Abstract
Thymic stromal lymphopoietin (TSLP) plays important roles in the pathogenesis of allergic diseases. Whether and how TSLP is involved in the initial priming of Th2 differentiation against harmless antigen remains unclear. Using an intranasal sensitization protocol with OVA and LPS, we showed that TSLP signaling is required for low-dose LPS induced Th2 inflammation, but not for high-dose LPS induced Th1 immunity. We further demonstrated that low-dose LPS-activated bone marrow derived dendritic cells expressed relatively high Tslp but low Il12a, and were able to prime naïve DO11.10 T cells to differentiate into Th2 in a TSLP dependent manner. After transfer into wild type recipient mice, the low-dose LPS-activated OVA-loaded DC induced airway eosinophilia, but primed neutrophil-dominated airway inflammation when TSLP-deficient DC were used. These studies demonstrated that TSLP released by DC in response to a low concentration of LPS plays a role in priming Th2 differentiation and thus may serve as a polarizing third signal, in addition to antigen/MHC II and costimulatory factors, from antigen presenting DC to direct effector T cell differentiation.
Keywords: Th2, allergic airway inflammation, TSLP
Introduction
Asthma is a chronic pulmonary disease of dysregulated immune responses against commonly inhaled innocuous antigens, which may be caused by a combination of environmental and genetic factors [1]. Studies of patients and animal models demonstrated that T helper type-2 (Th2) effector cells producing cytokines IL-4, IL-5 and IL-13 contribute to many of the pathophysiological features of asthma, including airway inflammation, mucus hypersecretion and airway hyperresponsiveness (AHR) [2].
Adaptive immune responses depend on signals from innate immune cells, particularly the professional antigen presenting dendritic cells (DC). In addition to provide TCR ligands and costimulation, DC are able to secrete IL-12 upon activation of pattern recognition receptors by pathogen-associated molecular patterns, priming naïve CD4+ T cells to differentiate into Th1 [2, 3]. How DC control Th2 differentiation, however, is not well understood. IL-4 is the most important cytokine for Th2 differentiation in vivo and in vitro [4–7]. Unlike the Th1 polarizing cytokine IL-12, the early source of IL-4 that initiates Th2 differentiation has been debated. Natural killer T cells [8] and basophils [9, 10] produce large amounts of IL-4 when activated and are essential for some allergic responses in vivo [11, 12], though the importance of these cells in Th2 cell induction or merely orchestrating/mediating the effector response in vivo is unclear.
Recent studies showed that thymic stromal lymphopoietin (TSLP) is critical for allergic inflammation in humans [13] and mice [14]. TSLP is highly expressed in acute and chronic atopic dermatitis lesions, and airways of allergic asthma patients [13, 15, 16]. Over-expression of TSLP in lung leads to eosinophilic airway inflammation and hyperactivity [14], while in skin results in skin inflammation characteristic of atopic dermatitis [17, 18]. TSLP receptor-deficient (Tslpr−/−) mice is protected from developing allergic airway inflammation [14, 19] and allergic skin inflammation [20]. Human TSLP strongly activated peripheral blood derived DCs in vitro to up-regulate MHC II and other co-stimulatory molecules leads to Th2 differentiation of allogeneic CD4+ T cells in vitro [13, 21]. In mice, TSLP is able to directly act on naïve CD4+ T cells to promote Th2 differentiation and/or IL-4 secretion in vivo and in vitro [11, 20, 22]. Transferring wild-type CD4+ T cells into Tslpr−/− mice restores their allergic responses in murine asthma and AD models [19, 20], supporting a function of TSLP through a direct action on CD4+ T cells. However, TSLP is believed to be mainly produced by epithelial cells of peripheral tissues [20, 23–26]. Thus it is not clear whether and how TSLP is involved in primary immune response (i.e. sensitization) which would require the presence of TSLP in the draining lymph nodes at the time of T cell activation.
LPS, a cell wall component of Gram-negative bacteria, is ubiquitously present in the environment, and known to induce DC maturation and production of IL-12 and IFN-γ to drive Th1 immunity [27]. Several studies demonstrated that airway Th1 or Th2 responses to inhaled antigen are determined by levels of LPS [26, 28, 29]. While inhalation of high concentration of LPS (HiLPS) induces IL-12 expression leading to Th1 response, animals sensitized with low-dose LPS (LoLPS) fail to induce IL12 in DC resulting in Th2 airway inflammation after challenge. In this study, we showed that TSLP signaling was only required for LoLPS-induced Th2 biased airway inflammation, but not HiLPS-induced Th1 inflammation. Our data further demonstrated that LPS activated bone marrow-derived dendritic cells (BMDC) to express TSLP, which provided a polarizing signal to prime Th2 differentiation in vitro. When transferred intranasally to naïve mice, the LoLPS-activated, OVA-loaded DC primed Th2 sensitization dependent on TSLP derived from the DC. Thus, in addition to an established role for DC in Th1 polarization, our data suggest that DC-derived TSLP could act as a polarizing cytokine to initiate Th2 differentiation and allergic sensitization in vivo, and further define the Th1/Th2 paradigm of airway mucosal immunity in response to the natural adjuvant LPS.
