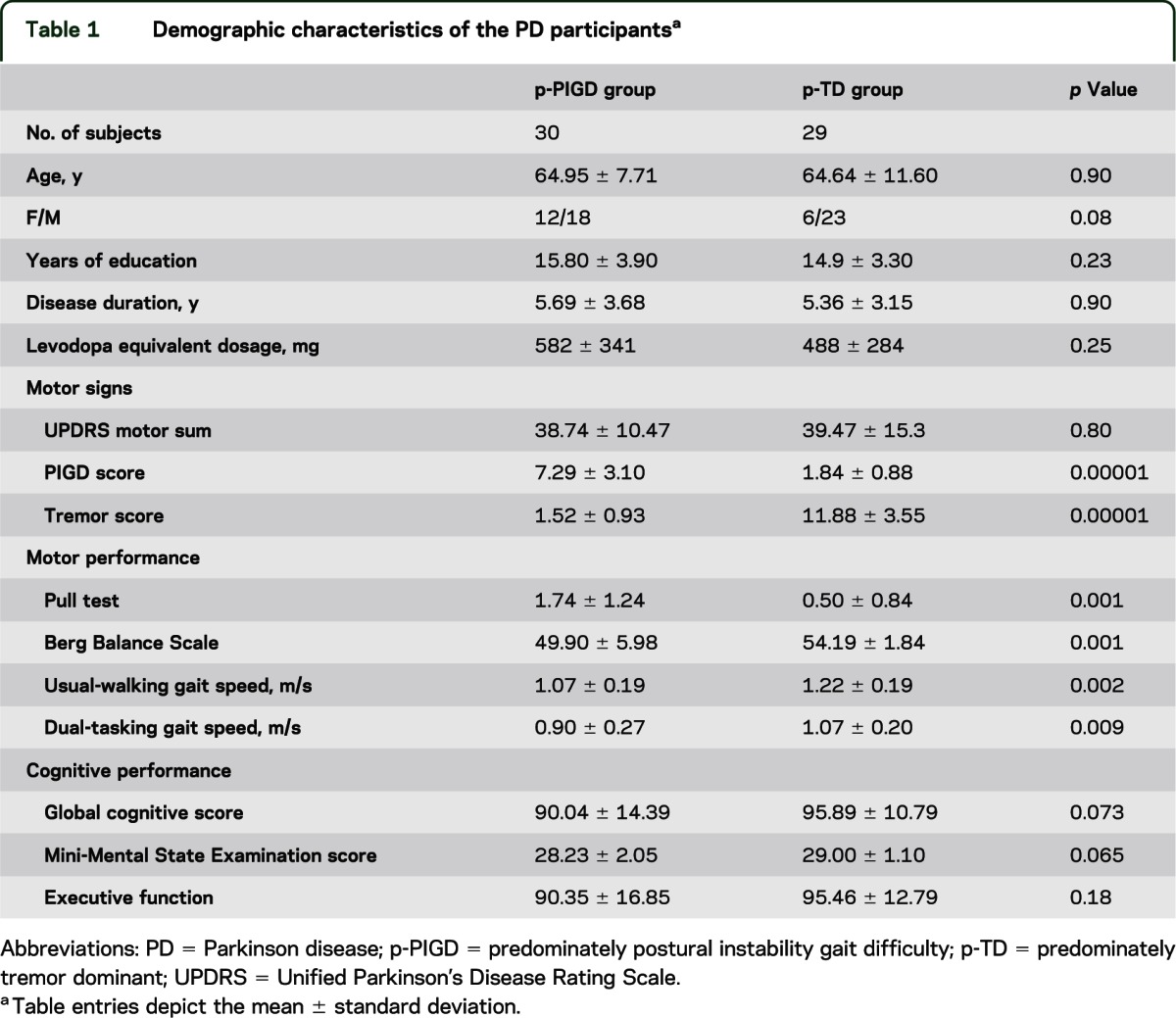
Table 1 summarizes the patient characteristics of the p-PIGD and p-TD groups. There were no significant differences between the p-PIGD and p-TD groups in their demographic characteristics including age, sex, years of education, UPDRS motor scores, disease duration, and levodopa equivalent dosage. Vascular risk factors were also similar between groups (table e-1). As expected, axial motor impairments were more severe in the p-PIGD group than in the p-TD group, as measured by the pull test (p < 0.0001), the Berg Balance Scale (p < 0.001), and gait speed (p < 0.009). The executive function index (p < 0.18), Mini-Mental State Examination score (p < 0.065), and global cognitive score (p < 0.073) tended to be lower in the p-PIGD group than in the p-TD group; however, these group differences were not significant (table 1).
