Abstract
Late-life minor depression (miD) is a prevalent but poorly understood illness. Verbal learning and memory profiles have commonly been used to characterize neuropsychiatric disorders. This study compared the performance of 27 older adults with miD on the California Verbal Learning Test (CVLT) with 26 age-matched individuals with Major Depressive Disorder (MDD) and 36 non-depressed controls. Results revealed that the miD group performed comparably with controls and significantly better than the MDD group on several CVLT indices. Moreover, cluster analysis revealed three distinct groups, consistent with theoretical representations of “normal,” “subcortical,” and “cortical” verbal learning and memory profiles. The majority of the miD group showed “normal” profiles (74%), whereas most individuals with MDD displayed “subcortical” profiles (54%). The findings suggest that depression in the elderly is a heterogeneous entity and that the CVLT may be a useful tool for characterizing learning and memory in late-onset depressive disorders.
Keywords: Depression, Minor, Elderly, Late-onset, CVLT, Verbal learning and memory
Introduction
Depression is a common symptom and disorder among elderly individuals. Within the spectrum of mental health disorders in older adults, it is second only to dementia in its incidence (Alexopoulos, 2005) and is often a better predictor of overall functioning than demographic and medical variables (Denihan et al., 2000; Sharma, Copeland, Dewey, Lowe, & Davidson, 1998). Epidemiological surveys have revealed that depressive syndromes not meeting criteria for Major Depressive Disorder (MDD) are most prevalent in elderly populations, and range between 15% and 23% (see Lavretsky, Kurbanyan, & Kumar, 2004, for a review), with 10% meeting criteria for minor depression (miD) (Beekman, Copeland, & Prince, 1999). In contrast, the most recent WHO mental health survey (Kessler et al., 2009) reported a point prevalence of 2.6% for MDD in individuals older than 64 in developed countries. Similarly, approximately 5% of community-dwelling elderly have reported a lifetime diagnosis of MDD, whereas 32% indicated a history of either minor or recurrent brief depression (per ICD-10 and Diagnostic and Statistical Manual of Mental Disorders-IV, Text Revision [DSM-IV-TR], intermittent major depressive episodes lasting <14 days) (Heun, Papassotiropoulos, & Ptok, 2000).
According to the DSM-IV-TR (American Psychiatric Association, 2000), miD is classified under the “Criteria Sets and Axes Provided for Further Study” as a depressive disorder of heterogeneous etiology with less severity than MDD (2–4 symptoms for miD compared with at least five symptoms for MDD) and less chronicity than Dysthymic Disorder (DyD; 2 weeks for miD vs. at least 2 years for DyD). DSM-V proposes a re-classification of miD as a “subsyndromal depression that meets duration criteria but not symptom count criteria for Major Depressive Episode” (American Psychiatric Association, 2010). Despite conceptualizations of miD as a subsyndromal form of major depression, the public health impact of miD is significant, as miD is associated with substantial functional disability and reduced productivity (Cuijpers et al., 2007; Judd & Akiskal, 2002), worse social and role functioning (Howland et al., 2008; Rapaport et al., 2002), poor quality of life (Ruo et al., 2003), increased mortality (Penninx et al., 1999), and greater bodily pain than individuals with major chronic medical conditions (Wells et al., 1989). Furthermore, untreated miD is a significant risk factor for future onset of MDD (Cuijpers, Smit, & Willemse, 2005; Judd & Akiskal, 2002), with one recent study reporting a 7-fold risk for developing MDD at 1-year follow-up compared with non-depressed controls (Lyness, Chapman, McGriff, Drayer, & Duberstein, 2009).
The limited number of studies that have examined miD in older adults have sought to better clarify, define, and distinguish miD as an affective diathesis through the examination of cognitive and neuroimaging patterns. Although normal aging alone contributes to attention and executive functioning difficulties (e.g., working memory, encoding, free recall, organizational strategies, memory for source, and temporal order) (Craik & Bialystok, 2006; Keys & White, 2000), these and other cognitive difficulties are exacerbated by miD. In a study examining a broad range of cognitive abilities in older adults with late-onset miD and MDD (Elderkin-Thompson et al., 2003), only executive functioning differentiated miD and control groups, with the miD group performing significantly worse on executive function measures. However, their performance was similar to controls and significantly better than those with MDD on tests of verbal recall (California Verbal Learning Test [CVLT] indices and semantic fluency) and maintenance of set (scores from the Modified Card Sorting Test); scores typically fell intermediate between control and MDD groups. Better performance generally correlated with less severe depression, although significant correlations were not observed for verbal recall when each patient group was analyzed separately, possibly due to the restricted range.
More recently, in an in-depth probe of the findings from Elderkin-Thompson and colleagues (2003), CVLT performance was found to be similarly impaired in those with late-life miD and MDD on selected learning indices, though the groups did not perform significantly worse than controls on delayed recall trials and recognition hits; however, at least half of both patient groups had prior depressive episodes (Elderkin-Thompson, Mintz, Haroon, Lavretsky, & Kumar, 2007). In addition, CVLT scores appeared to be mediated by executive functioning (i.e., semantic clustering) with the most robust mediation occurring on Trial 5 among late-onset patients. MRI studies have revealed significantly reduced prefrontal cortex volumes of elderly miD compared with age-matched, non-depressed controls, with volumes falling intermediate between controls and those with MDD (Kumar et al., 1997; Kumar, Jin, Bilker, Udupa, & Gottlieb, 1998).
In contrast to elderly miD, the neuropsychological sequelae of late-life MDD have been the focus of considerable research. Elderly individuals with MDD tend to exhibit the most difficulty on free recall measures of memory that require effortful processing (Lockwood, Alexopoulos, & van Gorp, 2002; see McClintock, Husain, Greer, & Cullum, 2010, for a broad recent review of cognition in MDD, including late-life MDD). While several neuroanatomical regions have been implicated in the pathophysiology of elderly MDD, including the hippocampus (Ballmaier et al., 2008), evidence from neuroimaging and neuropathological studies suggests a subcortical-frontal network syndrome (Coffey et al., 1993; Cummings, 1993; Elderkin-Thompson, Hellemann, Pham, & Kumar, 2009; Kumar et al., 2002). In addition to these neurobiologic markers, it is noteworthy that depression frequently coexists with subcortical dementia and that depressive symptoms often precede the onset of motor disability (Caine & Shoulson, 1983; Mayeux, Stern, Rosen, & Leventhal, 1981).
Moreover, the performance of patients with MDD on neuropsychological tasks often resembles that of frontal-subcortical dementias such as Parkinson's disease and Huntington's disease (HD), including psychomotor slowing, poor attention, problem-solving difficulties, and a general encoding and retrieval deficit that is typified by impaired recall but not retention and recognition deficits (Cummings, 1986; Kramer, Levin, Brandt, & Delis, 1989; Massman, Delis, Butters, Levin, & Salmon, 1990). Implicit memory, which is also associated with subcortical networks, does not appear to be impacted by depression (Elderkin-Thompson, Moody, Knowlton, Hellemann, & Kumar, 2011). In contrast, individuals who suffer from “cortical dementias,” such as Alzheimer's disease (AD) and Korsakoff's disease, typically demonstrate profound memory disruptions that predominantly affect encoding and storage, with deficits in recall, retention, and recognition performance (Butters, Granholm, Salmon, Grant, & Wolfe, 1987; Paulsen et al., 1995). They also exhibit highly elevated intrusion rates, which are posited to be a consequence of a bottom-up deterioration of semantic knowledge (Paulsen et al., 1995).