Results
TSLP is required for LoLPS induced Th2 sensitization
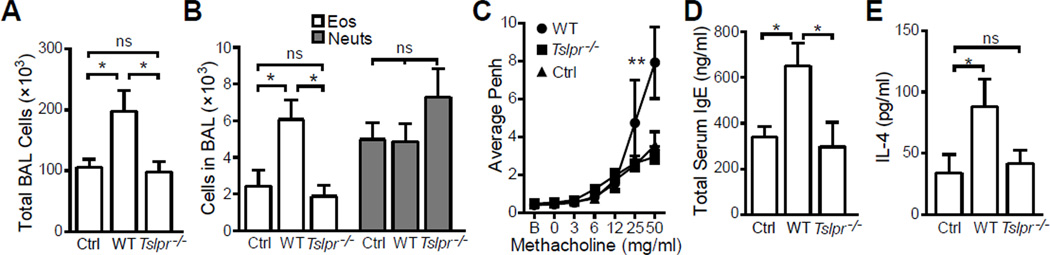
Several studies have shown that generation of Th1- or Th2-mediated inflammatory responses to inhaled antigen is dependent on the concentration of LPS administered with antigen [26, 28, 29]. Specifically, a high concentration of LPS (HiLPS) induces a Th1 response and a low concentration (LoLPS) induces Th2. Since TSLP is critical for Th2-mediated allergic inflammation [30], we set out to determine whether TSLP plays a role in LoLPS induced allergic airway inflammation. Balb/c mice and Tslpr−/− mice were sensitized intranasally (i.n.) with 100 µg OVA + 20 ng LPS for three days. After i.n. OVA challenge, Balb/c mice developed airway inflammation with significantly increased total cell numbers (Fig. 1A) and eosinophils (Fig. 1B) in BAL as compared to non-sensitized mice (OVA only without LPS at sensitization). The sensitized wild type mice also exhibited airway hyperresponsiveness (Fig. 1C). In contrast to wild type mice, Tslpr−/− mice showed no significant airway inflammation and AHR, compared to non-sensitized mice (Fig. 1A–C). Increased serum IgE, whose synthesis heavily relies on combined effects of Th2 cytokines [31], is closely related to allergic or atopic diseases. Consistent to their much reduced allergic airway inflammation, Tslpr−/− mice showed no increase in total serum IgE compared to control mice (Fig. 1D). We next isolated cells from lung draining lymph nodes and stimulated with anti-CD3/CD28. Only cells from wild type animals produced significantly increased IL-4 (Fig. 1E).
Figure 1. Induction of allergic airway inflammation in mice sensitized with OVA + 20 ng LPS requires TSLP signaling.
Wild type (WT) and Tslpr-deficient (Tslpr−/−) Balb/c mice were sensitized with 100 µg OVA + 20 ng LPS for three times on days 0, 1, and 2. Control (Ctrl) mice were treated with OVA only. All mice were challenged 4 times with 50 µg OVA on days 14, 15, 18 and 19. Lung function was analyzed on day 20 and animals were sacrificed on day 21 for rest of the analysis. (A) Total BAL cell counts. Only WT mice exhibited significant airway inflammation. (B) Eosinophil (Eos) and neutrophil (Neuts) counts in BAL. Only WT mice exhibited significant airway eosinophilia. (C) Airway responsiveness to increasing dose of methacholine analyzed by unrestrained whole body plethysmography and presented as average enhanced pause (Penh) over a three minute period. (D) Total serum IgE concentration. (E) IL-4 production by anti-CD3 restimulated cells isolated from lung draining lymph nodes. Data represent mean ± SEM (n = 4 mice/group). *: p < 0.05; **: p < 0.01; ***: P < 0.001; ns: not significant by analysis of variance with Bonferroni’s post-hoc tests.
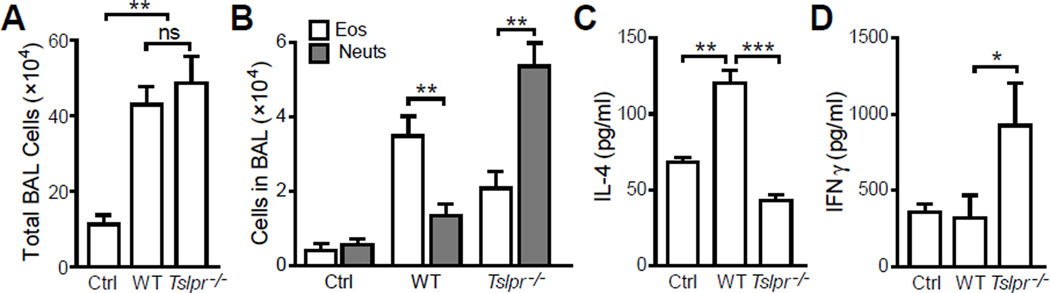
Since even wild type animals sensitized with 20 ng LPS showed only a modest increase in total BAL cells (Fig. 1A) and eosinophils (Fig. 1B), we increased the LPS dose used at sensitization. When sensitized with 100 µg OVA in the presence of 50 ng LPS, Tslpr−/− mice indeed showed significant airway inflammation with similar total cell counts in BAL to that seen in WT mice (Fig. 2A). Surprisingly, we found that types of airway inflammation in Tslpr−/− mice and WT mice were completely different. While wild type mice displayed typical eosinophilic airway inflammation, Tslpr−/− mice showed neutrophil-dominated infiltration in the airways with significantly higher neutrophils than eosinophils in the BAL (Fig. 2B). These data suggested that instead of the Th2-biased immune response in wild type mice, 50 ng LPS at sensitization primed a Th1-biased immune response when TSLP signaling was interrupted. To further confirm the Th2 to Th1 shift in Tslpr−/− mice, we examined cytokine expression by restimulated cells isolated from lung draining lymph nodes. Consistent with the composition of airway infiltrating leukocytes, cells from wild type animals produced more IL-4 (Fig. 2C) while Tslpr−/− cells secreted a higher concentration of IFNγ (Fig. 2D).
Figure 2. TSLP signaling determines the nature of airway inflammation in mice sensitized with OVA + 50 ng LPS.
Balb/c (WT) and TSLPR-deficient (Tslpr−/−) mice were treated as described in Fig. 1 except 50 ng LPS was used. (A) Total BAL cell counts. No significant difference in the severity of airway inflammation was seen in WT and Tslpr−/− mice. (B) Eosinophil (Eos) and neutrophil (Neuts) counts: Eosinophil-dominated airway inflammation in WT mice vs neutrophil-dominated airway inflammation in Tslpr−/− mice. (C) and (D) IL-4 and IFNγ production by cells isolated from lung draining lymph nodes and restimulated with anti-CD3. Data represent mean ± SEM (n = 4 mice/group). *: p < 0.05; **: p < 0.01; ***: P < 0.001; ns: not significant by analysis of variance with Bonferroni’s post-hoc tests.
