Abstract
Objective:
To study 5-HT transport and 5-HT1A receptors in temporal lobe epilepsy (TLE) and depression.
Methods:
Thirteen patients had PET with [11C]DASB for 5-HTT and [18F]FCWAY for 5-HT1A receptor binding, MRI, and psychiatric assessment. Sixteen healthy volunteers had [11C]DASB, 19 had [18F]FCWAY, and 6 had both PET studies. We used a reference tissue model to estimate [11C]DASB binding. [18F]FCWAY volume of distribution was corrected for plasma-free fraction. Images were normalized to common space. The main outcome was the regional asymmetry index. Positive asymmetry indicates relative reduced binding (reflecting transporter activity) ipsilateral to epileptic foci.
Results:
Mean regional [11C]DASB binding and asymmetry did not differ between patients and controls. [18F]FCWAY asymmetry was significantly greater for patients than controls in hippocampus, amygdala, and fusiform gyrus. On analysis of variance with region as a repeated measure, depression diagnosis had a significant effect on [11C]DASB asymmetry, with significantly higher [11C]DASB asymmetry in insular cortex (trend for fusiform gyrus). In insular cortex, patients had a significant correlation between [18F]FCWAY asymmetry and [11C]DASB asymmetry.
Conclusions:
Our study showed increased [11C]DASB asymmetry in insula and fusiform gyrus, and relatively reduced transporter activity, in subjects with both TLE and depression, as compared to subjects with TLE alone, implying reduced reuptake and thus increased synaptic 5-HT availability. This finding may represent a compensatory mechanism for 5-HT1A receptor loss. Altered serotonergic mechanisms have an important role in TLE and concomitant depression.
Serotonin (5-HT) exerts antiseizure effects in experimental models mediated by 5-HT1A receptors, by hyperpolarizing hippocampal CA3 membranes.1 Some antiepileptic drugs (AEDs) may exert antiseizure effects by blocking 5-HT reuptake.2
PET studies showed reduced 5-HT1A receptor binding ipsilateral to temporal lobe epilepsy (TLE) foci.3–5 Not due to hippocampal atrophy, reductions are more pronounced in seizure onset than secondary spread regions.5,6
Epidemiologic and clinical data support relationships between epilepsy and depression, one of the most common epilepsy comorbidities.7 A history of depression increases risk for later epilepsy development.8 Otherwise healthy patients with major depressive disorders (MDD) may have limbic 5-HT1A receptor binding reductions.9–11 5-HT1A receptor PET studies in patients with both TLE and depression showed additional binding alterations compared to TLE alone.5,7,12,13
In addition to documented 5-HT receptor abnormalities, studies in patients with mood disorders suggested a role for the serotonin transporter (5-HTT), which modulates 5-HT reuptake in the synaptic cleft.14–18 Subjects with 1 or 2 copies of the short allele of the 5-HTT promoter polymorphism had more depressive symptoms, diagnosable depression, and suicidality in relation to stressful life events than those homozygous for the long allele.19
We used PET in patients and healthy controls to test the hypothesis that 5-HT transporter is reduced in TLE, with greater reductions in concomitant depression.
METHODS
Subjects.
We included 13 patients (9 left focus, 4 right; 7 female; age 34.5 ± 8.4, mean ± SD) referred to the National Institute of Neurological Disorders and Stroke, Clinical Epilepsy Section, for uncontrollable seizures (table e-1 on the Neurology® Web site at www.neurology.org). All had focal seizures, with or without secondary generalization, as established by ictal video-EEG monitoring. All patients had a standard 1.5 or 3 T clinical MRI including T1- and T2-weighted, fluid-attenuated inversion recovery, and 3D volume sequences. Patients were taking a variety of AEDs; one had been taking 20 mg/day fluoxetine until 2 months before the study. We also scanned 29 healthy volunteers (12 female; age 33.7 ± 8.5, mean ± SD) who had never met DSM-IV criteria for a psychiatric disorder. Healthy volunteers received a general physical examination and routine laboratory tests. One control subject was taking Synthroid at a stable dose with thyroid-stimulating hormone levels within normal limits. No patient or healthy volunteer reported a history of substance abuse. All subjects had to abstain from smoking and ethanol intake for at least 2 weeks before the study.
All subjects completed Beck Depression Inventory–II as well as a Structured Clinical Interview for DSM-IV Axis I Disorders performed by a trained psychologist. The NIH PET Department prepared the 5-HTT ligand [11C]-3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile ([11C]DASB) and the 5-HT1A ligand 18F-trans-4-fluoro-N-2-[4-(2-methoxyphenyl) piperazin-1-yl]ethyl-N-(2-pyridyl) cyclohexane carboxamide ([18F]FCWAY).