Table 1.
Demographic characteristics of the PD participantsa
VBM results.
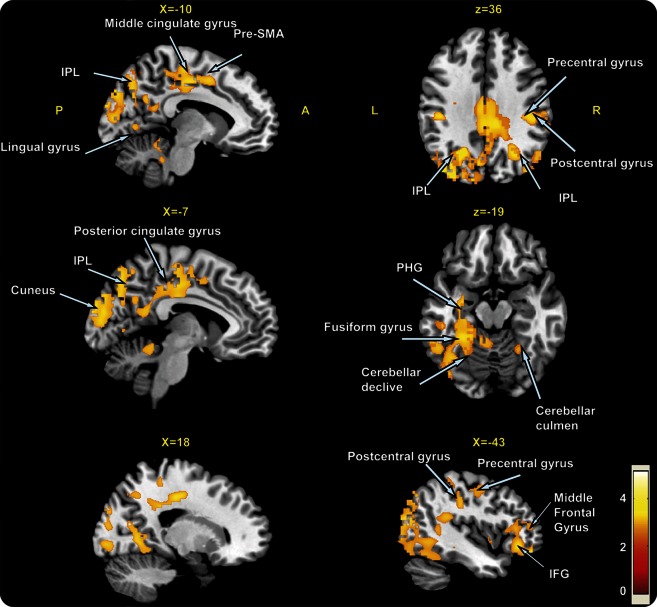
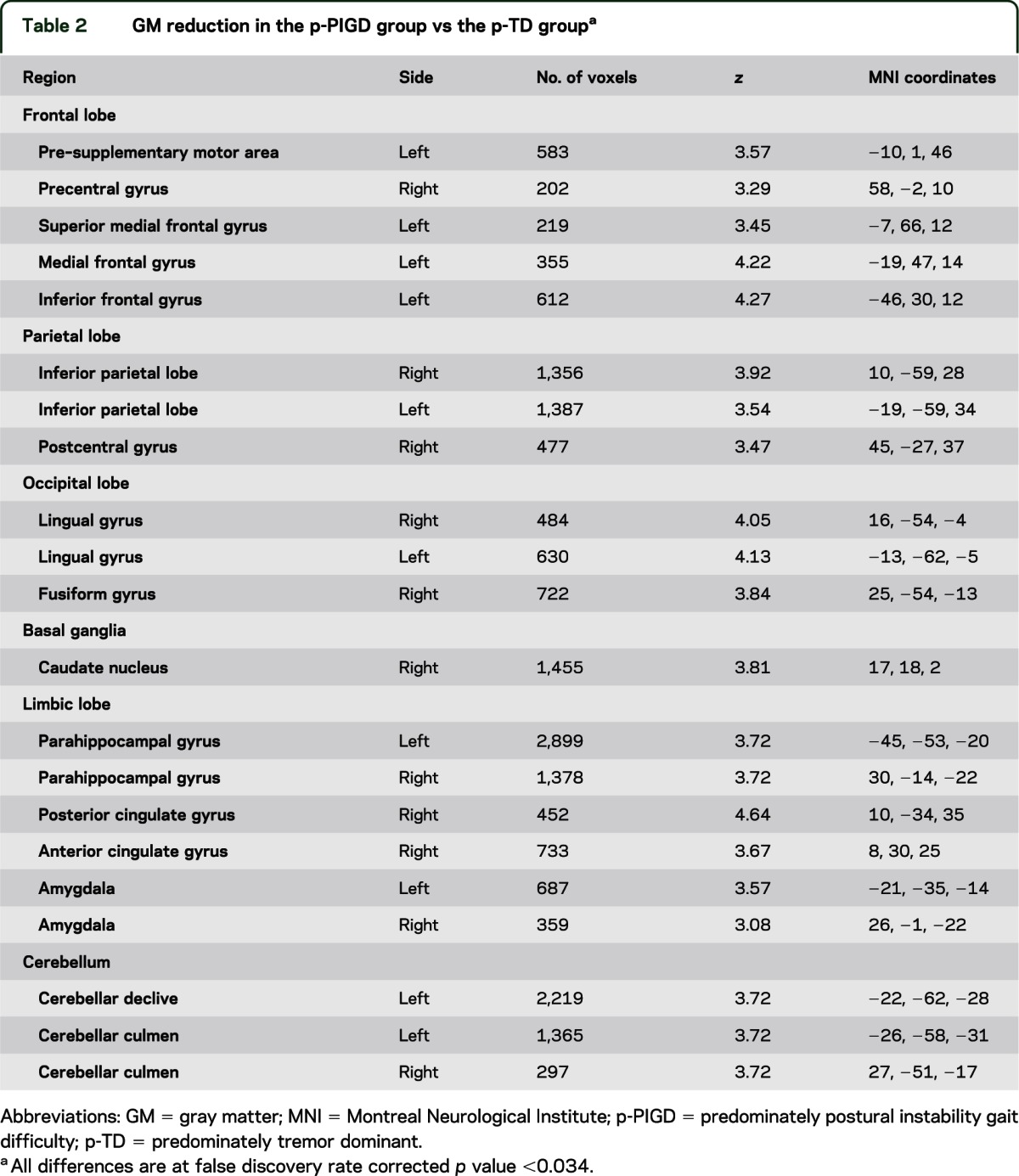
The percent of whole-brain GM was similar in the 2 groups (p-PIGD: 32.86% ± 2.57%; p-TD: 32.48% ± 2.45%; p = 0.57). This similarity suggests that any GM differences between the PD subtypes are attributable to site-specific tissue changes and not general brain GM atrophy. To further explore this possibility, we performed whole-brain VBM analysis. The whole-brain comparison between the groups using a 1-way analysis of covariance test, with age and disease duration added as covariates, showed distributed GM atrophy in the p-PIGD group as compared with the p-TD group. As summarized in figure 1 and table 2, significantly lower values of GM in the p-PIGD compared with the p-TD group were observed in all major brain lobes including the frontal lobe, parietal, occipital, and temporal (p < 0.005, FDR corrected). In addition, GM atrophy for the p-PIGD group could also be found in subcortical areas such as the caudate nucleus and the cerebellar culmen and declive (p < 0.005, FDR corrected) (see figure 1 and table 2). Because there was a trend for sex differences between the groups, the whole-brain analysis was repeated while adjusting for sex; similar results were observed at p < 0.001, cluster-level corrected. GM atrophy in patients of the TD subtype, relative to the PIGD subtype, showed no significant differences at p < 0.005 FDR corrected.
Figure 1. Areas of gray matter atrophy derived from a voxel-based morphometric direct comparison between patients with p-TD (n = 29) and p-PIGD (n = 30) subtypes.
The results are superimposed in representative sagittal and axial sections of a customized gray matter template, at a threshold of p < 0.05, false discovery rate corrected. IFG = inferior frontal gyrus; IPL = inferior parietal lobe; PHG = parahippocampal gyrus; p-PIGD = predominately postural instability gait difficulty; p-TD = predominately tremor dominant; SMA = supplementary motor area.