TSLP is not required for HiLPS induced Th1 sensitization
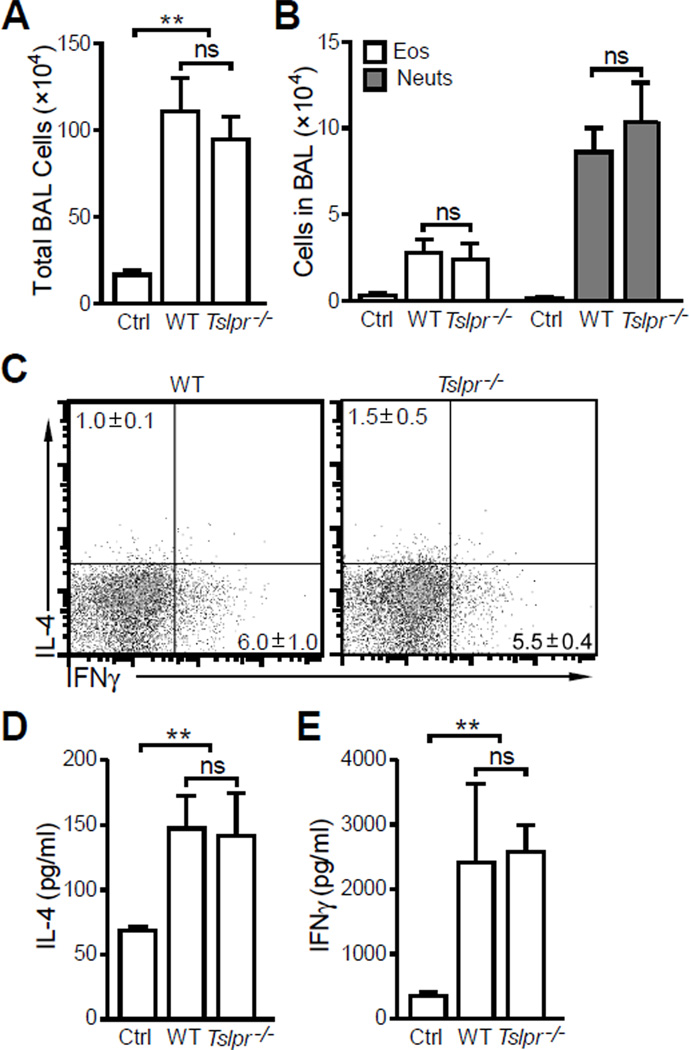
Next, we tested roles of TSLP in high-dose LPS primed Th1 response. Mice were sensitized i.n. with 100 µg OVA + 10 µg LPS and challenged i.n. with 50 µg OVA. Consistent with published data [26, 28, 29], high-dose LPS during sensitization primed a Th1-biased airway immune response dominated by neutrophils regardless of TSLP signaling (Fig. 3A and B). Intracellular staining of total BAL cells demonstrated that CD4+ T cells in the airways were Th1 polarized in both WT and Tslpr−/− mice (Fig. 3C). Consistent with intracellular staining of the BAL cells, anti-CD3 restimulation of cells isolated from lung draining lymph nodes also showed a low IL-4 (Fig. 3D) and high IFNγ (Fig. 3E) cytokine expression profile in HiLPS induced allergic response in both WT and Tslpr−/− mice.
Figure 3. TSLP signaling is not required for HiLPS induced neutrophilic airway inflammation.
Wild type (WT) and TSLPR-deficient (Tslpr−/−) Balb/c mice were treated as described in Fig. 1 except 10 µg LPS was used. (A) Total BAL cell counts. (B) Eosinophil (Eos) and neutrophil (Neuts) counts in BAL showing no significant differences in BAL eosinophils and neutrophils were seen in WT and Tslpr−/− mice. (C) Intracellular cytokine staining of BAL cells. (D) and (E) IL-4 and IFNγ production by cells isolated from lung draining lymph nodes and re-stimulated with anti-CD3. Data represent mean ± SEM (n = 4 mice/group). **: p < 0.01; ns: not significant by analysis of variance with Bonferroni’s post-hoc tests.
DC-derived Tslp expression promotes Th2 differentiation in vitro
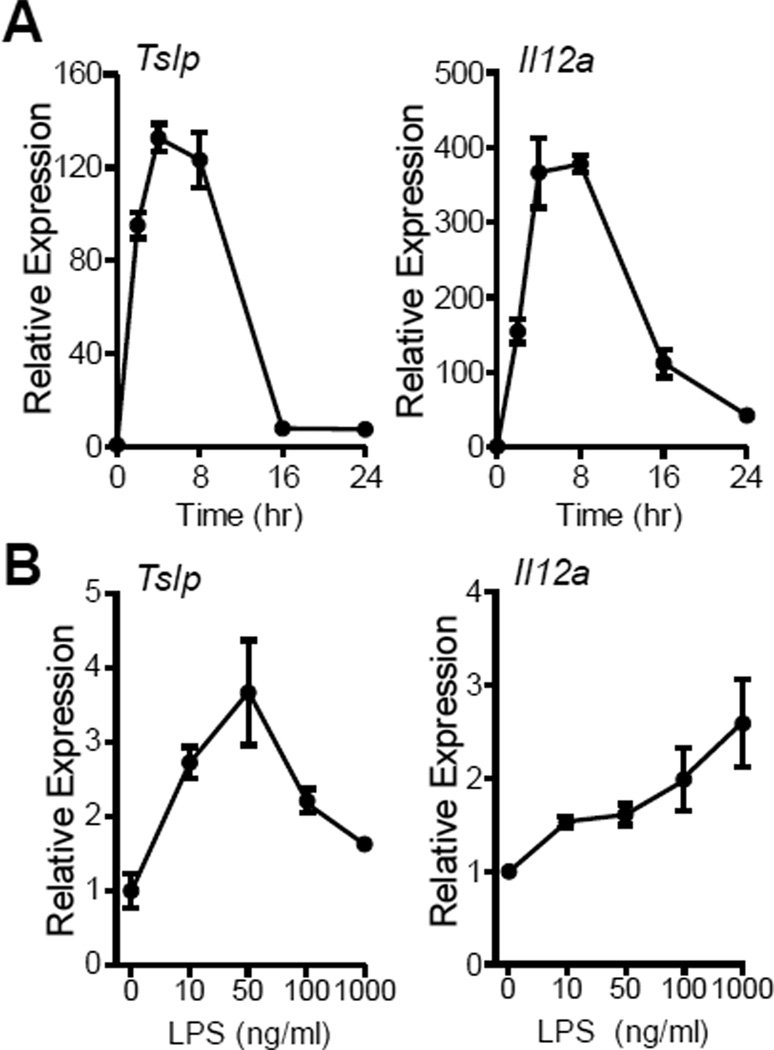
Airway DC are essential for controlling effector T-cell response in allergen sensitization [3, 28]. To delineate the mechanism by which TSLP primes Th2 differentiation, we examined the ability of BMDC to express TSLP when stimulated by LPS. Consistent to a recent finding that TSLP can be produced by mouse and human DC [32, 33], we also found that BMDC were able to express TSLP in response to LPS stimulation. In response to 10 ng/ml LPS, BMDC quickly upregulated Tslp expression, reaching a peak around 4-8 hr (Fig. 4A), a time-course similar to expression of the IL-12 components Il12a and Il12b expression (Fig. 4A and data not shown). Surprisingly, unlike Il12a whose expression increased with increasing dose of LPS, Tslp expression showed a bell curve pattern and its peak expression was seen between 10 – 100 ng/ml LPS (Fig. 4B).