All patients completed a structural MRI and [11C]DASB and [18F]FCWAY PET scans. All control subjects completed a structural MRI. Sixteen of the 29 control subjects completed [11C]DASB, 19 [18F]FCWAY PET, and 6 both the [11C]DASB and the [18F]FCWAY PET scans.
Standard protocol approvals, registrations, and patient consents.
The NIH Combined Neurosciences Institutional Review Board and the Radiation Safety Committee approved the protocol. We obtained informed consent from all protocol participants.
Data acquisition and processing.
T1-weighted MRI were acquired on a GE 1.5 or 3 T scanner using a 3D spoiled gradient-recalled acquisition or magnetization-prepared rapid acquisition with gradient echo (voxel size: 0.9375 × 0.9375 × 1.5 mm).
PET images were acquired on a GE Advance scanner in 3D mode with a reconstructed 3D spatial resolution = 6 mm full-width at half maximum, scanning 35 simultaneous slices with 4.25-mm slice separation. [11C]DASB was synthesized as previously described.20 After IV bolus administration of [11C]DASB (patients 18.5 ± 3.3, volunteers 18.7 ± 2.8 mCi) a 120-minute dynamic emission scan was acquired as 33 frames of increasing length (number × frame duration in minutes: 6 × 0.5; 3 × 1; 2 × 2; 22 × 5). We corrected for head motion by aligning later frames of longer duration (11 to 33) to a mean image of all frames using Statistical Parametric Mapping 2 (SPM2) software (www.fil.ion.ucl.ac.uk/spm)21 and MEDx (Sensor Systems, Sterling, VA). The motion-corrected PET image was coregistered to the corresponding subject's structural MRI using the Oxford Centre for Functional MRI of the Brain Linear Imaging Registration Tool (FLIRT) using a mutual information cost function.22
[11C]DASB images were processed and analyzed as previously described.15 Briefly, we used a 2-parameter multilinear reference tissue model (MRTM2)23 to estimate voxel-wise 5-HTT binding parameters. The MRTM2 allows voxel-wise estimation of the nondisplaceable receptor binding parameter BPND by using a receptor-free reference region, in this case the cerebellum, to estimate binding kinetics without using arterial data. The estimation is computationally less intensive and nearly identical to nonlinear one-tissue kinetic analysis, which requires arterial sampling. We generated parametric images of tracer delivery (R1) and binding from the dynamic [11C]DASB PET data using MRTM2 as described previously23,24 in PMOD 2.5 (PMOD Technologies, Ltd., Zurich, Switzerland). [18F]FCWAY was synthesized as previously described.6 After an IV bolus injection of 10 mCi of [18F]FCWAY over 10 seconds, a series of dynamic frames (1 to 5 minutes) was acquired for 120 minutes. Thirty radial arterial blood samples were taken to quantify [18F]FCWAY concentration and the metabolite [18F]fluorocyclohexanecarboxylic acid ([18F]FC) measured.6 Brain tissue activity frames were corrected for brain acid metabolite [18F]FC, vascular radioactivity, and F-18, fluoride metabolite spillover into the skull. An MRI-based partial volume correction (PVC) was applied.6
Patients were monitored clinically by an epilepsy nurse practitioner for ictal activity during scans. None reported experiencing seizures for at least 2 days before PET scans. All except one patient had the 2 scans no more than 2 days apart. In one case they were separated by 5 months as the FCWAY had to be rescheduled due to technical difficulties.
Data analysis.
To transform separate [18F]FCWAY and [11C]DASB binding images into a single stereotactic space for comparison, each MRI was spatially normalized to a common stereotactic array, the Montreal Neurological Institute (MNI) template, and that transform applied to the PET images; all images were resampled into 2 × 2 × 2 mm voxels using SPM2. Masks expressing the proportion of gray matter (GM) for each voxel were extracted from each MRI using SPM2 (GM mask factor 0.3).
Regions of interest (ROI) were drawn manually on a mean image of control subjects' MRI that had been normalized to MNI space. Template ROIs included the insula, hippocampus, amygdala, parahippocampal gyrus, fusiform gyrus, and cingulate cortex (figure e-1). Spatially normalized [18F]FCWAY and [11C]DASB PET images were multiplied by GM masks generated from respective MRI images. Template ROIs were applied to each subject's PET × GM image and mean voxel values were extracted for each ROI. [18F]FCWAY volume of distribution (VT) was corrected for tracer plasma-free fraction.25 For subsequent analysis, images of patients with right hemisphere foci were reversed to make the left side ipsilateral to the seizure focus. The main outcome measure was the asymmetry index [AI = 2 × (right VT – left VT)/(right VT + left VT)] calculated for each region. A positive asymmetry indicates relative reduced binding ipsilateral to the epileptic focus.