Table 2.
GM reduction in the p-PIGD group vs the p-TD groupa
A similar whole-brain analysis was also conducted for the PIGD (n = 60) and TD (n = 37) groups, as determined using the classification based on the ratio between symptoms.1 This analysis was performed to evaluate the generalizability of the findings and to infer about the differences between the patient groups as they may be diagnosed routinely in the clinic. As figure 2 shows, this analysis yielded very similar results with respect to the regions that showed GM atrophy for the p-PIGD group compared with the p-TD group. The differences between groups were observed although at a lower statistical threshold (p < 0.05, cluster-level corrected); this makes sense given that more symptoms are shared by the PIGD and TD groups as compared with the p-PIGD and p-TD groups.
Figure 2. Areas of gray matter atrophy derived from a direct comparison between patients with TD (n = 37) and PIGD subtypes (n = 60) using a ratio of symptoms to determine the group classification1.
The results are superimposed in representative sagittal and axial sections of a customized gray matter template, at a threshold of p < 0.05, cluster-level corrected. The results shown here are very similar to those shown in figure 1, supporting the idea that the results for the p-TD and p-PIGD comparisons are generalizable. IFG = inferior frontal gyrus; IPL = inferior parietal lobe; PHG = parahippocampal gyrus; p-PIGD = predominately postural instability gait difficulty; p-TD = predominately tremor dominant; SMA = supplementary motor area.
As indicated in the Methods section, all of the VBM analyses reported above were adjusted for age and disease duration. Not surprisingly, the PIGD score was moderately correlated with disease duration (r = 0.274, p < 0.05). To assess our findings relative to the stage of the disease, we evaluated a subgroup of patients with less than 4 years of motor symptoms (n = 21). A comparison of this group of p-PIGD (n = 11) and p-TD (n = 10) still revealed GM atrophy that was very similar to what was reported in figure 1 (even with FDR correction), consistent with the only mild correlation between PIGD score and disease duration.
ROI results: Correlations between GM and clinical measurements.
Table 3 summarizes the associations that were observed between GM volumes in the predefined ROIs and the measures of symptoms. Higher PIGD scores were associated with lower GM volume in the pre-SMA (r = −0.424; p < 0.001, Bonferroni corrected), and with lower GM volume in the precentral gyrus (r = −0.382; p < 0.003, Bonferroni corrected). In addition, poorer performance on the pull test (represented by a higher score) was associated with lower GM volumes in the precentral gyrus (r = −0.574; p < 0.0001, Bonferroni corrected). Higher GM volumes in the left IFG were associated with higher tremor scores (r = 0.436; p < 0.001). Higher gait speed during dual tasking was associated with higher GM volumes in the cerebellar tonsil (r = 0.347; p < 0.007, uncorrected). No correlations were found between GM volume in the selected regions and the global cognitive score, executive function index, or the Berg Balance Scale.
Table 3.
Correlations between GM volumes and PIGD and TD symptomsa

Functional connectivity results: Anatomical-functional relationships.
The precentral gyrus and pre-SMA were further evaluated in relation to their functional connectivity with the motor network, defined as connectivity with the putamen on the more affected side. Higher GM volumes in the pre-SMA were associated with higher level of connectivity between this region and the putamen in the p-PIGD group (n = 30, r = 0.42, p < 0.025), but not in the p-TD group (n = 29, r = −0.20, p = 0.302). No significant correlation was found between GM volumes in the precentral gyrus and the level of connectivity between the affected precentral and putamen in either group.