Several investigators have found good discriminability between subcortical and cortical disorders based on CVLT performance (e.g., Kramer et al., 1989; Paulsen et al., 1995). Of note is the application of a subcortical dysfunction hypothesis of verbal learning and memory deficits to a subgroup of middle-aged adults with MDD (n = 40) and Bipolar I disorder (n = 9) (Massman, Delis, Butters, Dupont, & Gillin, 1992). CVLT profiles of 49 depressed patients were compared with mildly demented patients with a prototypical cortical dementia (AD), a prototypical subcortical dementia (HD), and healthy controls. Discriminant function analysis yielded three key verbal learning and memory variables that differentiated the HD, AD, and healthy control groups: (a) total recall on Trials 1–5 of List A, (b) intrusion errors produced on cued recall of List A, and (c) the difference between recognition discriminability and Trial 5 free recall. This analysis classified 49% of depressed patients as “normal,” 29% as HD, and none as AD; 22% were not well-classified. Age, years of education, medication status, prior alcohol use, previous hospitalizations, and severity of depression were not related to the classification group. The findings of this study suggested that there is heterogeneity in the verbal learning and memory abilities of people with depression and that only a subgroup exhibited learning and memory deficits indicative of subcortical dysfunction.
The present work sought to better characterize miD in the elderly by examining its impact on verbal learning and memory processes and discriminating their pattern of performance from MDD and non-depressed control groups. We were also interested in further exploring heterogeneity in verbal learning and memory functions, both between and within the three groups. Moreover, we investigated whether a subcortical dysfunction hypothesis of verbal learning and memory deficits in MDD extended to elderly adults with late-onset miD and MDD. While CVLT performance has been studied in elderly miD (Elderkin-Thompson et al., 2003, 2007), several issues remain unresolved regarding the characteristics and heterogeneity of verbal learning and memory profiles in late-onset miD and MDD. For example, the earlier study (Elderkin-Thompson et al., 2003) found no significant differences in performance between miD participants and controls, with scores for the miD group falling intermediate between the control and MDD groups. The later study (Elderkin-Thompson et al., 2007) reported similarly deficient scores between miD and MDD groups on selected learning indices, though the groups did not perform significantly worse than controls on delayed recall trials and recognition hits. At least half of both patient groups in the Elderkin-Thompson and colleagues (2007) study had previous depressive episodes, whereas the index episode was the first mood episode in the Elderkin-Thompson and colleagues (2003) sample. In the Elderkin-Thompson and colleagues (2003) study, individual CVLT test scores were not examined; rather a “verbal recall” composite score was analyzed, which consisted of seven selected CVLT indices (which only partially overlap with this study) and a semantic fluency measure. Neither study examined all the selected CVLT variables in this study nor included memory profile analyses.
We hypothesized that CVLT performance would be significantly worse in the MDD group in comparison to the miD and healthy control groups. While we hypothesized that performance in the miD group would be somewhat reduced compared with controls, we did not expect the differences in scores to be significant. We expected that most persons in the MDD group would demonstrate a “subcortical” verbal learning and memory profile, while the majority of the miD group would show “normal” learning and memory with comparable, though somewhat lower, scores than controls. Few, if any, participants from any of the study groups were expected to show CVLT scores consistent with a “cortical” profile.
Method
Participants
Participants in this study were a subset of a larger sample recruited at the University of Pennsylvania Medical Center for neuropsychological, pharmacological, and neuroanatomical studies of late-onset depression (operationally defined as onset ≥60 years of age) (Elderkin-Thompson et al., 2003). These studies were approved by the University of Pennsylvania's Committee on Studies involving Human Beings. Twenty-seven elderly individuals with miD, 26 with MDD, and 36 controls participated. The MDD participants were recruited from ambulatory and inpatient geropsychiatry programs. The miD and control groups were volunteers who responded to community advertisements.
Both controls and patients underwent a screening evaluation consisting of medical, psychiatric, and mental status examinations. The three groups included individuals with stable comorbid medical disorders of comparable severity, such as hypertension, diabetes, and arthritis, as assessed by the Cumulative Illness Rating Scale (Linn, Linn, & Gurel, 1968). A psychiatric diagnostic evaluation was conducted with the Structured Clinical Interview for the DSM-IV (First, Spitzer, Gibbon, & Williams, 1996), including completion of a DSM-IV Research Diagnostic Criteria (RDC) checklist for miD (American Psychiatric Association, 1994). Exclusion criteria for all participants included: any new prescription or significant change in the regimen of an existing prescription in the 3 months prior to study participation for medications with potential psychotropic action (e.g., thyroxine, propranolol); history of alcohol or other substance use disorder; any history of schizophrenia or other psychotic disorder; history of CNS disease (e.g., dementia, tumor, stroke); history of ECT treatment; hospitalization for depression or any other psychiatric disorder in the past 12 months; a change in a medical condition requiring urgent re-evaluation or hospitalization in the prior 3 months; or the presence of a life-threatening condition (e.g., renal or hepatic failure).
In the two patient groups, information about depressive episodes was obtained from participants and caregivers. While all participants in the miD sample met the DSM-IV RDC criteria of low mood and/or loss of interest in activities and at least one additional depressive symptom from the DSM-IV checklist, the duration criterion was increased from 2 weeks to 1 month to enhance the likelihood that patients were not merely experiencing a transient dysphoria. Six patients who concurrently met DSM-IV criteria for DyD (minor depressive symptoms of at least 2 years duration) were included in the miD group. However, those with a history of earlier major depressive episodes were excluded. All miD patients reported the index episode as their first episode of a mood disturbance, and their scores on the Hamilton Depression Rating Scale (HDRS; Hamilton, 1967) were required to be within the 8–16 range. Most patients with miD were drug naïve, and all were free of psychotropic medications for at least 2 weeks prior to study participation.
In addition to meeting the standard DSM-IV diagnostic criteria for MDD, MDD participants obtained an HDRS score that was ≥15. Diagnosis of patients scoring 15 or 16 on the HDRS was decided based on clinical evaluation by a board-certified geriatric psychiatrist (AK) per DSM-IV research criteria. About one third of the patients in the MDD group were on a combination of low-dose anxiolytics and/or antidepressants in therapeutic dosages at the time of the study. The healthy elderly controls were screened using criteria similar to those for the patient groups except for no presently observed depressive symptoms and no history of depression.
Demographic characteristics of the three groups are summarized in Table 1. Results of univariate analyses of variance (ANOVAs) with subsequent Tukey's HSD analyses revealed a diagnosis-by-age interaction; the mean age of MDD participants was significantly higher than healthy controls (p= .01). A diagnosis-by-education interaction was also observed, with MDD patients having significantly less years of formal schooling than those in both the miD (p= .03) and control (p= .001) groups. Age and years of education were thus used as covariates in all subsequent analyses.
Table 1.
