Abstract
Objective:
To describe the presentation and management of encephalitis due to human herpes 6 virus (HHV-6) in patients who underwent allogeneic hematopoietic stem cell transplant (alloHSCT), via retrospective chart review.
Methods:
Of the 243 patients who underwent alloHSCT at the NIH Clinical Center during 2009 to 2011, we retrospectively analyzed 9 diagnosed with HHV-6 encephalitis post-alloHSCT.
Results:
Eight men and 1 woman (aged 19–60 years) met diagnostic criteria for study inclusion. The median time from HSCT to initial symptoms was 21 days. All patients presented with altered mental status and headaches. Seven patients had amnesia and 2 presented with fever of unknown etiology. Four patients had clinical seizures during the disease course. Brain MRI within 7 days was normal in all patients. Repeat MRI after 7 days showed hyperintensity in the limbic area in 3 patients. On initial testing, CSF analysis indicated acellularity and normal or minimally elevated protein; presence of HHV-6 was detected by PCR. After 7 days, mildly elevated protein and minimal pleocytosis were noted. Ganciclovir, foscarnet, or valganciclovir alone or in combination was initiated with subsequent improvement. Four patients remained alive at 1 year posttransplant; 2 had persistent memory deficits. Presence of encephalitis was associated with higher mortality post-alloHSCT.
Conclusion:
High clinical suspicion and CSF PCR testing are important for early diagnosis of HHV-6 encephalitis post-HSCT. Abnormalities on brain MRI or CSF testing may be minimal and delayed. Diagnosis and management of HHV-6 encephalitis is challenging, and a larger prospective study is needed for further research.
Primary infection with human herpes 6 virus (HHV-6) is generally acquired in early childhood, manifesting as a febrile illness or benign rash, with estimated seroprevalence of >95% after age 2 years.1 The majority of infections are caused by HHV-6 subtype B.2,3 Similar to other herpes viruses, HHV-6 remains latent in organs such as brain, kidneys, and salivary glands, and cells such as T-lymphocytes, bone marrow progenitor cells, and microglia.1,4 In approximately 2% of the population, viral DNA sequences are integrated into chromosomes.5 Viral reactivation occurs in severely immune-compromised states such as hematopoietic stem cell transplantation, solid organ transplantation, and HIV/AIDS, causing diverse clinical manifestations including encephalitis, pneumonitis, thrombocytopenia, hepatitis, and fever. Interestingly, symptomatic reactivation is more frequently noted in patients receiving hematopoietic stem cell transplantation compared with patients with HIV/AIDS.1 Neurologic infections due to HHV-6 can be relatively unique and therefore difficult to diagnose.
Hematopoietic stem cell transplants (HSCT) are increasingly used to treat hematologic, oncologic, autoimmune, immunodeficiency, and genetic diseases.6 Up to 25% of patients develop moderate to severe neurologic complications after allogeneic (allo)HSCT.7,8 Exposure to neurotoxic medications and disruption of immunity as a part of the HSCT procedure contribute to nervous system complications.9 In general, CNS infections post-alloHSCT have been associated with higher mortality and rejection rates, whereas long-term neurologic morbidity remains unknown.5,6 Hence, in addition to characterizing the clinical presentation of encephalitis due to HHV-6 infection, we investigated whether early diagnosis and treatment influences mortality in alloHSCT recipients.
METHODS
Study population.
We identified a total of 243 patients (109 women and 134 men) who underwent HSCT at the Clinical Center from 2009 to 2011. Indications for HSCT were acute and chronic hematologic malignancies in 160 patients (65.8%), bone marrow failure states or anemia in 34 (13.9%), primary immunodeficiency conditions in 25 (10.2%), and solid organ tumors in 24 (9.8%). Two hundred ten patients (86.4%) received alloHSCT, 7 (2.8%) received cord stem cell transplants, and 26 (10.6%) received autologous HSCT.
Of these 243 patients, we identified and performed retrospective chart review of 9 individuals (3.7%) who fulfilled the following inclusion criteria for the diagnosis of HHV-6 encephalitis: 1) presence of clinical symptoms of encephalitis, i.e., altered mental status, amnesia, or seizures; 2) presence of HHV-6 in the CSF detected by PCR; and 3) exclusion of other primary neurologic etiologies that could explain clinical symptoms or findings. We recorded their demographic information, neurologic symptoms, CSF and imaging findings, as well as treatment and prognosis. An additional 2 patients had HHV-6 identified by PCR in the CSF but did not meet the criteria for HHV-6 encephalitis and were excluded from the study.