Statistical analysis.
We used Student t tests to compare regional binding values ipsilateral and contralateral to the epileptic focus (for controls, left-sided regions were arbitrarily considered ipsilateral for calculation of asymmetry). For patients, single linear regression models were used to assess the effect of age at epilepsy onset and epilepsy duration on [11C]DASB BPND and [18F]FCWAY V/f1 as well as the relation between [11C]DASB and [18F]FCWAY asymmetry in selected regions. We used analysis of variance (ANOVA) with region as a repeated measure to assess the effects of sex, MDD diagnosis, and the presence of mesial temporal sclerosis (MTS). We only included main effects in our model. Analyses were performed in SPSS version 19 (IBM Inc., Armonk, NY). All reported significance values are 2-tailed. Using the Dunn-Sidak correction for multiple comparisons, significance was set at p = 0.01.26
RESULTS
All patients completed both [18F]FCWAY and [11C]DASB PET. Thirteen male and 6 female controls (mean age 34.7 ± 8.5) completed [18F]FCWAY, and 9 male and 7 female controls (mean age 31.3 ± 8.2) completed [11C]DASB PET. The difference in the sex distributions between patients and controls for each scan was not significant.
Four patients had a history of MDD diagnosed on the Structured Clinical Interview for DSM-IV.7 Two of these reported depressive symptoms at the time of the scan. Three of the 4 had a right temporal focus and 1 a left temporal focus.
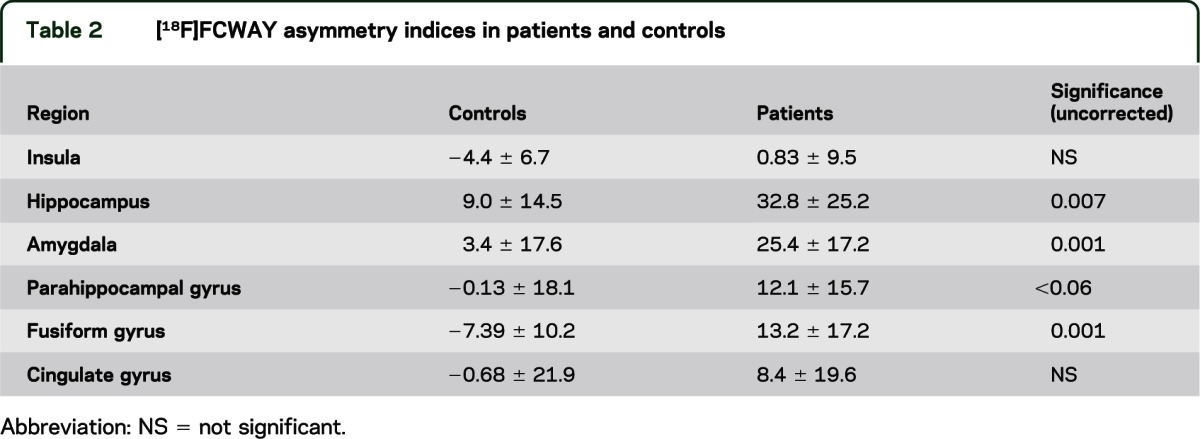
Sex did not affect [11C]DASB binding or [18F]FCWAY binding in either patients or controls. Mean regional [11C]DASB binding and asymmetry did not differ between patients and controls (table 1). Mean [18F]FCWAY binding was decreased in ipsilateral hippocampus (t = 4.4, p < 0.001) and amygdala (t = 4.9, p < 0.001), compared to contralateral regions. There were no side to side differences in [11C]DASB binding. [18F]FCWAY asymmetry was greater for patients than controls in hippocampus (p < 0.01), amygdala (p = 0.01), and fusiform gyrus (p = 0.001) (table 2).
Table 1.