Demographic characteristics of the miD, MDD, and control groups
| Characteristic | miD | MDD | Controls | Test statistic |
|---|---|---|---|---|
| n | 27 | 26 | 36 | |
| Age | ||||
| M | 71.63 | 74.69 | 69.53 | F (2, 86) = 4.29, p = .017 |
| SD | 7.89 | 5.59 | 6.83 | |
| Range | 60–87 | 66–84 | 60–85 | |
| Gender | ||||
| Women | 17 | 17 | 25 | χ2 (2) = 0.30, p = .859 |
| Men | 10 | 9 | 11 | |
| Years of education | ||||
| M | 14.04 | 12.17 | 14.69 | F (2, 86) = 7.40, p = .001 |
| SD | 2.30 | 3.04 | 2.44 | |
| Range | 10–20 | 8–20 | 9–20 | |
| Ethnicity | ||||
| African American | 1 | 4 | 3 | χ2 (2) = 4.45, p = .348 |
| Hispanic | 1 | 0 | 0 | |
| Non-Hispanic Caucasian | 25 | 22 | 33 | |
| # Right-Handed | 26 | 25 | 36 | χ2 (2) = 1.39, p = .499 |
| Age of onset | ||||
| M | 69.63 | 71.38 | — | t (51) = 0.91, p = .367 |
| SD | 8.06 | 5.75 | — | |
| Range | 60–84 | 63–84 | ||
| Illness duration (years) | ||||
| M | 2.00 | 3.31 | — | t (51) = 1.50, p = .140 |
| SD | 2.65 | 3.64 | — | |
| Range | 1 month–13 years | 1 month–15 years | ||
| HDRS | ||||
| M | 12.04 | 19.62 | — | t (39.5) = 9.44, p < .001 |
| SD | 2.05 | 3.57 | — | |
| Range | 8–16 | 15–26 | ||
Notes: miD = minor depression; MDD = Major Depressive Disorder; HDRS = Hamilton Depression Rating Scale.
Measures
The CVLT (Delis, Kramer, Kaplan, & Ober, 1987) provides an assessment of multiple processes involved in learning and remembering orally presented verbal material. Sixteen shopping items (List A) which comprise four semantic categories of four words each are read to examinees five times. After each of the five trials, examinees attempt free recall of the shopping items. An interference trial is then presented in which the examiner reads another 16-item shopping list (List B), also comprising four semantic categories of four words each, and asks for recall. Examinees subsequently recall as much of List A as possible (short-delay free recall). Following this free recall, the examiner offers semantic cues for List A (e.g., “tell me the fruits”). Free and cued recalls are repeated after a 20-min delay (long-delay). Nonverbal tasks are administered during the delay interval. Finally, a recognition trial is presented in which participants must discriminate List A words from distractors.
Procedures
After determination of study eligibility, participants were administered the CVLT as part of a larger neuropsychological battery (Elderkin-Thompson et al., 2003). Neuropsychological testing was conducted by blinded psychological technicians trained in standardized neuropsychological test administration under the supervision of neuropsychology faculty. The CVLT protocols were scored with a computer program that calculated raw scores, semantic and serial clustering ratios (observed/expected), learning slope, discriminability and response bias for recognition memory, and other relevant indices (Fridlund & Delis, 1987).
Statistical Analyses
Raw scores on these indices were converted to z-scores by using the healthy controls as a reference group for the two patient groups. We first focused on the six CVLT indices most consistently used to discriminate between healthy individuals and those with cortical and subcortical disorders: (a) total recall on Trials 1–5 of List A (TOT), (b) rate of learning (Slope), (c) semantic clustering ratio (SEM), (d) short-delay free recall (SD), (e) the difference between recognition discriminability (accounting for false-positive errors) and Trial 5 recall (DIFF), and (f) intrusion errors produced on the cued recall of List A (CUE) (Kramer et al., 1989; Massman et al., 1992; Paulsen et al., 1995). As women typically outperform men on verbal learning and memory measures at all ages, both in healthy (Kramer, Delis, & Daniel, 1988; Kramer, Delis, Kaplan, O'Donnell, & Prifitera, 1997; Lamar, Resnick, & Zonderman, 2003) and depressed patient (Otto et al., 1994) samples, gender was included as an independent variable along with diagnostic classification in all analyses.
To investigate whether a subcortical dysfunction hypothesis of verbal learning and memory deficits in MDD extended to elderly adults with late-onset miD and MDD, we also performed a k-means cluster analysis on three CVLT variables: (a) TOT, (b) CUE, and (c) DIFF. These three indices have successfully differentiated among young healthy adults and those with cortical and subcortical pathology (Massman et al., 1992).
Effort and motivation are important factors to consider in interpreting cognitive findings, and have been the focus of consideration in individuals with depression for some time (Millis, Putnam, Adams, & Ricker, 1995; Richards & Ruff, 1989; Rohling, Green, Allen, & Iverson, 2002). To address the potential role of reduced effort as an explanation for some of the lower CVLT scores in the MDD and miD groups, we applied a prediction formula for the CVLT developed by Millis and Volinsky (2001). Millis and Volinsky developed their prediction formula based on Bayesian model averaging for use in individuals with traumatic brain injury. We also included recognition discriminability in the detection algorithm, because it has been specifically associated with effort and applied established cutoff scores (Millis et al., 1995; S.R. Millis, personal communication, April 21, 2011). This post hoc method identified five individuals (four from the MDD group and one from the miD group) whose CVLT performance may have been lowered due to reduced effort. We thus re-ran all of our analyses to determine if any differences would emerge between the full and attenuated samples. Re-analysis revealed no statistically significant or meaningful differences between the results obtained with the full and attenuated clinical samples. Given the absence of differences in the results and the absence of specific effort assessment methods for the CVLT in late-life depression (D. Delis, personal communications, October 16 and 21, 2011), we chose to report findings based on the full clinical sample.
Results
Multivariate analysis of covariance (MANCOVA), controlling for age and education with diagnosis and gender entered as predictor variables, was conducted to assess for differences among the three participant groups and between genders on CVLT z-scores of (a) TOT, (b) Slope, (c) SEM, (d) SD, (e) DIFF, and (f) CUE. Results revealed a significant effect for diagnosis, F(12, 152) = 2.23, p = .01, but no main effect for gender and no diagnosis-by-gender interaction. Follow-up ANOVAs revealed significant differences among the three groups on (a) TOT, F(2, 81) = 4.20, p < .05, (b) Slope, F(2, 81) = 6.29, p < .01, (c) SD, F(2, 81) = 3.31, p < .05, and (d) DIFF, F(2, 81) = 3.75, p < .05, but not SEM or CUE. Post hoc Tukey's HSD procedures demonstrated that the MDD group performed significantly higher than the control group on DIFF (p< .001; better recognition performance compared with Trial 5 free recall), and significantly lower than both the miD and control groups on TOT (p = .003 compared with miD and p< .001 compared with controls), Slope (p< .001 compared with miD and p= .01 compared with controls), and SD (p = .002 compared with miD and p< .001 compared with controls). The miD and control groups did not significantly differ from each other on any of these indices. Table 2 provides the means and standard deviations of z-scores on the six CVLT indices.
Table 2.