DISCUSSION
In this study, we directly assessed GM differences between the PIGD and TD subtypes of patients with PD using 3 different MRI-based approaches: whole-brain GM volume differences, an ROI approach, and functional connectivity. The results of all 3 methods support the possibility that GM atrophy is increased among patients with PIGD compared with patients with TD. In fact, we observed GM atrophy in the p-PIGD group in several brain areas including motor as well as cognitive, associative, and limbic regions. This pattern was also detected using the traditional classification into PIGD and TD groups, although at a lower statistical threshold. Those results further strengthen our findings and suggest that there was minimal bias in the selection of the p-PIGD and p-TD subgroups. Lower GM was not found for the tremor group compared with the PIGD group. The idea that these findings are related to clinical symptoms is supported by the correspondence to decreased functional connectivity between cortical and subcortical motor-planning regions. Indeed, some of the regional atrophy was also correlated with increased severity of motor symptoms and reduced cognitive performance. Altogether, our findings support the idea that distributed GM atrophy is associated with the 2 different PD motor subtypes.
Among the p-PIGD patients, reduced GM volume was found in motor-related regions such as the pre-SMA, postcentral gyri, the cerebellar declive and culmen, as well as the caudate nucleus. The motor symptoms of the PIGD subtype, as represented by the PIGD score, were associated with decreased GM volume in the pre-SMA and in the precentral gyrus. The pre-SMA is well known to be involved in motor initiation and planning, and is activated preceding movement onset.23–25 The difficulties with the initiation of movement that are often present among patients with PIGD may be related to atrophy in this region. This interpretation is supported by the functional connectivity findings; GM atrophy in this region was associated with lower functional connectivity with the motor network. Reduced GM volumes in the pre-SMA were previously reported in patients with PD compared with controls26; however, the earlier work did not directly relate the atrophy to the PIGD subtype.
Reduced GM volumes in the p-PIGD group compared with the p-TD group were also detected in the primary motor area. This suggests that GM atrophy in this region is also related to the gait difficulties presented by the p-PIGD group because the lower GM volumes in this region were related to higher PIGD scores and decreased postural control as assessed by the pull test. The involvement of the precentral gyrus in posture control is supported by a recent study in which transcranial pulsed current stimulation in this region improved balance and gait parameters in patients with PD.27
Lower GM volumes in the PIGD group were detected in the cerebellum declive and culmen. This finding is consistent with the hypothesis that the cerebellum plays an important role in gait, by regulating posture and balance and by adjusting the feedforward control of locomotor output through error-feedback learning.28,29 Consistent with our findings, a study focusing on the associations between brain morphology and rest tremor reported decreased GM volume in the IFG of the patients with nontremor PD. However, they also reported lower GM volumes in the right quadrangular lobe and declive of the cerebellum in patients with tremor.30 These inconsistencies may be related to the different classification methods that were used.
Additional GM atrophy for the p-PIGD group was found in frontal regions including the medial frontal gyrus (Brodmann area 10) and left IFG. GM atrophy in the left IFG was associated with greater PIGD symptoms and, conversely, with fewer tremor symptoms. The reduction of GM in these frontal regions might partially explain the cognitive decline that is generally more common among patients with PIGD.2,3 Cognitive decline may also explain in part the gait difficulties presented in this group, as gait difficulties and fall risk were previously related to deficits in executive function,31–33 a reflection of motor-cognitive interdependence. A recent meta-analysis that included the above-mentioned VBM studies12 also reported GM reductions in the left IFG. We can speculate that the patients with PIGD in those studies contributed to this observation but no specific data were given with respect to the motor subtypes of PD.
The differences observed between the 2 PD subtypes might explain the conflicting findings among previous VBM studies that compared patients with PD to controls. Some of these investigations reported distributed GM reduction in the patients with PD.34–36 A few studies found more local reduction of GM in PD, although the regions were inconsistent across the studies.37,38 In addition, some reports showed no differences between PD and controls.39,40 Differences in the relative representation of the PIGD and TD subtypes may explain the disparate findings in these earlier reports. Our findings highlight the need to distinguish among the clinical subtypes when investigating the neural correlates underlying PD.