[11C]DASB asymmetry indices in patients and controlsa
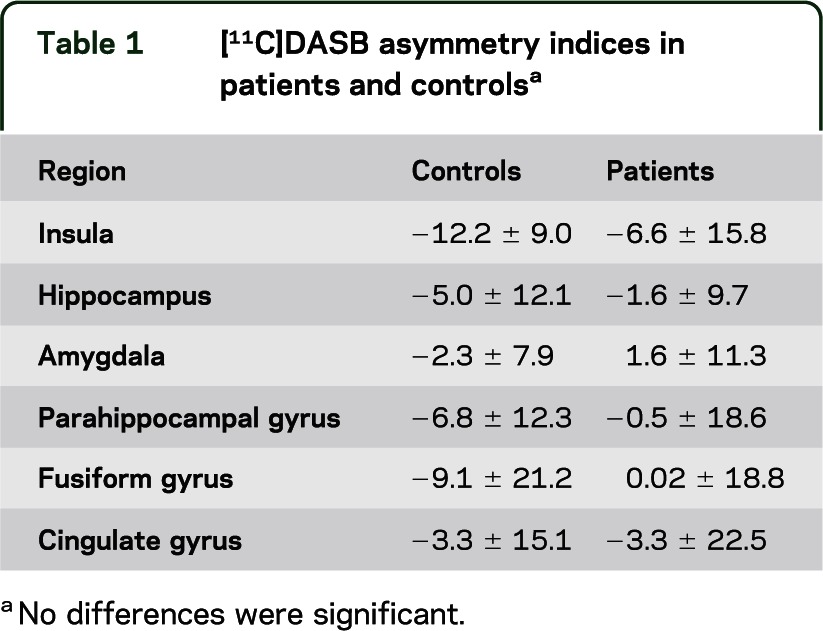
Table 2.
[18F]FCWAY asymmetry indices in patients and controls
On ANOVA with region as a repeated measure, MDD diagnosis had a significant effect on [11C]DASB AI (F = 9.3, p < 0.01). Post hoc analysis showed patients with a history of depression had higher [11C]DASB asymmetry in insular cortex (t = 4.3, p < 0.004) with a trend for fusiform gyrus (p < 0.05) (table 3 and figure 1). Adding side of seizure focus to the model did not affect the results (F = 0.397 for focus side; F = 1.69 for interaction with history of depression). There was a trend (0.05 < p < 0.10) for higher Beck Depression Inventory score to be correlated with increased insular [11C]DASB asymmetry.
Table 3.
[11C]DASB asymmetry index in patients with and without major depressive disorder
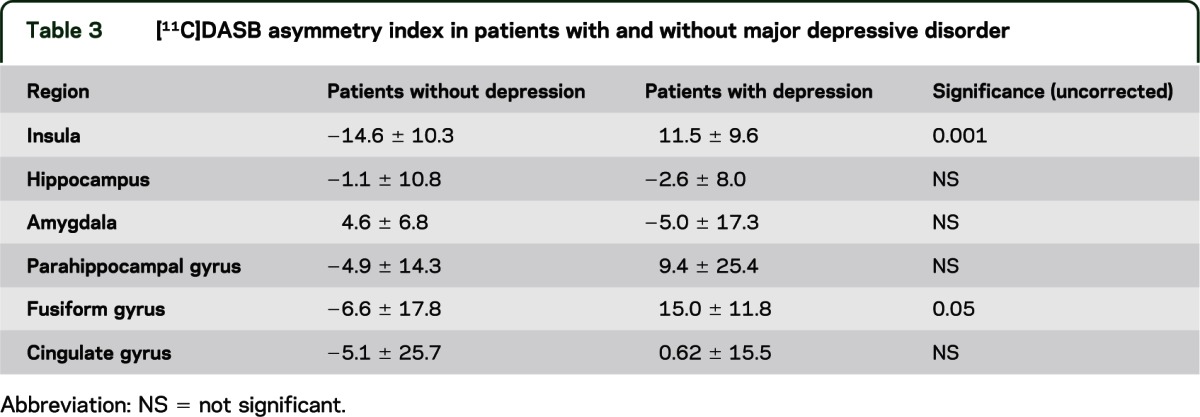
Figure 1. [11C]DASB PET.
(A) [11C]DASB PET in a patient with no history of depression shows symmetrical insula binding. (B) [11C]DASB PET in a patient with a history of depression shows relatively reduced right insula binding.
Age at epilepsy onset, epilepsy duration, side of focus, the presence of MTS, or lamotrigine, carbamazepine, or oxcarbazepine did not have significant effects on [11C]DASB binding in patients with TLE. We did not find any significant effects for depression history or MTS on [18F]FCWAY asymmetry.
In insular cortex, there was a correlation between [18F]FCWAY AI and [11C]DASB asymmetry for patients (R2 = 0.52; F = 12.0; p = 0.005) but not controls, suggesting that greater loss of 5-HT1A receptors may lead to reduced transport as a compensatory mechanism (figure 2). A trend was present for controls (R2 = 0.39; F = 2.5).