Figure 4. LPS induction of Tslp and Il12a expression in bone marrow derived dendritic cells.
After wash with RPMI media, triplicates of 1 × 106 BMDC were cultured in 1 ml DC media without GM-CSF and treated with LPS as indicated below. (A) Time course of Tslp and Il12a expression in DC cultured with 10 ng/ml LPS for varying time. (B) Dose response Tslp and Il12a expression in DC culture with varying LPS dose for 8 h. Gene expression was assessed by realtime PCR and presented relative to DC at 0 h (A) or cultured with 0 ng/ml LPS (B). Data represent mean ± SEM (n = 3).
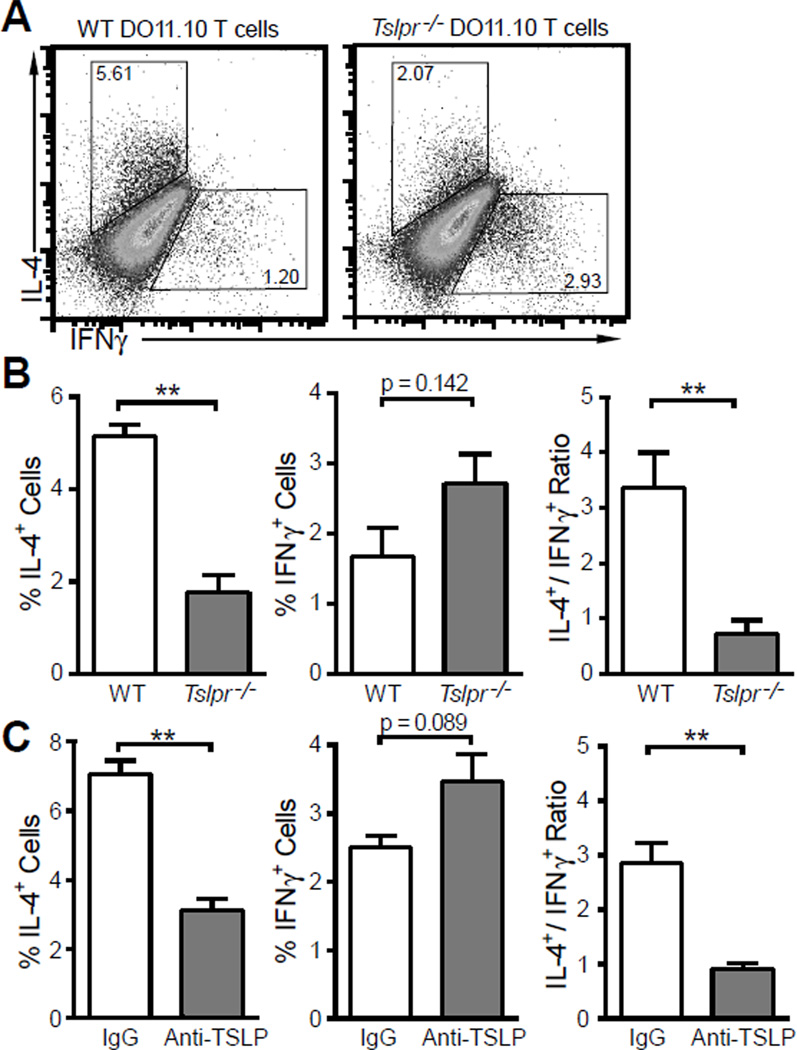
Next, we examined the role of DC-derived TSLP in Th2 differentiation in vitro. BMDC were pulsed with 10 ng/ml LPS and 100 µg OVA overnight, washed and cultured with wild type or Tslpr−/− DO11.10 CD4+ T cells in the presence of 100 µg OVA or 10 µg OVA323–339 peptide for five days. After the culture, helper T cell polarization was determined by intracellular cytokine staining. As shown in Figure 5, wild type DO11.10 CD4+ T cells showed strong Th2 polarization with IL-4+/IFNγ+ cell ratio of 3.4 ± 0.6 whereas Tslpr−/− cells exhibited modest Th1 polarization with IL-4+/IFNγ+ cell ratio of 0.7 ± 0.2. To rule out the possibility that Tslpr−/− cells were developmentally predisposed to Th1 differentiation rather than the role of DC-derived TSLP, we cultured wild type DO11.10 CD4+ T cells with conditioned BMDC as above in the presence of anti-TSLP neutralization antibody [11] and observed that neutralizing TSLP reduced IL-4+ cells and increased IFNγ+ cells in the culture (Fig. 5C).