Standard protocol approvals, registrations, and patient consents.
This retrospective study was exempted from full review by the Office of Human Subjects Research Protections at the NIH because the data were extracted in de-identified form using the Biomedical Translational Research Information System. However, all patients received HSCT as a part of an Institutional Review Board–approved research protocol at the Clinical Center of the NIH and had consented for using their research data or records.
HHV-6 testing in the blood and CSF.
HHV-6 (subtypes A and B) quantitative, real-time PCR was performed by extracting DNA from a 200-µL aliquot of whole blood or CSF and using primers and probes that amplify and detect a portion of the viral DNA polymerase gene as described previously.7 The assay is able to differentiate between subtypes A and B based on melt curve analysis. The limit of detection of the PCR assay is 50 genome equivalents per 1.0 mL with a lower limit of the quantifiable range of 250 genome equivalents per 1.0 mL.
Statistical analysis.
Continuous variables were summarized using descriptive statistics such as mean, median, minimum, and maximum. Categorical data were summarized using frequencies and percentages. A Kaplan-Meier survival analysis (or Kaplan-Meier product limit estimate) was performed to estimate survival curve. The date at which the patients were diagnosed with HHV-6 encephalitis was defined as entry date. The survival time was defined as the difference between the entry date and the date at which the patient died. If a patient was alive at the time of this study, the patient's survival time was censored. Log-rank test was used to compare survival curves of 2 groups: myeloablative vs nonmyeloablative pre-HSCT conditioning, HHV-6 viral load <100,000 and >100,000 copies/mL, use of a single antiviral agent, and combination of antiviral agents for treatment. Cox regression was used to assess the association between survival time and patient age as well as duration of treatment.
RESULTS
The 9 patients included 8 men and 1 woman aged 19 to 60 years. The male predisposition was not statistically significant with a p value of 0.0687. In 7 patients, HSCT was performed for hematologic malignancies (leukemia and lymphomas) and in 2 patients, for hereditary immune disorders (dedicator of cytokinesis 8 [DOCK8] deficiency and chronic granulomatous disease). Eight patients received stem cells from matched unrelated donors and 1 patient from a related (sibling) donor. The patient with DOCK8 deficiency received double umbilical cord stem cells, whereas others received alloHSCT. Five patients received reduced intensity or nonmyeloablative conditioning and 4 patients received ablative conditioning pretransplantation.
The median time from date of transplant to onset of symptoms was 21 days (range 6–145 days); median time from onset of symptoms to diagnosis (detection of virus in the CSF) was 7 days (range 1–13 days) (table 1).
Table 1.
Patient demographics and clinical presentation
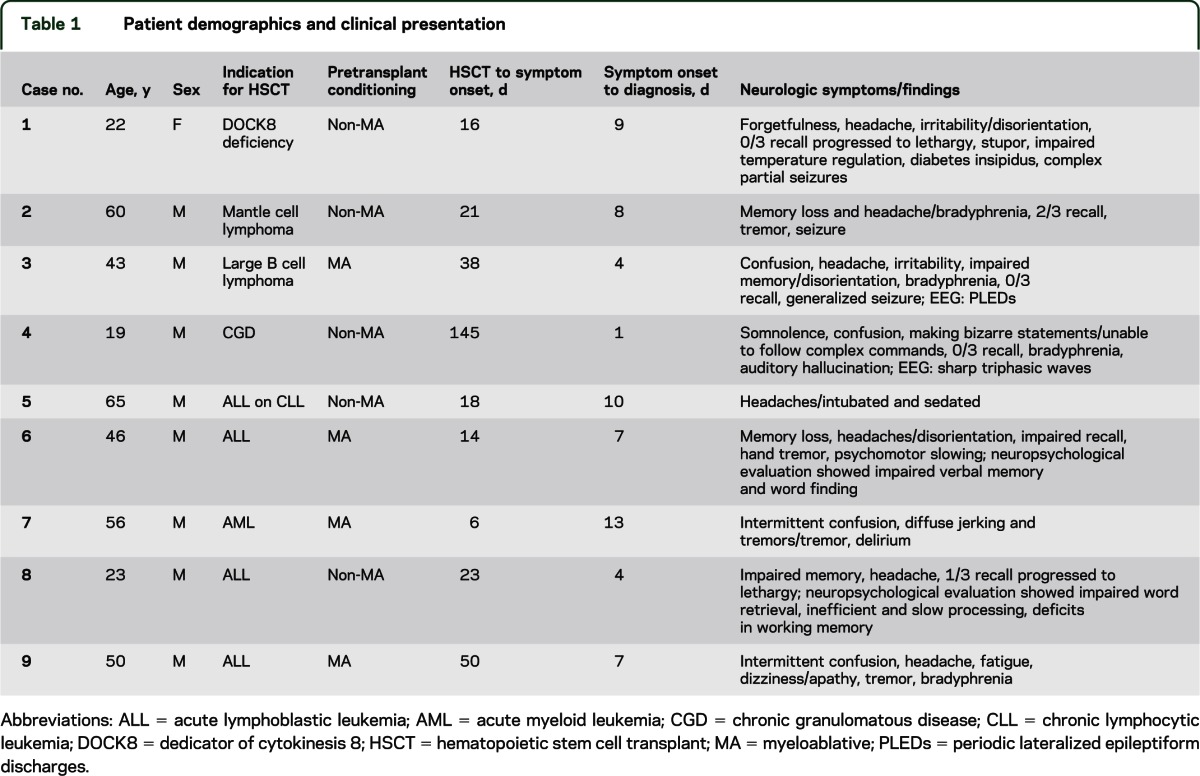
Clinical presentation.