Figure 2. Relation of [11C]DASB asymmetry index to [18F]FCWAY asymmetry index in patients.
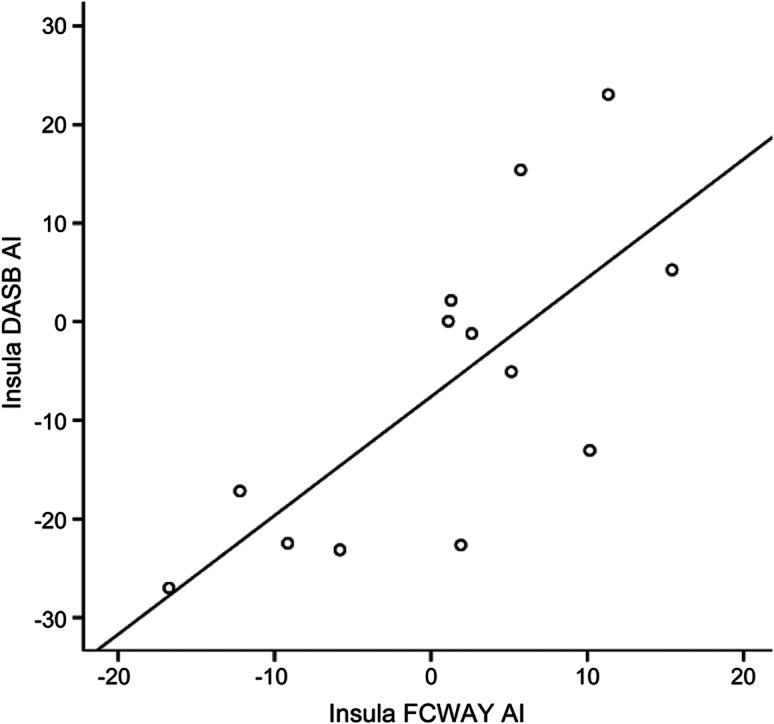
R2 = 0.522. AI = asymmetry index.
DISCUSSION
Our study provides additional evidence for involvement of serotonin in epilepsy and its association with depression, supporting results from previous clinical and imaging studies. We found an increase in [11C]DASB asymmetry in insula and fusiform gyrus, and thus relatively reduced transporter activity, in subjects with both TLE and depression, as compared to subjects with TLE alone, implying reduced reuptake and thus increased synaptic 5-HT availability. This finding may represent a compensatory mechanism for reduced 5-HT receptor binding. We found the strongest effect for depression on [11C]DASB binding in the insula, with lesser effects in mesial temporal regions. In patients with MDD, some studies have shown reduced17,18 and others increased [11C]DASB binding.15,16 Both MDD and TLE may be more heterogeneous than understood currently.
Patients with TLE and depression studied with PET using the highly selective 5-HT1A receptor ligands [18F]FCWAY and [11C]WAY had greater 5-HT1A receptor binding reductions than those with TLE alone.5,7,12 One study using another 5-HT1A ligand, [18F]MPPF, found patients with TLE and depression had relatively increased binding in some regions compared to patients with epilepsy alone.13 These dichotomous findings may be due to differences in receptor affinity between the 2 tracers. [18F]MPPF is more sensitive to synaptic 5-HT levels than [18F]FCWAY, potentially leading to relatively increased exogenous radioligand binding when endogenous serotonin availability is reduced.13
Using the PET ligands [11C](+)McN5652 and [11C]DASB, elevations in 5-HTT availability were found in subjects with MDD and bipolar disorder compared to controls in structures including thalamus, insula, prefrontal and cingulate cortex.14–16 Other investigators reported decreased 5-HTT availability in midbrain, amygdala, hippocampus, thalamus, putamen, and anterior cingulate cortex.17,18
We did not find any effects of TLE itself on [11C]DASB binding. However, our study was too small to rule out a relationship, or assess any potential clinical application of [11C]DASB PET. Several previous studies have suggested a potential role for 5-HTT in epilepsy. An association analysis found that patients with TLE had a higher frequency of the 10 repeat for the 44-bp insertion/deletion polymorphism in the 5′ regulatory region of the 5-HTT promoter second intron compared with controls.27 A study combining analysis of the 5-HTT gene-linked polymorphic region (5-HTTLPR) biallelic and 5-HTT-variable number of tandem repeats (VNTR) allele variants found that neither alone differed between patients and controls, but a combination of low-efficiency genotypes was more common in patients.28 Homozygous carriers for the 12-repeat VNTR allele had higher risk for mesial TLE with hippocampal sclerosis not responding to AEDs compared to carriers of the 10-repeat VNTR allele.29 A similar study also found that patients with TLE with the combination of more efficient genotypes (5-HTTLPR L/L and VNTR-212/12) had worse response to AEDs.30 Thus, some studies suggest high (leading to increased reuptake and reduced synaptic 5-HT) and others low 5-HTT expression (implying decreased reuptake and thus increased synaptic 5-HT) could be related to TLE itself, and that higher 5-HTT activity, and presumably lower synaptic 5-HT availability, could be related to poor AED response. One study showed no relation between 5-HTT variants and TLE.31 Preclinical data suggest that selective serotonin reuptake inhibitors (SSRIs) may be most active in partial seizure models, although not all the effects may be related to 5-HT directly.32 Limited clinical data suggesting that SSRIs may have antiseizure effects also support a role for 5-HT in seizure susceptibility.2,33 However, the wide variety of 5-HT receptors and their varying physiologic effects complicates interpretation of study results.2