Figure 5. BMDC matured with 10 ng/ml LPS polarize Th2 differentiation in a TSLP dependent manner.
BMDC were pulsed with 100 µg/ml OVA and 10 ng/ml LPS overnight. Naïve CD4+ T cells were isolated from wild type (WT) or Tslpr-deficient (Tslpr−/−) DO11.10 mice and cocultured with BMDC in the presence of 100 µg/ml OVA or 10 µg/ml OVA peptide for 5 days. Th1/Th2 polarization of CD4+ T cells were assessed by flow cytometry after intracellular staining for IL-4 and IFNγ. (A) Representative dot plots of flow cytometry. (B) Polarization of wild type (WT) and Tslpr-deficient (Tslpr−/−) DO11.10 T cells primed by LoLPS matured DC. (C) Polarization of wild type DO11.10 T cell when DC-derived TSLP was neutralized by anti-TSLP antibody. IgG: normal rat IgG as control antibody. Data represent mean ± SEM (n = 3). **: p < 0.01 by t - test.
Priming of Th2 response by LoLPS conditioned DC relies on DC-derived TSLP in vivo
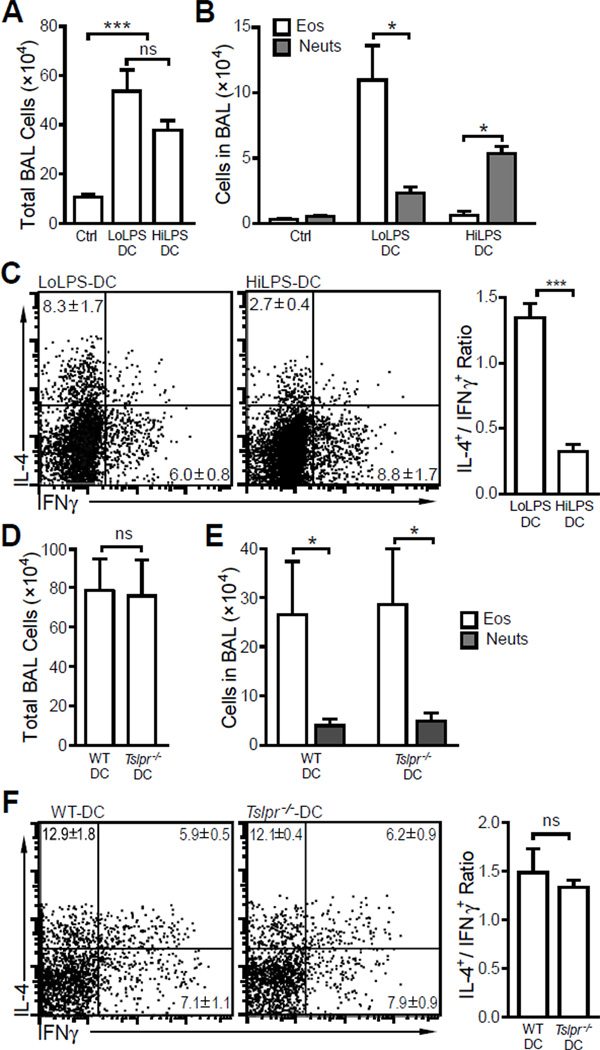
Based upon our in vitro results (Fig. 4 and Fig. 5), we hypothesized that BMDC stimulated with 10 ng/ml LPS, expressing high TSLP and low IL-12, would prime Th2 sensitization when transferred into recipient mice. To test this hypothesis, BMDC were pulsed overnight with 10 ng/ml or 1 µg/ml LPS in the presence of 100 µg/ml OVA, washed three times and intranasally transferred into wild type Balb/c animals (2 × 106 cells/mouse). Ten days after the cell transfer, mice were challenged i.n. with OVA for three times and airway inflammation examined. We observed that transferring BMDC matured with 10 ng/ml LPS (Low LPS-DC) or 1 µg/ml LPS (High LPS-DC) induced similar airway inflammation after challenge (Fig. 6A). However, differential cell counts indicated that Low LPS-DC induced eosinophilic airway inflammation while transfer of High LPS-DC led to neutrophilic airway inflammation (Fig. 6B). Consistent with the BAL cell composition, CD4+ T cells in BAL were polarized into Th2 or Th1 determined by LPS doses used to mature BMDC (Fig. 6C). These results indicated that varying concentrations of LPS acting on DC alone are sufficient to determine Th1/Th2 polarization in the airways.
Figure 6. LPS doses used to pulse BMDC determine the nature of airway inflammation.
(A – C) BMDC were pulsed with 10 ng/ml LPS (LL-DC) or 1 µg/ml LPS (HL-DC) overnight in the presence of 100 µg OVA, and were intranasally transferred into wild type Balb/c mice. Control mice received PBS. Ten days after the DC transfer, mice were challenged i.n. with 50 µg OVA for 3 times. (A) Total BAL cell counts. (B) Eosinophil (Eos) and neutrophil (Neuts) counts showed the ratios of eosinophils and neutrophils in BAL were determined by LPS doses used to pulse the DC. (C) Intracellular cytokine staining of BAL cells. (D – F) Wild type (WT) or Tslpr-deficient (Tslpr−/−) BMDC were pulsed with 10 ng/ml LPS overnight in the presence of 100 µg OVA, intranasally transferred into wild type Balb/c mice. Ten days after the DC transfer, mice were challenged i.n. with 50 µg OVA for 3 times. (D) Total BAL cell counts. (E) Eosinophil (Eos) and neutrophil (Neuts) counts. (F) Intracellular cytokine staining of BAL cells showing Th2 biased airway inflammation. Data represent mean ± SEM (n = 4 mice/group). *: p < 0.05; ** p < 0.01; ***: p < 0.001; ns: not significant by analysis of variance with Bonferroni’s post-hoc tests (A) or by t-test (B – F).