Standard (z) scores for the six selected CVLT indices
| CVLT index | Diagnostic classification |
df | F | |||||
|---|---|---|---|---|---|---|---|---|
| miD |
MDD |
Controls |
||||||
| M | SD | M | SD | M | SD | |||
| Total recall on Trials 1–5, A | −0.54 | 0.91 | −1.47 | 1.23 | 0.00 | 1.00 | 2, 81 | 4.20* |
| Learning slope | 0.48 | 1.29 | −0.91 | 1.31 | 0.00 | 1.00 | 2, 81 | 6.29** |
| Semantic clustering ratio | −0.16 | 0.82 | −0.20 | 1.04 | 0.00 | 1.00 | 2, 81 | 0.30 |
| Short-delay FR | −0.16 | 0.94 | −1.07 | 0.85 | 0.00 | 1.00 | 2, 81 | 3.31* |
| Discriminability versus Trial 5 FR | 0.49 | 0.63 | 0.94 | 0.92 | 0.00 | 1.03 | 2, 81 | 3.75* |
| Intrusions on cued recall, A | −0.26 | 0.86 | 0.15 | 1.06 | 0.00 | 1.00 | 2, 81 | 1.83 |
| Six indices combined (MANCOVA) | 12, 152 | 2.23** | ||||||
| Gender |
||||||||
| Malesa |
Femalesa |
|||||||
| M | SD | M | SD | |||||
| Total recall on Trials 1–5, A | −0.91 | 0.78 | −0.43 | 1.35 | 1, 81 | 7.53** | ||
| Learning slope | −0.31 | 1.15 | −0.02 | 1.36 | 1, 81 | 1.69 | ||
| Semantic clustering ratio | −0.41 | 0.90 | 0.05 | 0.95 | 1, 81 | 3.47 | ||
| Short-delay FR | −0.62 | 0.76 | −0.23 | 1.14 | 1, 81 | 7.06** | ||
| Discriminability versus Trial 5 FR | 0.67 | 0.87 | 0.30 | 1.00 | 1, 81 | 3.04 | ||
| Intrusions on cued recall, A | 0.01 | 1.11 | −0.06 | 0.92 | 1, 81 | 0.01 | ||
| Six indices combined (MANCOVA) | 6, 76 | 1.66 | ||||||
Notes: All z-scores indicate the level of performance relative to the control group. miD = minor depression; MDD = Major Depressive Disorder; CVLT = California Verbal Learning Test; FR = free recall; MANCOVA = multivariate analysis of covariance.
aAs no significant diagnosis-by-gender interaction was observed, z-scores for each gender represent scores across three groups.
*p ≤ .05.
**p ≤ .01.
A k-means cluster analysis was used to classify the miD and MDD patients into three verbal learning and memory subgroups based on their z-scores on three CVLT variables of (a) TOT, (b) CUE and (c) DIFF. These indices represent measures of initial learning, susceptibility to intrusions, and improvement in recall when provided with recognition prompts, respectively. The three CVLT indices and the three clusters were set a priori in order to reflect theoretical distinctions between cortical dementia, subcortical dementia, and normal verbal learning and memory profiles (Massman et al., 1992). The cluster analysis sorted the patients into three distinct subgroups based on their pattern of performance on the CVLT indices; that is, each cluster was defined by those scores that exhibited the least possible variability from each other and maximal variability between scores from the other two clusters.
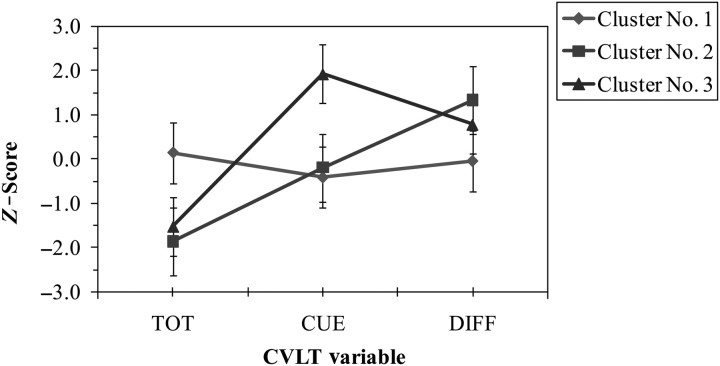
As shown in Fig. 1, three clusters emerged that resembled theoretical representations of normal, subcortical, and cortical verbal learning and memory profiles. Cluster 1 represents a prototypical “normal” verbal learning and memory profile, as performance on all three CVLT variables was average. In contrast, persons classified to Cluster 2 exhibited a classic “subcortical” verbal learning and memory profile. Although their acquisition and learning were poor, they demonstrated an average number of cued intrusions and an above average ability to benefit from recognition cues. Cluster 3 is characteristic of a “cortical” verbal learning and memory profile. Individuals assigned to this cluster showed reduced acquisition and learning, a considerably large number of cued intrusions and a lower ability to benefit from recognition prompts than persons grouped in Cluster 2. The cluster means and standard deviations of z-scores for each CVLT variable are presented in Table 3. ANOVAs revealed significant differences among the three clusters on all three CVLT variables of interest: (a) TOT, F(2, 86) = 65.9, p < .001, (b) CUE, F(2, 86) = 75.7, p < .001, and (c) DIFF, F(2, 86) = 27.5, p < .001.
Fig. 1.
Mean z-scores (±SD) for each cluster. CVLT = California Verbal Learning Test; TOT = Total recall on Trials 1–5 of List A; CUE = cued recall of List A; DIFF = difference between recognition discriminability and Trial 5 recall.
Table 3.
Standard (z) scores for the three selected CVLT indices on the three clusters
| CVLT index | Cluster No. 1 (“normal”; n = 54) |
Cluster No. 2 (“subcortical”; n = 23) |
Cluster No. 3 (“cortical”; n = 12) |
|||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| TOT | 0.15 | 0.75 | −1.86 | 0.84 | −1.52 | 0.65 |
| CUE | −0.41 | 0.56 | −0.19 | 0.61 | 1.92 | 0.74 |
| DIFF | −0.04 | 0.77 | 1.33 | 0.82 | 0.78 | 0.58 |
Notes: All z-scores indicate the level of performance relative to the control group. CVLT = California Verbal Learning Test; TOT = Total recall on Trials 1–5 of List A; CUE = cued recall of List A; DIFF = difference between recognition discriminability and Trial 5 recall.
As shown in Table 4, cluster assignments for the three participant groups differed significantly:χ2(4) = 16.76, p = .002. Cluster assignments for the miD and control groups were very similar, with the majority of participants in each group assigned to Cluster 1 (“normal” profile). In contrast, MDD participants largely fell into Cluster 2 (“subcortical” profile).
Table 4.
Cluster classification rates for each participant group
| Group | Cluster No. 1 (“normal”; n = 54) | Cluster No. 2 (“subcortical”; n = 23) | Cluster No. 3 (“cortical”; n = 12) |
|---|---|---|---|
| miD | 74.1% (n = 20) | 14.8% (n= 4) | 11.1% (n = 3) |
| MDD | 30.8% (n = 8) | 53.8% (n = 14) | 15.4% (n = 4) |
| Controls | 72.2% (n = 26) | 13.9% (n = 5) | 13.9% (n = 5) |
Notes: miD = minor depression; MDD = Major Depressive Disorder.
Finally, we examined the presence of any cluster-by-diagnosis interactions in each of the patient groups on gender and severity of depression (as defined by HDRS scores). Within the miD and MDD groups, MANOVAs revealed no significant differences between the three clusters in severity of depression. Chi-square analysis showed that gender did not significantly differ by cluster assignment.