Because this is a cross-sectional investigation and not a prospective study, we can only speculate about the temporal relationship between GM atrophy and the clinical symptoms and whether the observed changes reflect transient or long-term changes. Cause and effect cannot be conclusively determined. Another factor may lead to GM atrophy and the PIGD symptoms; however, a likely candidate is not readily apparent. Thus, one interesting possibility is that the specific GM degeneration reflects the pathologic changes in the gait and posture networks that are known to be widespread in the brain and involve cortical and subcortical areas. The possibility that early GM degeneration leads to the PIGD subtype of PD should be tested in early naive patients as well as in a longitudinal study. The present findings indicate that relatively widespread GM atrophy is apparently a characteristic feature of a distinct subset of patients with PD. Nonetheless, the source of this atrophy and its role in the development of PIGD and tremor remain to be determined more fully.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to all the patients who participated in this study. The authors thank Marina Brozgol from the Movement Disorders Unit for her help in clinical evaluation, and Guy Heiman from the Functional Brain Center, Wohl Institute for Advanced Imaging, for support in data acquisition.
GLOSSARY
- FDR
false discovery rate
- GM
gray matter
- IFG
inferior frontal gyrus
- PD
Parkinson disease
- PIGD
postural instability gait difficulty
- p-PIGD
predominately postural instability gait difficulty
- p-TD
predominately tremor dominant
- ROI
region of interest
- SMA
supplementary motor area
- TD
tremor dominant
- UPDRS
Unified Parkinson's Disease Rating Scale
- VBM
voxel-based morphometry
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Rosenberg-Katz: drafting the manuscript for content, analysis and interpretation of data, acquisition of data, statistical analysis. Mrs. Herman: study design, acquisition of data, revising the manuscript for content. Miss Jacob: acquisition of data, statistical analysis, revising the manuscript for content. Prof. Giladi: study concept and design, revising the manuscript for content. Prof. Hendler: analysis and interpretation of data, revising the manuscript for content. Prof. Hausdorff: study concept or design, revising the manuscript for content, study supervision, obtaining funding.
STUDY FUNDING
This study was supported by the Michael J. Fox Foundation for Parkinson's Research.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990;40:1529–1534 [DOI] [PubMed] [Google Scholar]
- 2.Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry 2005;76:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burn DJ, Rowan EN, Allan LM, Molloy S, O'Brien JT, McKeith IG. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:585–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003;60:387–392 [DOI] [PubMed] [Google Scholar]
- 5.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 2007;130:1787–1798 [DOI] [PubMed] [Google Scholar]
- 6.Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci 2009;29:15223–15231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging 2004;25:377–396 [DOI] [PubMed] [Google Scholar]
- 8.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 2005;15:1676–1689 [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Sachdev PS, Wen W, et al. Gray matter atrophy patterns of mild cognitive impairment subtypes. J Neurol Sci 2012;315:26–32 [DOI] [PubMed] [Google Scholar]
- 10.Ferreira LK, Diniz BS, Forlenza OV, Busatto GF, Zanetti MV. Neurostructural predictors of Alzheimer's disease: a meta-analysis of VBM studies. Neurobiol Aging 2011;32:1733–1741 [DOI] [PubMed] [Google Scholar]
- 11.Dumurgier J, Crivello F, Mazoyer B, et al. MRI atrophy of the caudate nucleus and slower walking speed in the elderly. Neuroimage 2012;60:871–878 [DOI] [PubMed] [Google Scholar]
- 12.Pan PL, Song W, Shang HF. Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson's disease. Eur J Neurol 2011;19:199–206 [DOI] [PubMed] [Google Scholar]