All patients initially presented with confusion and headaches. Only 2 had concomitant fever of unknown etiology. Common symptoms reported by patients or family included personality changes, irritability, apathy, intermittent confusion, and forgetfulness. The neurologic examination in 7 patients revealed prominent anterograde amnesia, confusion, variable levels of disorientation, inability to follow complex commands, and bradyphrenia. Initial neurologic assessment in 2 patients was limited because of intubation and/or sedation secondary to cardiac condition. Four patients had postural hand tremor on assessment (table 1).
Disease course.
Seven of 9 patients were alert and awake at the initial neurologic assessment, but advanced to lethargy and somnolence with disease progression. One patient was mildly lethargic at the time of presentation and another patient remained intubated and sedated. Four patients experienced clinical seizures and concomitant epileptiform changes on EEG lateralizing to the temporal area. Two patients developed postural hand tremor during the disease course. One patient developed impaired homeostasis and demonstrated wide variations in temperature between 30°C and 38°C, fluctuations of blood pressure and heart rate, as well as diabetes insipidus, suggesting hypothalamic involvement.
Seven patients were treated successfully with antiviral agents, as demonstrated by improvement in their neurologic examination and serial HHV-6 titers from the CSF. One patient (case 5) died during the active phase of encephalitis. Two patients (cases 2 and 6) developed a relapse or second episode of encephalitis 2 to 3 months after adequate treatment of the initial episode. These patients had shown improvement in their neurologic examination and serial HHV-6 CSF titers, but several months later had the recurrence of neurologic symptoms. Repeat lumbar puncture showed HHV-6 viremia in the CSF, requiring another course of treatment.
Neuroimaging.
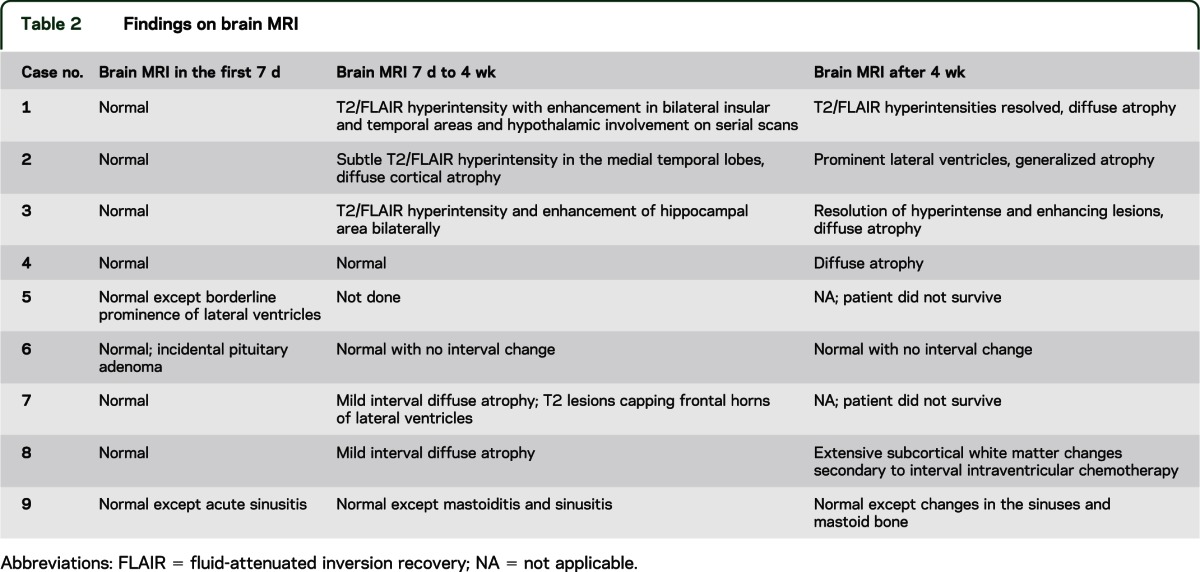
Brain MRI performed within the first week after developing symptoms was normal in all 9 patients. Repeat imaging after 7 to 10 days of progression of clinical symptoms showed nonenhancing, hyperintense lesions in the limbic structures on T2-weighted images consistent with limbic encephalitis in 3 of 9 patients. Repeat imaging in 4 patients remained unremarkable up to 4 weeks. One patient had nonspecific periventricular T2 lesions capping the frontal horns of the lateral ventricles. Another patient had borderline prominence of lateral ventricles on repeat imaging. Serial MRIs after 4 weeks demonstrated development of interval diffuse atrophy in 7 of 9 patients (figure 1, table 2).
Figure 1. Serial MRIs at <7 days, 7 days to 4 weeks, and after 4 weeks, with limbic encephalitis (A) and without limbic encephalitis (B).
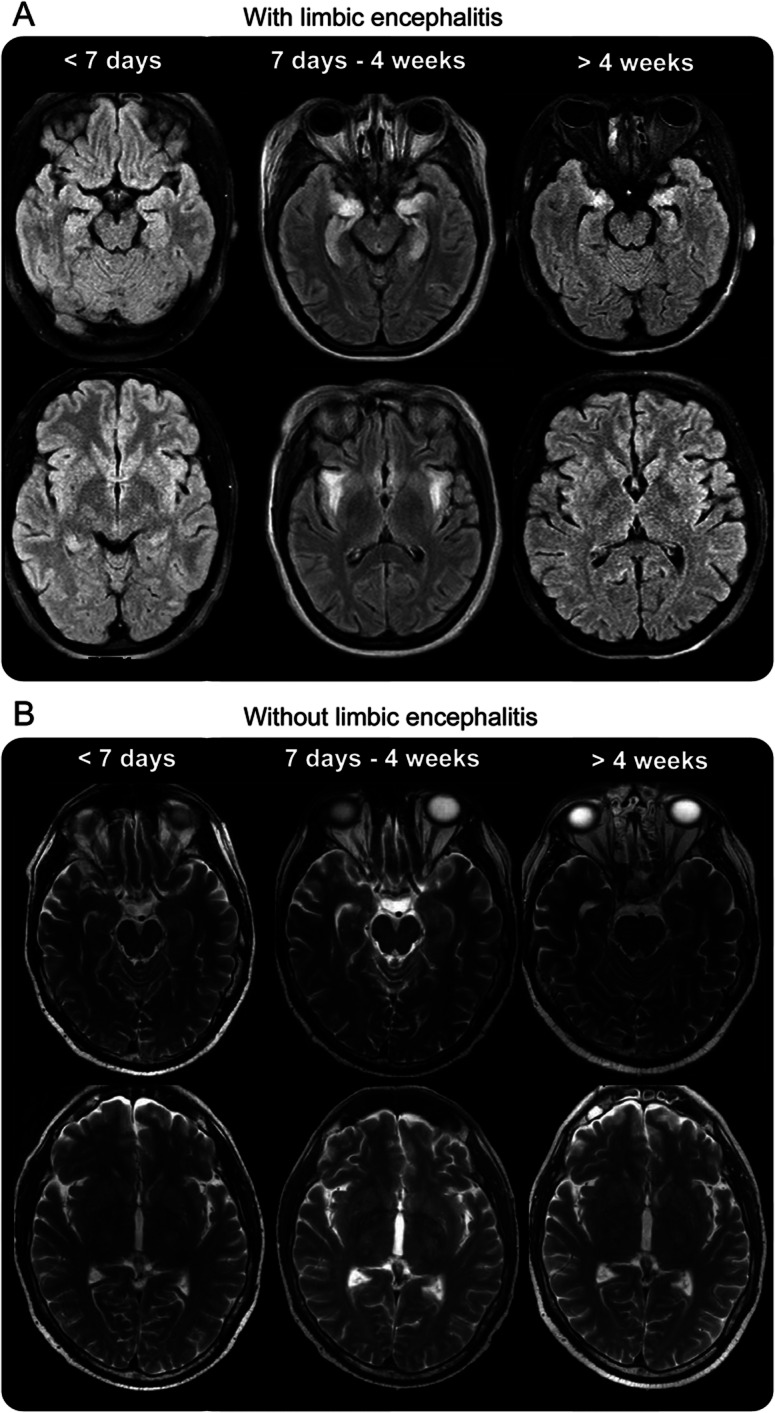
Table 2.