Discussion
While depression is a heterogeneous entity, findings from this study suggest that the CVLT may be a useful tool for characterizing and differentiating between learning and memory processes in late-onset MDD and the more prevalent miD in older adults. As expected, those with miD performed comparably with controls and significantly better than those with MDD on the six CVLT indices most consistently used to differentiate among healthy individuals and those with cortical and subcortical pathologies.
Specifically, the MDD group scored significantly lower than both the miD and control groups in acquisition (total recall on Trials 1–5 of List A), rate of learning, and short-delay free recall. Compared with controls, the MDD group also showed significantly greater improvement in recall when provided with recognition prompts. This latter finding supports a retrieval deficit in MDD and bolsters the notion that subcortical pathology is likely to result in reduced performance on more effortful recall tasks while recognition is intact. Semantic clustering and intrusion errors on cued recall of List A were the only two of the six CVLT indices that did not differ significantly among the three groups. The lack of significant differences on semantic clustering is consistent with the results of two other studies (Elderkin-Thompson et al., 2003, 2007) examining CVLT performance in late-life miD and MDD when the index episode was the first mood episode, as it was in the current study; in Elderkin-Thompson and colleagues (2007), semantic clustering did significantly differ between the groups when individuals with a history of depressive episodes were also included in the analyses. Intrusions on cued recall were not specifically examined in these studies (Elderkin-Thompson et al., 2003, 2007). Since the semantic clustering ratio did not discriminate among the groups in this or the two prior studies by Elderkin-Thompson and colleagues (2003, 2007), it is possible that the CVLT findings from this study may readily be generalized to other verbal list learning tests that do not measure semantic organization (e.g., Rey Auditory Verbal Learning Test). However, this warrants further study with similar samples, particularly in light of the findings from Elderkin-Thompson and colleagues (2007) that semantic clustering appeared to mediate scores on some CVLT indices.
The findings also provide support for the use of verbal learning and memory classification groups to characterize the heterogeneity among elderly individuals with depressive disorders. Three distinct verbal learning and memory profiles emerged from cluster analysis of the three CVLT indices previously used to discriminate among “normal” abilities and subcortical and cortical pathologies (total recall on Trials 1–5 of List A, the difference between recognition discriminability and Trial 5 recall, and intrusion errors produced on the cued recall of List A). Within the miD group, roughly 74% were classified as normal, nearly 15% were categorized as subcortical, and approximately 11% were classified as cortical. The majority of MDD patients (∼54%) were assigned to the subcortical verbal learning and memory profile, almost 31% were classified as normal, and about 15% were categorized as cortical. The proportion of individuals assigned to each cluster differed significantly, and the three clusters resembled theoretical representations of normal, cortical, and subcortical profiles, as hypothesized.
Consistent with this study's hypotheses, most individuals in the miD group demonstrated normal verbal learning and memory profiles. While MRI studies have documented that prefrontal lobe volumes in elderly individuals with miD fall between age-matched controls and those with MDD (Kumar et al., 1997, 1998), such findings do not appear to translate into performance differences in verbal memory between those with miD and controls. Although Elderkin-Thompson and colleagues (2007) found similarly deficient CVLT performance between miD and MDD groups, at least half of both of these groups had previous depressive episodes, and the study did not examine cued intrusions or the difference between recognition discriminability and free recall.
The pattern of performance exhibited by the MDD group is also consistent with the study hypotheses, as well as a subcortical dysfunction hypothesis of verbal learning and memory deficits for a subgroup of depressed patients (Massman et al., 1992). Compared with Massman's group, this study's MDD sample showed a higher proportion of subcortical and cortical profiles. This discrepancy may be due to the compounding effects of age and depression on cognition of MDD patients in this study (e.g., King, Caine, Conwell, & Cox, 1991). The finding that classification status for both patient groups was not significantly influenced by severity of depression was also congruent with findings from Massman and colleagues (1992) and other investigators (Sweeney, Wetzler, Stokes, & Kocsis, 1989).
It is notable that while there were significant differences in verbal learning and memory profile cluster assignments (“normal,” “subcortical,” or “cortical”) between the groups, there was heterogeneity in profile assignments within each group. While some variability may be due to moderating influences not examined in this study (e.g., use of estrogen replacement therapy [ERT] in women, non-psychotropic medications, psychotherapy, previous hospitalizations), it should also be considered that elderly individuals with miD are at a significant risk for subsequent development of MDD (Cuijpers et al., 2005; Judd & Akiskal, 2002; Lyness et al., 2009). Furthermore, MDD may present as an early sign of a later developing dementia (e.g., Agbayewa, 1986; Kral & Emery, 1989; Nussbaum, Kaszniak, Allender, & Rapcsak, 1995). Indeed, Lyness and colleagues (2009) reported that untreated miD conferred a 7-fold risk for developing MDD at 1-year follow-up compared with non-depressed controls. Thus, within both patient groups, perhaps neurobiologically, those who showed normal profiles were experiencing miD, while those with subcortical profiles had MDD and those with cortical profiles had an incipient dementing illness.
This study found no significant gender differences on the six selected CVLT indices as a group. There were also no significant gender differences in cluster assignments to normal, subcortical, or cortical profiles. Reduced power due to the modest sample size in this study may have limited the potential of detecting an interaction with gender. Direct comparison with gender effects in the Massman and colleagues (1992) study, on which this study's classification strategy was based, was not possible as nearly all depressed participants from the Massman study were male. However, when examining the six CVLT indices individually, women performed significantly better than men on TOT and SDFR. Other studies have reported similar sex differences on TOT and SDFR indices, both in healthy (Kramer et al., 1988, 1997; Lamar et al., 2003; Saykin et al., 1995) and depressed samples (Otto et al., 1994). While females have also been found to use semantic clustering strategies to a significantly greater extent than males, findings have either been restricted to children and adolescents (Kramer et al., 1997) or represented a broad age range and were not specific to older adults (Kramer et al., 1988). In the two previous studies of CVLT performance in late-life miD (Elderkin-Thompson et al., 2003, 2007), the influence of gender has been equivocal as it was either not specifically examined (Elderkin-Thompson et al., 2007) or no gender effects were found on several CVLT indices when analyzed as a group (effects were not assessed on individual indices) (Elderkin-Thompson et al., 2003).
These results should also be interpreted in the context of limitations related to sample size and characteristics, and the potential influence of moderator variables. As this study focused on community-dwelling elderly patients who were predominantly Caucasian and had a variety of comorbid medical illnesses, the findings can only be generalized to similar populations. However, the study may provide clinical utility by the inclusion of outpatients with diverse medical problems as clinicians are often asked to treat such individuals (Lichtenberg, Ross, Millis, & Manning, 1995).
Another limitation is that the effect of psychotropic medication (low-dose antidepressants and/or anxiolytics in therapeutic dosages) on one third of the MDD group is unknown. Improvement in cognitive functioning resulting from antidepressant medications has been documented (Van den Berg, Oldehinkel, Brilman, Bouhuys, & Ormel, 2000); however, any such substantive effects in this sample seem unlikely because the pattern of cognitive findings fits the hypotheses. Although these medications, particularly the anxiolytics, may have contributed to reduced CVLT performance of MDD participants (Mintzer & Griffiths, 2000; Settle, 1998), most studies have found such effects to be small (Berg & Dellasega, 1996). Notably, the scatterplots of CVLT scores did not show subsets of MDD participants who were outliers in either direction, although medication effects cannot be entirely ruled out. Participants in the miD group were drug-free, and most were drug naïve.