Next, we transferred low dose LPS (10 ng/ml) conditioned, OVA-pulsed WT and TSLPR-deficient BMDC into wild type Balb/c mice and challenged these mice with OVA for three times. The mice exhibited similar airway eosinophilia and Th2 polarization of CD4+ T cells in BAL (Fig. 6D – F). The fact that both WT and TSLPR-deficient BMDC primed Th2 sensitization suggest that Th2 differentiation of CD4+ T cells was not determined by the ability of antigen-presenting DC to respond to TSLP in this experimental model.
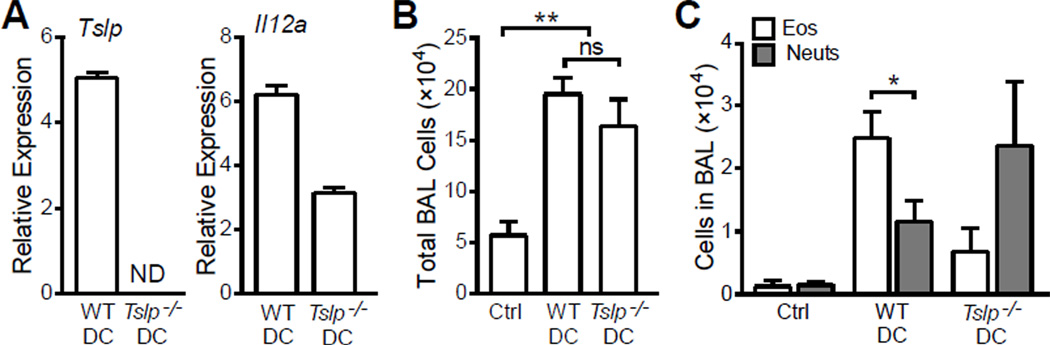
To directly examine the role of DC-derived TSLP in priming Th2 sensitization in vivo, we generated TSLP knockout (Tslp−/−) mice (C57BL/6 background) with targeted embryonic stem cells purchased from the Knock-Out Mouse Program (KOMP). BMDC from these mice expressed Il12a but no detectable Tslp expression, after culture for 8 h with 20 ng/ml LPS (Fig. 7A). After being pulsed with 20 ng/ml LPS and 100 µg/ml OVA, wild type or Tslp−/− DC were washed three times and transferred i.n. into wild type C57BL/6 mice. Since C57BL/6 mice were used in this experiment, they were challenged 6 times with 50 µg OVA 10 days after DC transfer. Under these conditions, both WT DC and Tslp−/− DC were able to induced mild airway inflammation without significant difference in total BAL cell counts (Fig. 7B). While WT DC induced eosinophilic airway infiltration with significantly higher eosinophils than neutrophils in BAL, DC transfer induced neutrophilic airway inflammation with more neutrophils than eosinophils in BAL (Fig. 7C). The composition of the inflammatory cells infiltrating the airways suggested that the ability of low LPS-stimulated DC to prime Th2 differentiation in vivo was dependent on DC-derived TSLP.
Figure 7. Eosinophilic airway inflammation induced by LoLPS pulsed BMDC is dependent on DC-derived TSLP.
Wild type DC (WTDC) and Tslp-deficient DC (Tslp−/−DC) were pulsed with 20 ng/ml LPS and 100 µg/ml OVA overnight, washed and transferred i.n. into wild type C57BL/6 mice. Control mice received PBS. Ten days after DC transfer, mice were challenged with 50 µg OVA for 6 times. (A) Relative expression of Tslp and Il12a in DC cultured with 20 ng/ml LPS for 8 h. Gene expression was assessed by realtime PCR and presented relative to DC cultured with 0 ng/ml LPS. (B) Total BAL cell counts. (C) Eosinophil (Eos) and neutrophil (Neuts) counts in BAL. Without DC-derived TSLP, eosinophil-dominated airway inflammation shifted to neutrophil-dominated airway inflammation. Data represent mean ± SEM (n = 3 for A and n = 5 – 7 mice/group for B, C). ND: not detectable; *: p < 0.05; ns: not significant by analysis of variance with Bonferroni’s post-hoc tests.
Discussion
Antigens presented by mature DCs result in the activation of naïve CD4+ T cells which differentiate into helper T subsets in the presence of polarizing cytokines. Although the DC-derived polarizing signals such as IL-12 involved in Th1 differentiation are well defined, much less is known about those required for the generation of Th2 responses. DCs activated and matured by LPS or other TLR ligands do not produce the primary Th2 polarizing cytokine IL-4. Thus it has been proposed that Th2 development occurred by default in the absence of IL-12 from DCs activated by low dose of LPS [28]. In this report, we present evidence showing that DC-derived TSLP in response to low LPS might serve as a polarizing cytokine to induce Th2 differentiation during primary immune responses.
While the requirement for TSLP in the pathogenesis of allergic airway inflammation has been documented [14, 19], the use of peritoneal OVA/Alum to initiate strong Th2 responses is not the optimal model for examining T cell-APC interactions. Sensitization with antigen and LPS through the respiratory system, the anatomic site that would normally encounter environmental allergens and LPS, provides a particular relevant model to investigate immune responses in the pathogenesis of asthma [34]. Here we demonstrated that TSLPR-deficient mice failed to initiate a Th2 response when i.n. sensitized with OVA + 20 ng LPS (Fig. 1), but exhibited normal Th1 immunity in response to OVA + 10 µg LPS sensitization (Fig. 3). The skewing from Th2 to Th1 immunity by Tslpr−/− mice in response to 50 ng LPS (Fig. 2) strongly supports a critical role of TSLP in promoting Th2 polarization in vivo.