Findings on brain MRI
CSF analysis.
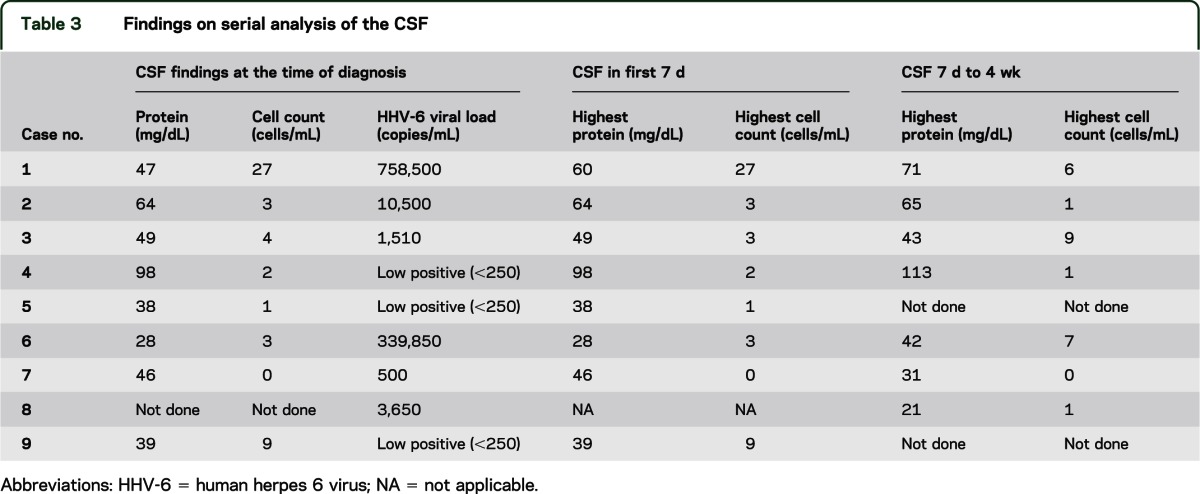
The CSF of 5 patients showed protein levels of >45 mg/dL, with the highest level at 98 mg/dL. Two patients had mild pleocytosis of 9 and 27 cells/mL3 (predominantly lymphocytes) at diagnosis. In 2 patients with normal cell counts on initial testing, serial CSF analysis showed mild subsequent elevation with a maximal cell count of 9 cells/mL3. CSF glucose was not fully analyzed because of inconsistent recordings of concomitant serum glucose levels; overall, minimal serum glucose changes were noted and were considered to be noninformative (table 3). In all patients presenting with encephalitis, CSF is tested for cytomegalovirus, Epstein-Barr virus, JC virus, HHV-6 and -7, herpes simplex virus, varicella-zoster virus, toxoplasmosis, and Cryptococcus. Because HHV-6 subtype B is the only form routinely associated with symptomatic disease, in patients with very high levels of HHV-6 subtype A, blood is tested for chromosomal integration of the virus, which was not the case for any of the patients reported here. This is done by comparing viral titers between blood and plasma; in cases in which there is chromosomal integration, the whole blood titer will be significantly higher.
Table 3.
Findings on serial analysis of the CSF
Viral load.
Eight of the 9 patients had HHV-6 viremia in the peripheral blood as well as CSF. In 8 patients, peripheral viremia preceded virus detection in the CSF. There was no correlation between viral load in the peripheral blood and the CSF. One patient (case 9) had virus present in the CSF with negative viremia. Another patient (case 2) had no detectable virus in the peripheral blood during the course of treatment, but continued to demonstrate virus in the CSF. There was no statistically significant correlation between the viral loads at the time of diagnosis with adverse clinical outcome of death (table 3).
Treatment.