Our study has some limitations. The analysis in SPM2 degraded native 3D image resolution from 6 mm to 8 mm. Although we found an effect of MDD history on [11C]DASB asymmetry, the small number of subjects in our study suggests cautious interpretation. Our sample size was too small to assess effects of seizure frequency, and might account for failure to find effects of epilepsy duration or onset age. The small sample size may also account for failure to find an effect of depression on [18F]FCWAY binding. Only 6 subjects completed both PET scans, making it more difficult to compare the relation of [11C]DASB and [18F]FCWAY binding asymmetry between patients and control groups. Our results will need to be confirmed by additional studies.
Although 3 of 4 patients with a history of depression had a right temporal and 1 a left temporal focus, the side of focus did not affect our results. Studies of the effect of the side and location of epileptic foci, as well as the presence or absence of MRI lesions including hippocampal sclerosis, have not found consistent results.34
None of the patients was currently taking antidepressants, but all were on AEDs, and one had a history of past SSRI therapy. Studies in MDD have suggested that past SSRI exposure might affect 5-HT1A receptor binding even when patients are not on the drugs; patients who had never received antidepressants had higher 5-HT1A receptor binding than either healthy controls or nonmedicated patients with past exposure.10 There are no data on the effect of AEDs on 5-HTT binding. Carbamazepine and lamotrigine might affect 5-HT reuptake.2 However, in a previous study, after correction for free fraction, we found no significant effects of individual AEDs on 5-HT1A receptor binding measured with [18F]FCWAY, or differences in binding between patients taking AEDs and healthy volunteers in regions outside the epileptic focus.25 We did not find any AED effects in the present study.
We chose to use regions drawn on averaged healthy subject brains, rather than on individual subject images, to reduce data variability and operator bias. Although this approach might have had the effect of producing apparent greater binding in healthy subjects, our use of individual subject GM masks would have corrected for this potential confounder. The FCWAY data were processed with an initial additional PVC, while the DASB data had only the SPM2 GM mask applied. The initial PVC is important for FCWAY due to the potential for spill-in and spill-out from the fluoridated metabolite.6
For [18F]FCWAY, we chose to use Vt/f1 rather than BP_F, in order to avoid the possible errors that could be introduced by using the cerebellum as a measure of nonspecific binding. Since nonspecific binding is very low, this measure is equivalent to Vs/f1, and BP_F.35,36 However, since AI was our main outcome measure, the choice of binding parameter would not affect the results.
Depression has a severe effect on quality of life in people with epilepsy.37 It may be associated with poor AED response and outcome after temporal lobectomy.38,39 Moreover, postictal 5-HT neuronal dysfunction may be associated with respiratory depression, and potentially sudden unexpected death in epilepsy, both in patients and in DBA/2 mice, where postictal respiratory arrest is reduced by SSRI pretreatment.40 Our results support data from previous imaging, pharmacologic, and clinical studies suggesting that altered serotonergic transmission may play an important role in TLE and its comorbidities.