- 13.Linder J, Birgander R, Olsson I, et al. Degenerative changes were common in brain magnetic resonance imaging in patients with newly diagnosed Parkinson's disease in a population-based cohort. J Neurol 2009;256:1671–1680 [DOI] [PubMed] [Google Scholar]
- 14.Wu T, Wang L, Chen Y, Zhao C, Li K, Chan P. Changes of functional connectivity of the motor network in the resting state in Parkinson's disease. Neurosci Lett 2009;460:6–10 [DOI] [PubMed] [Google Scholar]
- 15.Ni Z, Pinto AD, Lang AE, Chen R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol 2010;68:816–824 [DOI] [PubMed] [Google Scholar]
- 16.Mito Y, Yoshida K, Yabe I, et al. Brain SPECT analysis by 3D-SSP and phenotype of Parkinson's disease. J Neurol Sci 2006;241:67–72 [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653 [DOI] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995;57:289–300 [Google Scholar]
- 19.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med 1997;16:2529–2542 [DOI] [PubMed] [Google Scholar]
- 20.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003;19:1233–1239 [DOI] [PubMed] [Google Scholar]
- 21.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537. [DOI] [PubMed] [Google Scholar]
- 22.Peltier SJ, Noll DC. T(2)(*) dependence of low frequency functional connectivity. Neuroimage 2002;16:985–992 [DOI] [PubMed] [Google Scholar]
- 23.Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage 2002;15:373–385 [DOI] [PubMed] [Google Scholar]
- 24.Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science 2004;303:1208–1210 [DOI] [PubMed] [Google Scholar]
- 25.Shima K, Mushiake H, Saito N, Tanji J. Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci USA 1996;93:8694–8698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meppelink AM, de Jong BM, Teune LK, van Laar T. Regional cortical grey matter loss in Parkinson's disease without dementia is independent from visual hallucinations. Mov Disord 2010;26:142–147 [DOI] [PubMed] [Google Scholar]
- 27.Alon G, Yungher DA, Shulman LM, Rogers MW. Safety and immediate effect of noninvasive transcranial pulsed current stimulation on gait and balance in Parkinson disease. Neurorehabil Neural Repair 2010;26:1089–1095 [DOI] [PubMed] [Google Scholar]
- 28.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 1992;15:403–442 [DOI] [PubMed] [Google Scholar]
- 29.Morton SM, Bastian AJ. Mechanisms of cerebellar gait ataxia. Cerebellum 2007;6:79–86 [DOI] [PubMed] [Google Scholar]
- 30.Benninger DH, Thees S, Kollias SS, Bassetti CL, Waldvogel D. Morphological differences in Parkinson's disease with and without rest tremor. J Neurol 2009;256:256–263 [DOI] [PubMed] [Google Scholar]
- 31.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2007;62:844–850 [DOI] [PubMed] [Google Scholar]
- 32.Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One 2012;7:e40297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord 2008;23:329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kostic VS, Agosta F, Petrovic I, et al. Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology 2010;75:857–863 [DOI] [PubMed] [Google Scholar]
- 35.Nishio Y, Hirayama K, Takeda A, et al. Corticolimbic gray matter loss in Parkinson’s disease without dementia. Eur J Neurol 2010;17:1090–1097 [DOI] [PubMed] [Google Scholar]
- 36.Pereira JB, Junqué C, Martí MJ, Ramirez-Ruiz B, Bargalló N, Tolosa E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson's disease. Mov Disord 2009;24:1193–1199 [DOI] [PubMed] [Google Scholar]
- 37.Cordato NJ, Duggins AJ, Halliday GM, Morris JG, Pantelis C. Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain 2005;128:1259–1266 [DOI] [PubMed] [Google Scholar]
- 38.Tir M, Delmaire C, le Thuc V, et al. Motor-related circuit dysfunction in MSA-P: usefulness of combined whole-brain imaging analysis. Mov Disord 2009;24:863–870 [DOI] [PubMed] [Google Scholar]
- 39.Feldmann A, Illes Z, Kosztolanyi P, et al. Morphometric changes of gray matter in Parkinson's disease with depression: a voxel-based morphometry study. Mov Disord 2008;23:42–46 [DOI] [PubMed] [Google Scholar]
- 40.Jubault T, Brambati SM, Degroot C, et al. Regional brain stem atrophy in idiopathic Parkinson's disease detected by anatomical MRI. PLoS One 2009;4:e8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.