TSLP is able to directly act on naïve CD4+ T cells to promote Th2 differentiation and/or IL-4 secretion [11, 20, 22]. TSLP treatment leads to immediate, direct Il4 gene transcription and drives Th2 differentiation in the absence of exogenous IL-4 [22]. However, IL-4 blockade inhibited TSLP-mediated Th2 differentiation, demonstrating endogenous IL-4 is involved via an autocrine mechanism. Yet it is not clear whether and how TSLP is involved in the initiation of Th2 sensitization in vivo since TSLP is produced by epithelial cells of peripheral tissues in response to TLR agonists and proinflammatory cytokines [20, 23–26]. Studies by Sokol et al [11] showed that basophils activated by papain were recruited to draining lymph nodes and expressed TSLP and IL-4. Unlike DCs which were recruited into draining lymph nodes 18 hours after papain immunization, significant basophil numbers were recruited to draining lymph nodes 3 days after immunization when substantial IL-4-eGFP+ Th2 cells already existed in the draining lymph nodes [11]. Thus TSLP and IL-4 expressing basophils are most likely augmenting, rather than initiating, Th2 differentiation in vivo. Such a notion was supported by a recent study showing that basophils did not interact with antigen-specific T cells in lymph nodes and were not required for Th2 priming in vivo [35].
Airway DC are essential for controlling effector T-cell response in sensitization, the primary immune response [3, 28]. DC isolated from OVA + HiLPS sensitized mice induced Th1 polarization and DC isolated from OVA + LoLPS sensitized mice induced Th2 polarization [26]. The fact that LoLPS induced stronger eosinophilic airway inflammation in mice with intact TLR4 on hematopoietic cells than in mice with intact TLR4 on lung stromal cells [26] supports the existence of the LoLPS induced signal(s) on airway DC to prime Th2 sensitization in vivo. A recent study showed that various types of DC produced TSLP in response to TLR agonist and allergen house dust mite extract [32, 33]. Importantly, lung DC expressed even higher TSLP than lung epithelial cells [32]. We show in this report that lower concentrations of LPS activated DC expressed relatively higher Tslp but lower Il12a (Fig. 4). Although Tslp mRNA expression peaked 2 – 10 hr and decreased to a relative low level (~10 fold increase over non-activated DC) 16 hr after LPS treatment, the LoLPS primed DC were capable of inducing Th2 polarization in a TSLP-dependent manner in vitro (Fig. 5). Arguably, these antigen-loaded, TSLP-expressing DC would be able to prime Th2 sensitization when they reach the draining lymph nodes. Indeed, mature TSLP-expressing DC were detected in the draining lymph nodes after immunization [11]. By i.n. transfer of OVA-loaded DC, we demonstrated that the dose of LPS used to induce DC maturation determines Th1/Th2 sensitization of the recipient mice (Fig. 6). More importantly, the ability of LoLPS-matured DC to induce Th2 sensitization in vivo was dependent on the ability of the DC to express TSLP (Fig. 7). Consistent to our finding that DC-derived TSLP directly acting on CD4+ T cells to direct T helper differentiation, a recent study demonstrated that DC-derived TSLP directly acting on CD4+ T cells to suppress Th17 differentiation while foster Foxp3+ Tregs in the gut [33].
Taken together, our data suggested parallel pathways for initiating Th1/Th2 polarization involving IL-12/IFNγ and TSLP/IL-4 action and the balance of DC-derived IL-12 and TSLP, depending on the dose of LPS, determines the nature of airway immune responses against harmless antigens. In sensing HiLPS, DC express relatively high IL-12 and low TSLP, which stimulate naïve CD4+ T cells to differentiate into IFNγ-producing Th1 cells [36]. Autocrine action of endogenous IFNγ would further amplify Th1 development [37]. In the LoLPS condition, DC express relatively high TSLP but low IL-12, which could stimulate naïve CD4+ T cells to differentiate into IL-4-producing Th2 cells [11, 22]. Autocrine action of endogenous IL-4 would further amplify Th2 development [22].
Materials and Methods
Animals
Balb/c, C57BL/6 wild type mice and DO11.10 TCR transgenic mice were purchased from Jackson Laboratories. Tslpr-deficient (Tslpr−/−)mice were described previously [14]. The Tslp targeted embryonic stem cells used to generate Tslp-deficient (Tslp−/−) ice were purchased from Knockout Mouse Project (KOMP). All mice were housed in specific pathogen-free conditions and all experiments were performed as approved by the Indiana University Institutional Animal Care and Use Committee.
Intranasal sensitization and challenge
Mice were anesthetized with ketamine/xylazine, and then sensitized intranasally with 100 µg OVA (Worthington Biochemicals) plus 20-50 ng low dose LPS (Sigma-Aldrich) (LoLPS) or 10 µg high dose LPS (HiLPS) in 40 µl PBS on days 0, 1, and 2. Mice received OVA were used as controls. Mice were challenged i.n. on day 14, 15, 18, and 19 with 50 µg OVA [26]. Mice were then analyzed for airway hyperresponsiveness (AHR) by whole body plethysmography [38] and sacrificed on day 21 for further analysis.
Evaluation of lung inflammation
Mice were euthanized, bronchoalveolar lavage were performed as described previously [39]. Briefly, lungs were washed three times with 1 ml cold PBS. BAL fluid fractions were centrifuged at 1400 × g for 5 min at 4°C. Pellets were resuspended and counted. Cytospin preparation were stained with modified Wright-Giemsa stain, and differential cell counts were evaluated by counting at least 200 cells for determination of relative percentage of each cell type in the BAL [14]
Isolation and restimulation of cells from lung draining lymph nodes
Mediastinal lymph node were dissected from sensitized and challenged mice, and single-cell suspensions were prepared and stimulated in vitro either with plate-bound 2 µg/ml anti-CD3 (17A2; Biolegend) for 24 h. Cell-free supernatants were analyzed for IFNγ and IL-4 using commercially available ELISA kit (Biolegend and eBioscience). Lower detection limits were 1.0 pg/ml (IL-4) and 4.0 pg/ml (IFNγ).
Generation of BMDC
Bone marrow-derived dendritic cells (BMDC) were generated as described [40]. RPMI 1640 medium was supplemented with 5% FBS, 1 mM sodium pyruvate, 10 mM HEPES buffer, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µM 2-ME, and 20 ng/ml recombinant mouse granulocyte macrophage-colony stimulating factor (GM-CSF; Biolegend, San Diego, CA). At day 9, they were pulsed in vitro overnight with OVA and LPS for in vivo experiment or directly used for in vitro experiment.