Supplementary Material
GLOSSARY
- 5-HTTLPR
5-HTT gene-linked polymorphic region
- AED
antiepileptic drug
- AI
asymmetry index
- ANOVA
analysis of variance
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- [18F]FC
[18F]fluorocyclohexanecarboxylic acid
- GM
gray matter
- MDD
major depressive disorder
- MNI
Montreal Neurological Institute
- MRTM2
2-parameter multilinear reference tissue model
- MTS
mesial temporal sclerosis
- PVC
partial volume correction
- ROI
region of interest
- SPM2
Statistical Parametric Mapping 2
- SSRI
selective serotonin reuptake inhibitor
- TLE
temporal lobe epilepsy
- VNTR
variable number of tandem repeats
Footnotes
Supplemental data at www.neurology.org
Editorial, page 1450
AUTHOR CONTRIBUTIONS
Ashley Martinez analyzed data and wrote the article. Andrey Finegersh analyzed data. Dara M. Cannon designed the study and analyzed data. Irene Dustin carried out the study. Alison Nugent designed the study and wrote the article. Peter Herscovitch designed the study and was responsible for PET scanning. William H. Theodore designed and carried out the study, analyzed data, and wrote the article. Statistical analysis was performed by Ashley Martinez and William H. Theodore.
STUDY FUNDING
Supported by Division of Intramural Research, National Institute of Neurological Disorders and Stroke, NIH.
DISCLOSURE
A. Martinez and A. Finegersh report no disclosures. D.M. Cannon received research support from GlaxoSmithKline. I. Dustin, A. Nugent, and P. Herscovitch report no disclosures. W.H. Theodore receives an honorarium from Elsevier for editing Epilepsy Research. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Clinckers R, Smolders I, Meurs A, et al. Anticonvulsant action of hippocampal dopamine and serotonin is independently mediated by D and 5-HT receptors. J Neurochem 2004;89:834–843 [DOI] [PubMed] [Google Scholar]
- 2.Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem 2007;100:857–873 [DOI] [PubMed] [Google Scholar]
- 3.Toczek MT, Carson RE, Lang L, et al. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology 2003;60:749–756 [DOI] [PubMed] [Google Scholar]
- 4.Merlet I, Ostrowsky K, Costes N, et al. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an F-18 MPPF-PET study. Brain 2004:127;900–913 [DOI] [PubMed] [Google Scholar]
- 5.Savic I, Lindstrom P, Gulyas B, Halldin C, Andree B, Farde L. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology 2004;62:1343–1351 [DOI] [PubMed] [Google Scholar]
- 6.Giovacchini G, Toczek MT, Bonwetsch R, et al. 5-HT1A receptors are reduced in temporal lobe epilepsy after partial-volume correction. J Nucl Med 2005;46:1128–1135 [PMC free article] [PubMed] [Google Scholar]
- 7.Hasler G, Bonwetsch R, Giovacchini G, et al. 5-HT1A receptor binding in temporal lobe epilepsy patients with and without major depression. Biol Psychiatry 2007;62:1258–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol 2000;47:246–249 [PubMed] [Google Scholar]
- 9.Sargent PA, Kjaer KH, Bench CJ, et al. Brain serotonin(1A) receptor binding measured by positron emission tomography with C-11 WAY-100635-Effects of depression and antidepressant treatment. Arch Gen Psychiatry 2000;57:174–180 [DOI] [PubMed] [Google Scholar]
- 10.Parsey RV, Oquendo MA, Ogden RT, et al. Altered serotonin 1A binding in major depression: a carbonyl-C-11 WAY100635 positron emission tomography study. Biol Psychiatry 2006;59:106–113 [DOI] [PubMed] [Google Scholar]
- 11.Drevets WC, Thase ME, Moses-Kolko EL, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol 2007;34:865–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theodore WH, Hasler G, Giovacchini G, et al. Reduced hippocampal 5-HT1A PET receptor binding and depression in temporal lobe epilepsy. Epilepsia 2007;48:1526–1530 [DOI] [PubMed] [Google Scholar]
- 13.Lothe A, Didelot A, Hammers A, et al. Comorbidity between temporal lobe epilepsy and depression: a F-18 MPPF PET study. Brain 2008;131:2765–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer JH, Houle S, Sagrati S, et al. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry 2004;61:1271–1279 [DOI] [PubMed] [Google Scholar]
- 15.Cannon DM, Ichise M, Fromm SJ, et al. Serotonin transporter binding in bipolar disorder assessed using C-11 DASB and positron emission tomography. Biol Psychiatr 2006;60:207–217 [DOI] [PubMed] [Google Scholar]