Third, an ongoing consideration in the assessment of memory and cognition in adults with depression is the issue of reduced effort, which itself might be reasonably regarded as a feature of depression (Rohling et al., 2002). Our study did not include embedded or stand-alone cognitive effort tests. However, in an attempt to address this important issue, we applied a post hoc detection method developed by Millis and colleagues for the CVLT with individuals with traumatic brain injury (Millis et al., 1995; Millis & Volinsky, 2001). Based on the results of this approach, reduced effort was determined to be an unlikely source of influence on our findings. Nonetheless, future studies, particularly those employing the CVLT-II, should take care to index effort in accord with emerging standards of practice (Heilbronner et al., 2009).
Finally, not all factors that may have influenced CVLT performance were analyzed. For example, psychotherapeutic interventions in the miD and MDD were not assessed. It was also not known whether women in this study were receiving ERT, and estrogen levels were not obtained. Additionally, variables such as comorbid medical problems, the use of multiple non-psychotropic medications, and hospitalizations prior to 1 year before the study may have the potential to affect CVLT scores. Although Massman and colleagues (1992) did not find any significant relationships between these variables and verbal learning and memory profile classification status, their depressive sample was younger than the elderly individuals who participated in this study.
To address these limitations, future investigations of cognition in late-onset miD should be replicated with a larger sample size, which is more ethnically and racially diverse, to confirm the findings and to consider the influence of aforementioned moderating variables including potential interactions with gender. It is also important that researchers operationally define miD explicitly. The current DSM-IV research criteria are tentative, and there are no consistent guidelines that researchers follow when investigating correlates of miD. As noted, the miD duration criterion in this study was increased from 2 weeks to 1 month to enhance the likelihood that patients were not merely experiencing a transient dysphoria. Additional efforts to examine the neurobiological (e.g., neuroimaging) and neuropsychological correlates of miD will likely reveal more specific characteristics that are common to this putative and heterogeneous disorder. Comparing cognitive profiles of miD to DyD, as well as longitudinal studies of cognition in miD, may be valuable for better understanding the functional impact of miD and the extent to which cognition may vary as a function of mood state.
In summary, the findings from this study suggest that although elderly individuals with miD may manifest similar mood symptoms to those with MDD (e.g., depressive mood, anhedonia), their verbal learning and memory profiles are different and have different functional implications. The miD group performed comparably with controls on the CVLT, both in terms of magnitude and patterns, and significantly better than the MDD group. Furthermore, the majority of participants in both the miD and control groups showed prototypical “normal” verbal learning and memory profiles, while the majority of those in the MDD group exhibited prototypical “subcortical” profiles. These findings highlight the value of assessing both mood symptoms and cognition in older adults with depression, particularly given the considerable public health impact of both miD and MDD, including evidence that untreated miD is a significant risk factor for developing MDD (Cuijpers et al., 2005; Judd & Akiskal, 2002; Lyness et al., 2009) and that MDD may be an early sign of progression to dementia (e.g., Agbayewa, 1986; Kral & Emery, 1989; Nussbaum et al., 1995). The results also raise important treatment considerations for clinicians. For example, therapeutic interventions for those with verbal learning and memory deficits would need to incorporate strategies to monitor medication adherence. Positive behavior supports that help individuals compensate for memory problems (e.g., prompts, cues, environmental modifications) would also be beneficial, as would education of family members about cognitive difficulties associated with the illness and compensatory adaptations.
While there is heterogeneity in learning and memory functions that is not sufficiently explained by diagnosis alone, clinicians can generally assume that mild or subsyndromal forms of depression in the elderly typically do not include memory problems, while memory problems do accompany MDD and require specific attention. However, 25% of the miD sample in this study exhibited either subcortical or cortical profiles, suggesting that a substantial minority of older adults with miD have memory problems at a rate exceeding expectations related to normal aging alone. Establishing the implications for risk of progression to MDD and dementia, as well as its functional impact and potential for compensation, require additional research attention with longitudinal investigations.
Funding
This work was supported by a grant from The Dana Foundation Consortium on Memory Loss and Aging; and, in part, by Massachusetts Department of Mental Health research support to the Commonwealth Research Center; and CIDAR grant P50MH080272 from the National Institute of Mental Health to Dr. Mesholam-Gately.
The work presented in this paper represents part of a larger study by the University of Pennsylvania Medical Center for neuropsychological, pharmacological and neuroanatomical studies of late-onset depression. This paper was based on research conducted for Dr. Mesholam-Gately's thesis at Drexel University.
Conflict of Interest
None declared.
References
- Agbayewa M. O. Earlier psychiatric morbidity in patients with Alzheimer's disease. Journal of the American Geriatrics Society. 1986;34:561–564. doi: 10.1111/j.1532-5415.1986.tb05759.x. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G. S. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. doi:10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed.) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed.), text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Depressive conditions not elsewhere classified. 2010. Retrieved from http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=47 .
- Ballmaier M., Narr K. L., Toga A. W., Elderkin-Thompson V., Thompson P. M., Hamilton L., et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. American Journal of Psychiatry. 2008;165(2):229–237. doi: 10.1176/appi.ajp.2007.07030506. doi:10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman A. T., Copeland J. R., Prince M. J. Review of community prevalence of depression in later life. British Journal of Psychiatry. 1999;174:307–311. doi: 10.1192/bjp.174.4.307. doi:10.1192/bjp.174.4.307. [DOI] [PubMed] [Google Scholar]
- Berg S., Dellasega C. The use of psychoactive medications and cognitive function in older adults. Journal of Aging and Health. 1996;8(1):136–149. doi: 10.1177/089826439600800107. doi:10.1177/089826439600800107. [DOI] [PubMed] [Google Scholar]