Asthma model using OVA+LPS-pulsed DC
BMDC were matured with 10 – 20 ng/ml low dose LPS (LL-DC) or 1 µg/ml high dose LPS (HL-DC) in the presence of 100 µg/ml OVA. After wash, 2 × 106 BMDC were transferred intranasally into the airways of naive anesthetized mice, as described previously [40]. 10 d after immunization, mice were challenged i.n. with 50 µg OVA for 3 times (Balb/c mice) or 6 times (C57BL/6 mice).
Co-culture of BMDC with naïve CD4+ T cells
Naïve CD4+CD62L+ T cells were isolated from spleens and lymph nodes of DO11.10 or Tslpr−/− DO11.10 mice (MACS isolation system; Miltenyi Biotec, Auburn, CA). BMDC were activated by 10 ng/ml LPS plus 100 µg/ml OVA. After overnight incubation, the cells were seeded (5 × 104 cells/well in 1 ml) in the complete medium with naïve T cells (1 × 105 cells/well) for 5 days in 48-well plates in the presence of 10 µg/ml OVA323-339 peptide (GenScript) or 100 µg/ml OVA protein [41]. After the coculture, CD4+ T cells were stained for expression of IL-4 and IFNγ.
Intracellular cytokine staining and Abs
The following mAbs were purchased from Biolegend: CD4 (clone GK1.5), DO11.10 TCR (clone KJ1-26), IL-4 (clone 11B11), and IFN-γ (clone XMG1.2). Rat IgG1 (clone RTK2071), rat IgG2a (clone RTK2758), and rat IgG2b (RTK4530) were included as isotype controls. For the detection of intracellular cytokines, cells were stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin for 6 h. Four hours prior to harvesting, monensin (Biolegend) was added to cultures to retain cytokines in the cytoplasm. Cells were then stained for IL-4 and IFN-γ production as described [38].
Acknowledgement
This work was supported by NIH grants R21 AI072617, R01 AI085046 to Dr. Baohua Zhou.
Abbreviations used
- AHR
airway hyperresponsiveness
- BMDC
bone marrow derived dendritic cells
- iTreg
inducible regulatory T cells
- TSLP
thymic stromal lymphopoietin.
Footnotes
Conflict of Interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 2.Georas SN, Guo J, De Fanis U, Casolaro V. T-helper cell type-2 regulation in allergic disease. Eur Respir J. 2005;26:1119–1137. doi: 10.1183/09031936.05.00006005. [DOI] [PubMed] [Google Scholar]
- 3.Lambrecht BN, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat Rev Immunol. 2003;3:994–1003. doi: 10.1038/nri1249. [DOI] [PubMed] [Google Scholar]
- 4.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 5.Coyle AJ, Le Gros G, Bertrand C, Tsuyuki S, Heusser CH, Kopf M, Anderson GP. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 1995;13:54–59. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- 6.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 8.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seder RA, Paul WE, Dvorak AM, Sharkis SJ, Kagey-Sobotka A, Niv Y, Finkelman FD, Barbieri SA, Galli SJ, Plaut M. Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc Natl Acad Sci U S A. 1991;88:2835–2839. doi: 10.1073/pnas.88.7.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 13.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 14.Zhou B, Comeau MR, Smedt TD, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 15.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen KD, Vanichsarn C, Nadeau KC. TSLP directly impairs pulmonary Treg function: association with aberrant tolerogenic immunity in asthmatic airway. Allergy Asthma Clin Immunol. 2010;6:4. doi: 10.1186/1710-1492-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Messaddeq N, Teletin M, Pasquali JL, Metzger D, Chambon P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci U S A. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 23.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, Soumelis V. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 25.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan AM, Chen HC, Pochard P, Eisenbarth SC, Herrick CA, Bottomly HK. TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J Immunol. 2010;184:3535–3544. doi: 10.4049/jimmunol.0900340. [DOI] [PubMed] [Google Scholar]
- 27.McAleer JP, Vella AT. Educating CD4 T cells with vaccine adjuvants: lessons from lipopolysaccharide. Trends Immunol. 2010;31:429–435. doi: 10.1016/j.it.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007;178:5375–5382. doi: 10.4049/jimmunol.178.8.5375. [DOI] [PubMed] [Google Scholar]
- 30.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402:B18–B23. doi: 10.1038/35037014. [DOI] [PubMed] [Google Scholar]
- 32.Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic Stromal Lymphopoietin Is Produced by Dendritic Cells. J Immunol. 2011;187:1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012 doi: 10.1038/mi.2011.64. [DOI] [PubMed] [Google Scholar]
- 34.Piggott DA, Eisenbarth SC, Xu L, Constant SL, Huleatt JW, Herrick CA, Bottomly K. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005;115:459–467. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr Opin Immunol. 2003;15:620–626. doi: 10.1016/j.coi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Bradley LM, Dalton DK, Croft M. A direct role for IFN-gamma in regulation of Th1 cell development. J Immunol. 1996;157:1350–1358. [PubMed] [Google Scholar]
- 38.Zhou B, Headley MB, Aye T, Tocker J, Comeau MR, Ziegler SF. Reversal of Thymic Stromal Lymphopoietin-Induced Airway Inflammation through Inhibition of Th2 Responses. J Immunol. 2008;181:6557–6562. doi: 10.4049/jimmunol.181.9.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei L, Zhang Y, Yao W, Kaplan MH, Zhou B. Thymic stromal lymphopoietin interferes with airway tolerance by suppressing the generation of antigen-specific regulatory T cells. J Immunol. 2011;186:2254–2261. doi: 10.4049/jimmunol.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruedl C, Bachmann MF, Kopf M. The antigen dose determines T helper subset development by regulation of CD40 ligand. Eur J Immunol. 2000;30:2056–2064. doi: 10.1002/1521-4141(200007)30:7<2056::AID-IMMU2056>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]