- 16.Cannon DM, Ichise M, Rollis D, et al. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and C-11 DASB: comparison with bipolar disorder. Biol Psychiatry 2007;62:870–877 [DOI] [PubMed] [Google Scholar]
- 17.Parsey RV, Hastings RS, Oquendo MA, et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry 2006;163:52–58 [DOI] [PubMed] [Google Scholar]
- 18.Oquendo MA, Hastings RS, Huang YY, et al. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry 2007;64:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386–389 [DOI] [PubMed] [Google Scholar]
- 20.Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S. Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, and in vitro and ex vivo evaluation of C-11-labeled 2-(phenylthio) araalkylamines. J Med Chem 2000;43:3103–3110 [DOI] [PubMed] [Google Scholar]
- 21.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992;16:620–633 [DOI] [PubMed] [Google Scholar]
- 22.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001;5:143–156 [DOI] [PubMed] [Google Scholar]
- 23.Ichise M, Liow JS, Lu JQ, et al. Linearized reference tissue parametric Imaging methods: application to C-11 DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 2003;23:1096–1112 [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Ichise M, Sangare J, Innis RB. PET imaging of serotonin transporters with C-11 DASB: test-retest reproducibility using a multilinear reference tissue parametric imaging method. J Nucl Med 2006;47:208–214 [PubMed] [Google Scholar]
- 25.Theodore WH, Giovacchini G, Bonwetsch R, et al. The effect of antiepileptic drugs on 5-HT1A receptor binding measured by positron emission tomography. Epilepsia 2006;47:499–503 [DOI] [PubMed] [Google Scholar]
- 26.Dunn OJ. Multiple comparisons among means. J Am Stat Assoc 1961;56:52–64 [Google Scholar]
- 27.Manna I, Labate A, Gambardella A, et al. Serotonin transporter gene (5-HTT): association analysis with temporal lobe epilepsy. Neurosci Lett 2007;421:52–56 [DOI] [PubMed] [Google Scholar]
- 28.Schenkel LC, Bragatti JA, Torres CM, et al. Serotonin transporter gene (5-HTT) polymorphisms and temporal lobe epilepsy. Epilepsy Res 2011;95:152–157 [DOI] [PubMed] [Google Scholar]
- 29.Kauffman MA, Consalvo D, Gonzalez-Morón D, Aguirre F, D’Alessio L, Kochen S. Serotonin transporter gene variation and refractory mesial temporal epilepsy with hippocampal sclerosis, Epilepsy Res 2009;85:231–234 [DOI] [PubMed] [Google Scholar]
- 30.Hrvoje H, Jasminka S, Lipa C-S, Vida D, Branimir J. Association of serotonin transporter promoter (5-HTTLPR) and intron 2 (VNTR-2) polymorphisms with treatment response in temporal lobe epilepsy. Epilepsy Res 2010;91:35–38 [DOI] [PubMed] [Google Scholar]
- 31.Stefulj J, Bordukalo-Niksic T, Hecimovic H, Demarin V, Jernej B. Epilepsy and serotonin (5-HT): variation of 5-HT-related genes in temporal lobe epilepsy. Neurosci Lett 2010;478:29–31 [DOI] [PubMed] [Google Scholar]
- 32.Igelstrom KM. Preclinical antiepileptic actions of selective serotonin reuptake inhibitors: implications for clinical trial design. Epilepsia 2012;53:596–605 [DOI] [PubMed] [Google Scholar]
- 33.Alper K, Schwartz KA, Kolts RL, Khan A. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) Summary basis of approval reports. Biol Psychiatr 2007;62:345–354 [DOI] [PubMed] [Google Scholar]
- 34.Hoppe C, Elger CE. Depression in epilepsy: a critical review from a clinical perspective. Nat Rev Neurol 2011;7:462–472 [DOI] [PubMed] [Google Scholar]
- 35.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007;27:1533–1539 [DOI] [PubMed] [Google Scholar]
- 36.Savitz JB, Drevets WC. Neuroreceptor imaging in depression. Neurobiol Dis 2012;52:49–65 [DOI] [PubMed] [Google Scholar]
- 37.Cramer JA, Blum D, Reed M, Fanning K; Epilepsy Impact Project Group The influence of comorbid depression on quality of life for people with epilepsy. Epilepsy Behav 2003;4:515–521 [DOI] [PubMed] [Google Scholar]
- 38.Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res 2007;75:192–196 [DOI] [PubMed] [Google Scholar]
- 39.Kanner AM, Byrne RW, Chicharro AV, Wuu J, Frey M. Is a lifetime psychiatric history predictive of a worse postsurgical seizure outcome following a temporal lobectomy? Neurology 2009;72:793–799 [DOI] [PubMed] [Google Scholar]
- 40.Richerson GB, Buchanan GF. The serotonin axis: shared mechanisms in seizures, depression, and SUDEP. Epilepsia 2011;52(suppl 1):28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