- Butters N., Granholm E., Salmon D. P., Grant I., Wolfe J. Episodic and semantic memory: A comparison of amnesic and demented patients. Journal of Clinical and Experimental Psychology. 1987;9:479–497. doi: 10.1080/01688638708410764. [DOI] [PubMed] [Google Scholar]
- Caine E. D., Shoulson I. Psychiatric syndromes in Huntington. American Journal of Psychiatry. 1983;140:728–733. doi: 10.1176/ajp.140.6.728. s disease. [DOI] [PubMed] [Google Scholar]
- Coffey C. E., Wilkinson W. E., Weiner R. D., Parshos I. A., Djang W. T., Webb M. C., et al. Quantitative cerebral anatomy in depression: A controlled magnetic resonance imaging study. Archives of General Psychiatry. 1993;50:7–16. doi: 10.1001/archpsyc.1993.01820130009002. [DOI] [PubMed] [Google Scholar]
- Craik F. I., Bialystok E. Cognition through the lifespan: Mechanisms of change. Trends in Cognitive Sciences. 2006;10(3):131–138. doi: 10.1016/j.tics.2006.01.007. doi:10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Cuijpers P., Smit F., Oostenbrink J., de Graaf R., Ten Have M., Beekman A. Economic costs of minor depression: A population-based study. Acta Psychiatrica Scandinavica. 2007;115(3):229–236. doi: 10.1111/j.1600-0447.2006.00851.x. doi:10.1111/j.1600-0447.2006.00851.x. [DOI] [PubMed] [Google Scholar]
- Cuijpers P., Smit F., Willemse G. Predicting the onset of major depression in subjects with subthreshold depression in primary care: A prospective study. Acta Psychiatrica Scandinavica. 2005;111(2):133–138. doi: 10.1111/j.1600-0447.2004.00416.x. doi:10.1111/j.1600-0447.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- Cummings J. L. Subcortical dementia: Neuropsychology, neuropsychiatry, and pathophysiology. British Journal of Psychiatry. 1986;149:682–697. doi: 10.1192/bjp.149.6.682. doi:10.1192/bjp.149.6.682. [DOI] [PubMed] [Google Scholar]
- Cummings J. L. The neuroanatomy of depression. Journal of Clinical Psychiatry. 1993;54(11):14–20. [PubMed] [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., Ober B. A. The California Verbal Learning Test. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Denihan A., Kirby M., Bruce I., Cunningham C., Coakley D., Lawlor B. A. Three-year prognosis of depression in the community-dwelling elderly. British Journal of Psychiatry. 2000;176:453–457. doi: 10.1192/bjp.176.5.453. doi:10.1192/bjp.176.5.453. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V., Hellemann G., Pham D., Kumar A. Prefrontal brain morphology and executive function in healthy and depressed elderly. International Journal of Geriatric Psychiatry. 2009;24(5):459–468. doi: 10.1002/gps.2137. doi:10.1002/gps.2137. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V., Kumar A., Bilker W. B., Dunkin J. J., Mintz J., Moberg P. J., et al. Neuropsychological deficits among patients with late-onset minor and major depression. Archives of Clinical Neuropsychology. 2003;18(5):529–549. doi: 10.1016/s0887-6177(03)00022-2. doi:10.1016/S0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V., Mintz J., Haroon E., Lavretsky H., Kumar A. Executive dysfunction and memory in older patients with major and minor depression. Archives of Clinical Neuropsychology. 2007;22(2):261–270. doi: 10.1016/j.acn.2007.01.021. doi:10.1016/j.acn.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V., Moody T., Knowlton B., Hellemann G., Kumar A. Explicit and implicit memory in late-life depression. American Journal of Geriatric Psychiatry. 2011;19(4):249–255. doi: 10.1097/JGP.0b013e3181e89a5b. [DOI] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. W. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Fridlund A., Delis D. C. CVLT research edition administration and scoring software. New York: The Psychological Corporation; 1987. [Google Scholar]
- Hamilton M. Development of a rating scale for primary care depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. doi:10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Heilbronner R. L., Sweet J. J., Morgan J. E., Larrabee G. J., Millis S. R. Conference Participants. American Academy of Clinical Neuropsychology Consensus Conference Statement on the neuropsychological assessment of effort, response bias, and malingering. The Clinical Neuropsychologist. 2009;23(7):1093–1129. doi: 10.1080/13854040903155063. doi:10.1080/13854040903155063. [DOI] [PubMed] [Google Scholar]
- Heun R., Papassotiropoulos A., Ptok U. Subthreshold depressive and anxiety disorders in the elderly. European Psychiatry. 2000;15(3):173–182. doi: 10.1016/s0924-9338(00)00228-5. doi:10.1016/S0924-9338(00)00228-5. [DOI] [PubMed] [Google Scholar]
- Howland R. H., Schettler P. J., Rapaport M. H., Mischoulon D., Schneider T., Fasiczka A., et al. Clinical features and functioning of patients with minor depression. Psychotherapy and Psychosomatics. 2008;77(6):384–389. doi: 10.1159/000151519. doi:10.1159/000151519. [DOI] [PubMed] [Google Scholar]
- Judd L. L., Akiskal H. S. The clinical and public health relevance of current research on subthreshold depressive symptoms to elderly patients. American Journal of Geriatric Psychiatry. 2002;10(3):233–238. [PubMed] [Google Scholar]
- Kessler R. C., Birnbaum H. G., Shahly V., Bromet E., Hwang I., McLaughlin K. A., et al. Age differences in the prevalence and co-morbidity of DSM-IV major depressive episodes: Results from the WHO world mental health survey initiative. Depression and Anxiety. 2009;27(4):351–364. doi: 10.1002/da.20634. doi:10.1002/da.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys B. A., White D. A. Exploring the relationship between age, executive abilities, and psychomotor speed. Journal of the International Neuropsychological Society. 2000;6(1):76–82. doi: 10.1017/s1355617700611098. [DOI] [PubMed] [Google Scholar]
- King D. A., Caine E. D., Conwell Y., Cox C. The neuropsychology of depression in the elderly: A comparative study of normal aging and Alzheimers disease. Journal of Neuropsychiatry and Clinical Neurosciences. 1991;3:163–168. doi: 10.1176/jnp.3.2.163. [DOI] [PubMed] [Google Scholar]
- Kral V. A., Emery O. B. Long-term follow-up of depressive pseudodementia of the aged. Canadian Journal of Psychiatry. 1989;34:445–446. doi: 10.1177/070674378903400515. [DOI] [PubMed] [Google Scholar]
- Kramer J. H., Delis D. C., Daniel M. H. Sex differences in verbal learning. Journal of Clinical Psychology. 1988;44(6):907–915. doi:10.1002/1097-4679(198811)44:6<907::AID-JCLP2270440610>3.0.CO;2-8. [Google Scholar]
- Kramer J. H., Delis D. C., Kaplan E., O'Donnell L., Prifitera A. Developmental sex differences in verbal learning. Neuropsychology. 1997;11(4):577–584. doi: 10.1037//0894-4105.11.4.577. doi:10.1037/0894-4105.11.4.577. [DOI] [PubMed] [Google Scholar]
- Kramer J. H., Levin B. E., Brandt J., Delis D. C. Differentiation of Alzheimers, Huntingtons, and Parkinsons disease patients on the basis of verbal learning characteristics. Neuropsychology. 1989;3:111–120. [Google Scholar]
- Kumar A., Jin Z., Bilker W., Udupa J., Gottlieb G. Late-onset minor and major depression: Early evidence for common neuroanatomical substrates detected by using MRI. Proceedings of the National Academy of Sciences of the USA. 1998;95(13):7654–7658. doi: 10.1073/pnas.95.13.7654. doi:10.1073/pnas.95.13.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Schweizer E., Jin Z., Miller D., Bilker W., Swan L. L., et al. Neuroanatomical substrates of late-life minor depression. A quantitative magnetic resonance imaging study. Archives of Neurology. 1997;54(5):613–617. doi: 10.1001/archneur.1997.00550170085018. [DOI] [PubMed] [Google Scholar]