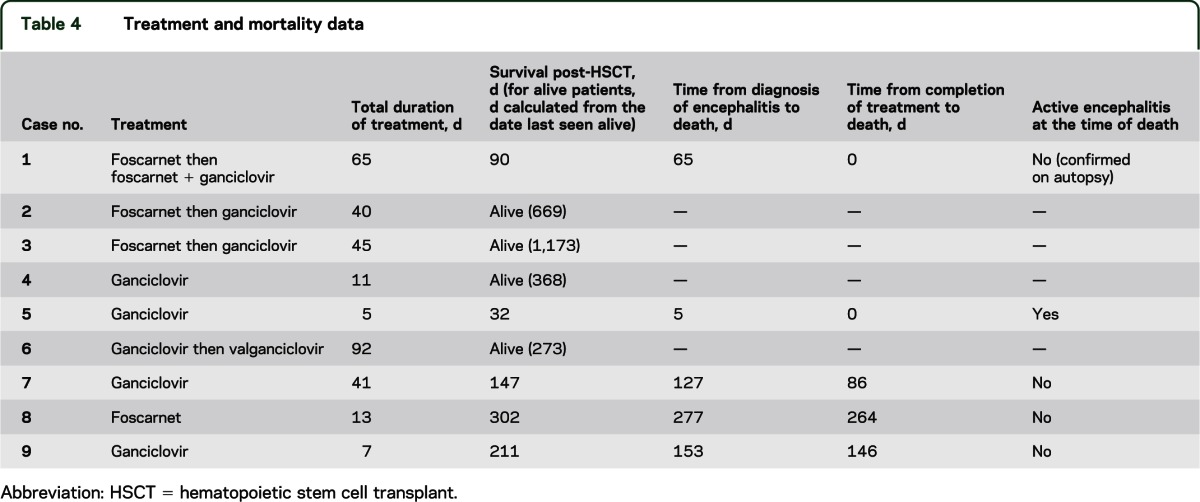
Antiviral agents, either ganciclovir or foscarnet, administered IV were used empirically in all patients as first-line therapy. Three patients who showed clinical progression together with suboptimal reduction of viral load in the CSF on single antiviral therapy were consequently treated with a combination of ganciclovir and foscarnet. One patient had HHV-6 viremia and clinical symptoms develop while receiving IV cidofovir therapy for severe warts. Therapy varied from 5 to 92 days (median 40 days) and depended on clinical response and reduction in viral load to undetectable levels in the CSF on serial monitoring. In general, patients with milder disease and lower viral load (low positive, <250 copies) received a shorter course of antiviral agents (5–11 days) (table 4). There was no statistically significant correlation between duration of therapy or use of single agent vs combination therapy and survival.
Table 4.
Treatment and mortality data
Prognosis and follow-up.
Five of 9 patients in the cohort did not survive as of August 2012. Median time from transplantation to death was 147 days (range 32–302 days) and the median time from the diagnosis of HHV-6 encephalitis to death was 127 days (range 5–277 days). The most common cause of mortality was bacterial sepsis in the context of bone marrow suppression or relapse of underlying malignancy or conditioning for retransplantation. Only one patient had ongoing encephalitis at the time of death. In the other 4 patients, a median of 86 days (range 0–264 days) was noted between the completion of treatment and resolution of encephalitis (defined by clinical improvement and no virus detection by PCR in the CSF) and death. Autopsy data were available for only one patient (case 1). In this patient, mental status had improved on antiviral treatment, which was continued until her death due to multiorgan failure related to fungal sinusitis and pneumonia. At autopsy, there was no evidence of active encephalitis in the brain, but instead gliosis was seen in the hippocampus, hypothalamus, and basal ganglia (table 4).
Using the Kaplan-Meier survival analysis, the probability of survival at 127 days is 67%, that at 153 days is 56%, and that over 277 days is 37% (figure e-1 on the Neurology® Web site at www.neurology.org). There was no correlation between mortality and age, pretransplant conditioning (myeloablative vs nonmyeloablative), high or low viral load in the CSF at the time of presentation (<100,000 vs >100,000 copies/mL), duration of antiviral therapy, or use of single antiviral agents vs the combination therapy (figure e-1).
Of the 4 patients who survived, 2 (50%) continued to have persistent memory deficits and anterograde amnesia for up to 2 years.
DISCUSSION
Establishing a diagnosis and making a decision to initiate treatment for HHV-6 encephalitis post-alloHSCT are challenging. Typical clinical presentation and radiologic findings of limbic encephalitis10,11 were seen only in one-third (3 of 9) of the patients in our cohort, and did not correlate with the viral load in the CSF. The majority of our patients presented with symptoms of nonspecific encephalopathy with minimal changes on neuroimaging (brain MRI) and CSF, but the diagnosis seemed likely because of detection of HHV-6 in the CSF by PCR, exclusion of other neurologic etiologies (e.g., toxic-metabolic encephalopathy or other viral encephalitis), and clinical improvement with antiviral agents. In cases with low CSF viral loads (<250 copies/mL), other CSF abnormalities such as high protein or cell count were considered while establishing a diagnosis. This suggests that there may be a broader spectrum of clinical presentations, not limited to limbic encephalitis, that are associated with this condition and need further characterization. Severe immunosuppression and inability to mount an adequate immune response to viral agents in the posttransplant period could potentially explain the paucity of findings on imaging and CSF. There was no correlation between clinical symptoms of limbic encephalitis or severity of alteration in consciousness and viral load in the CSF. This adds to the ambiguity and difficulty in making a diagnosis with certainty, especially in cases of low CSF viral loads, and raises the question on significance of the viral load in the CSF and whether it can be used as a reliable biomarker.
The rather “ubiquitous” nature of HHV-6 and the incidence of asymptomatic virus in the CSF also pose a diagnostic challenge. We found 2 posttransplant cases in which HHV-6 was detected in the CSF but these patients had neurologic manifestations that were implicated to other neurologic infections, and thus were excluded from this study. One patient had multiple cardioembolic strokes and suspected varicella-zoster virus vasculitis, which was treated with acyclovir, and received no treatment with the other above-mentioned antiviral agents. He died secondary to multiple systemic infections and sepsis. Another patient had cytomegalovirus meningitis characterized by neck pain, stiffness, and headache, but no evidence of encephalitis clinically or on autopsy. HHV-6 reactivation has been associated with other viral reactivations in patients who underwent HSCT.12,13 The role of HHV-6 in the pathophysiology of these viral infections is not clear.
Although effective for encephalitis, prolonged IV use of antiviral agents such as ganciclovir, valganciclovir, and foscarnet can cause significant bone marrow suppression and renal toxicity, which may affect mortality. The marrow toxicity contributes to graft failure and delayed engraftment. Hence, the decision to initiate these agents is challenging, and physicians must weigh the need to treat the infection against the risk of graft failure or renal toxicity and concern for developing resistance. Moreover, there are no guidelines or consensus on indications to initiate treatment with antiviral agents, impact of early vs delayed treatment, or optimal duration of treatment. Further data are also needed on the incidence or risk of relapse and role of secondary prophylaxis to prevent such a relapse, if any. The natural history of the disease largely remains unknown at this time. Thus, currently, the management of HHV-6 encephalitis complicating HSCT is challenging and the clinical decisions are best made considering the clinical presentation, toxicity profile, and experience of the treating clinician in conjunction with multidisciplinary input from all members of the transplant team.
Our series shows that 55.5% of the patients who developed HHV-6 encephalitis did not survive, compared with an overall 28.4% mortality among the 243 alloHSCT recipients. Thus, the mortality in patients who develop encephalitis during the post-alloHSCT period is much higher and is consistent with other reports.14,15 Eight patients in our cohort were adequately treated for viral encephalitis at the time of death. No statistical significance was noted on analysis of correlations between death and viral loads, duration of antiviral agents, or use of single or combination antiviral agents. This suggests that even though encephalitis was not the direct cause of death, there was an association with overall higher mortality, and the causal factor(s) influencing mortality in these patients remains an important unanswered question for further investigation.
Two major limitations of this study are its retrospective nature and small sample size. However, we attempted to explore and provide preliminary data to systematically characterize a phenotypic spectrum and address current challenges associated with diagnosis and management of this emerging infection. The unique observations made in this study are possible because at the NIH our clinical laboratory is set up to conduct viral loads for HHV-6 in blood and CSF and we have a large population of immunocompromised patients who get tested for HHV-6 if they have any neurologic manifestations. We also have excellent follow-up on these patients. However, a prospective study addressing these challenges, the development of appropriate clinical/neurologic outcome measures and sensitive biomarkers to detect viral pathogenicity in the CNS infection, as well as guidelines for treatment of patients undergoing alloHSCT affected with encephalitis secondary to HHV-6 are greatly needed at this time.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. James Cimino and Ms. Andrea Beri from the informatics division of the NIH, Ms. Michelle Henderson from the medical records at the Clinical Center for help with data collection, Dr. Tianxia Wu from the NINDS/NIH for help with statistical analysis, and Devera Schoenberg, MSc, for editing the manuscript for nonintellectual content.
GLOSSARY
- alloHSCT
allogeneic hematopoietic stem cell transplant
- DOCK8
dedicator of cytokinesis 8
- HHV-6
human herpes 6 virus
- HSCT
hematopoietic stem cell transplant
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Bhanushali contributed to the study concept and design, acquisition, analysis and interpretation of data, preparation and critical revisions for the intellectual content of the manuscript. Dr. Kranick contributed to study design, the acquisition of data, analysis and interpretation and critical revision of the manuscript for important intellectual content. Dr. Freeman, Dr. Cuellar-Rodriguez, and Dr. Battiwalla contributed to the acquisition of data, analysis and interpretation, and critical revision of the manuscript for important intellectual content. Dr. Gea-Banacloche, Dr. Hickstein, and Dr. Pavletic contributed to the acquisition of data, analysis and interpretation, and critical revision of the manuscript for important intellectual content. Mr. Fahle contributed to data acquisition, performed critical testing and analysis of the laboratory data, and added to important intellectual content of the manuscript. Dr. Nath contributed to the study design, analysis and interpretation of data, critical revisions of the manuscript for important intellectual content, and study supervision.
STUDY FUNDING
This research was supported by the Intramural Research Program of the NIH, NINDS, NIAID, NHLBI, and NCI.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev 2005;18:217–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zerr DM. Human herpesvirus 6: a clinical update. Herpes 2006;13:20–24 [PubMed] [Google Scholar]
- 3.Zerr DM. Human herpesvirus 6 and central nervous system disease in hematopoietic cell transplantation. J Clin Virol 2006;37(suppl 1:S52–S56 [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Haq NM, Asmar BI. Human herpesvirus 6 (HHV6) infection. Indian J Pediatr 2004;71:89–96 [DOI] [PubMed] [Google Scholar]
- 5.Ogata M. Human herpesvirus 6 in hematological malignancies. J Clin Exp Hematop 2009;49:57–67 [DOI] [PubMed] [Google Scholar]
- 6.Pasquini MC, Wang Z, Horowitz MM, Gale RP. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl 2010:87–105 [PubMed] [Google Scholar]
- 7.Denier C, Bourhis JH, Lacroix C, et al. Spectrum and prognosis of neurologic complications after hematopoietic transplantation. Neurology 2006;67:1990–1997 [DOI] [PubMed] [Google Scholar]
- 8.Sostak P, Padovan CS, Yousry TA, Ledderose G, Kolb HJ, Straube A. Prospective evaluation of neurological complications after allogeneic bone marrow transplantation. Neurology 2003;60:842–848 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Hieber M, Schwender J, Heinz WJ, et al. Viral encephalitis after allogeneic stem cell transplantation: a rare complication with distinct characteristics of different causative agents. Haematologica 2011;96:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorniak RJ, Young GS, Wiese DE, Marty FM, Schwartz RB. MR imaging of human herpesvirus-6-associated encephalitis in 4 patients with anterograde amnesia after allogeneic hematopoietic stem-cell transplantation. AJNR Am J Neuroradiol 2006;27:887–891 [PMC free article] [PubMed] [Google Scholar]
- 11.Seeley WW, Marty FM, Holmes TM, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology 2007;69:156–165 [DOI] [PubMed] [Google Scholar]
- 12.Tormo N, Solano C, de la Camara R, et al. An assessment of the effect of human herpesvirus-6 replication on active cytomegalovirus infection after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2010;16:653–661 [DOI] [PubMed] [Google Scholar]
- 13.Wang FZ, Larsson K, Linde A, Ljungman P. Human herpesvirus 6 infection and cytomegalovirus-specific lymphoproliferative responses in allogeneic stem cell transplant recipients. Bone Marrow Transplant 2002;30:521–526 [DOI] [PubMed] [Google Scholar]
- 14.Muta T, Fukuda T, Harada M. Human herpesvirus-6 encephalitis in hematopoietic SCT recipients in Japan: a retrospective multicenter study. Bone Marrow Transplant 2009;43:583–585 [DOI] [PubMed] [Google Scholar]
- 15.Sakai R, Kanamori H, Motohashi K, et al. Long-term outcome of human herpesvirus-6 encephalitis after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2011;17:1389–1394 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