- Kumar A., Thomas A., Lavretsky H., Yue K., Huda A., Curran J., et al. Frontal white matter biochemical abnormalities in late-life major depression detected with proton magnetic resonance spectroscopy. American Journal of Psychiatry. 2002;159(4):630–636. doi: 10.1176/appi.ajp.159.4.630. doi:10.1176/appi.ajp.159.4.630. [DOI] [PubMed] [Google Scholar]
- Lamar M., Resnick S. M., Zonderman A. B. Longitudinal changes in verbal memory in older adults: Distinguishing the effects of age from repeat testing. Neurology. 2003;60(1):82–86. doi: 10.1212/wnl.60.1.82. [DOI] [PubMed] [Google Scholar]
- Lavretsky H., Kurbanyan K., Kumar A. The significance of subsyndromal depression in geriatrics. Current Psychiatry Reports. 2004;6(1):25–31. doi: 10.1007/s11920-004-0034-8. doi:10.1007/s11920-004-0034-8. [DOI] [PubMed] [Google Scholar]
- Lichtenberg P. A., Ross T., Millis S. R., Manning C. A. The relationship between depression and cognition in older adults: A cross-validation study. Journal of Gerontology: Psychological Sciences. 1995;50B(1):P25–P32. doi: 10.1093/geronb/50b.1.p25. doi:10.1093/geronb/50B.1.P25. [DOI] [PubMed] [Google Scholar]
- Linn B. S., Linn B. W., Gurel L. Cumulative Illness Rating Scale. Journal of the American Geriatrics Society. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Lockwood K. A., Alexopoulos G. S., van Gorp W. G. Executive dysfunction in geriatric depression. American Journal of Psychiatry. 2002;159(7):1119–1126. doi: 10.1176/appi.ajp.159.7.1119. doi:10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- Lyness J. M., Chapman B. P., McGriff J., Drayer R., Duberstein P. R. One-year outcomes of minor and subsyndromal depression in older primary care patients. International Psychogeriatrics. 2009;21(1):60–68. doi: 10.1017/S1041610208007746. doi:10.1017/S1041610208007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massman P. J., Delis D. C., Butters N., Dupont R. M., Gillin J. C. The subcortical dysfunction hypothesis of memory deficits in depression: Neuropsychological validation in a subgroup of patients. Journal of Clinical and Experimental Neuropsychology. 1992;14(5):687–706. doi: 10.1080/01688639208402856. doi:10.1080/01688639208402856. [DOI] [PubMed] [Google Scholar]
- Massman P. J, Delis D. C., Butters N., Levin B. E., Salmon D. P. Are all subcortical dementias alike? Verbal learning and memory in Parkinson. Journal of Clinical and Experimental Neuropsychology s and Huntingtons disease patients. 1990;12(5):729–744. doi: 10.1080/01688639008401015. doi:10.1080/01688639008401015. [DOI] [PubMed] [Google Scholar]
- Mayeux R., Stern Y., Rosen J., Leventhal J. Depression, intellectual impairment, and Parkinsons disease. Neurology. 1981;31:64–650. doi: 10.1212/wnl.31.6.645. [DOI] [PubMed] [Google Scholar]
- McClintock S. M., Husain M. M., Greer T. L., Cullum C. M. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology. 2010;24(1):9–34. doi: 10.1037/a0017336. doi:10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- Millis S. R., Putnam S. H., Adams K. M., Ricker J. H. The California Verbal Learning Test in the detection of incomplete effort in neuropsychological evaluation. Psychological Assessment. 1995;7(4):463–471. doi:10.1037/1040-3590.7.4.463. [Google Scholar]
- Millis S. R., Volinsky C. T. Assessment of response bias in mild head injury: Beyond malingering tests. Journal of Clinical and Experimental Neuropsychology. 2001;23(6):809–828. doi: 10.1076/jcen.23.6.809.1017. doi:10.1076/jcen.23.6.809.1017. [DOI] [PubMed] [Google Scholar]
- Mintzer M. Z., Griffiths R. R. Acute effects of triazolam on false recognition. Memory and Cognition. 2000;28(8):1357–1365. doi: 10.3758/bf03211836. doi:10.3758/BF03211836. [DOI] [PubMed] [Google Scholar]
- Nussbaum P. D., Kaszniak A. W., Allender J., Rapcsak S. Depression and cognitive decline in the elderly: A follow-up study. The Clinical Neuropsychologist. 1995;9(2):101–111. doi:10.1080/13854049508401592. [Google Scholar]
- Otto M. W., Bruder G. E., Fava M., Delis D. C., Quitkin F. M., Rosenbaum J. F. Norms for depressed patients for the California Verbal Learning Test: Associations with depression severity and self-report of cognitive difficulties. Archives of Clinical Neuropsychology. 1994;9:81–88. [PubMed] [Google Scholar]
- Paulsen J. S., Butters N., Sadek J. R., Johnson S. A., Salmon D. P., Swerdlow N. R., et al. Distinct cognitive profiles of cortical and subcortical dementia in advanced illness. Neurology. 1995;45:951–956. doi: 10.1212/wnl.45.5.951. [DOI] [PubMed] [Google Scholar]
- Penninx B. W., Geerlings S. W., Deeg D. J., van Eijk J. T., van Tilburg W., Beekman A. T. Minor and major depression and the risk of death in older persons. Archives of General Psychiatry. 1999;56(10):889–895. doi: 10.1001/archpsyc.56.10.889. doi:10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- Rapaport M. H., Judd L. L., Schettler P. J., Yonkers K. A., Thase M. E., Kupfer D. J., et al. A descriptive analysis of minor depression. American Journal of Psychiatry. 2002;159(4):637–643. doi: 10.1176/appi.ajp.159.4.637. doi:10.1176/appi.ajp.159.4.637. [DOI] [PubMed] [Google Scholar]
- Richards P. M., Ruff R. M. Motivational effects on neuropsychological functioning: comparison of depressed versus nondepressed individuals. Journal of Consulting and Clinical Psychology. 1989;57(3):396–402. doi: 10.1037//0022-006x.57.3.396. doi:10.1037/0022-006X.57.3.396. [DOI] [PubMed] [Google Scholar]
- Rohling M. L., Green P., Allen L. M., 3rd, Iverson G. L. Depressive symptoms and neurocognitive test scores in patients passing symptom validity tests. Archives of Clinical Neuropsychology. 2002;17(3):205–222. [PubMed] [Google Scholar]
- Ruo B., Rumsfeld J. S., Hlatky M. A., Liu H., Browner W. S., Whooley M. A. Depressive symptoms and health-related quality of life: The heart and soul study. Journal of the American Medical Association. 2003;290(2):215–221. doi: 10.1001/jama.290.2.215. doi:10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin A. J., Gur R. C., Gur R. E., Shtasel D. L., Flannery K. A., Mozley L. H., et al. Normative neuropsychological test performance: Effects of age, education, gender and ethnicity. Applied Neuropsychology. 1995;2:79–88. doi: 10.1207/s15324826an0202_5. doi:10.1207/s15324826an0202_5. [DOI] [PubMed] [Google Scholar]
- Settle E. C. Antidepressant drugs: Disturbing and potentially dangerous adverse effects. Journal of Clinical Psychiatry. 1998;59(Suppl. 16):25–42. [PubMed] [Google Scholar]
- Sharma V. K., Copeland J. R., Dewey M. E., Lowe D., Davidson I. Outcome of the depressed elderly living in the community in liverpool: A 5-year follow-up. Psychological Medicine. 1998;28(6):1329–1337. doi: 10.1017/s0033291798007521. doi:10.1017/S0033291798007521. [DOI] [PubMed] [Google Scholar]
- Sweeney J. A., Wetzler S., Stokes P., Kocsis J. Cognitive functioning in depression. Journal of Clinical Psychology. 1989;45(6):836–842. [PubMed] [Google Scholar]
- Van den Berg M. D., Oldehinkel A. J., Brilman E. I., Bouhuys A. L., Ormel J. Correlates of symptomatic, minor and major depression in the elderly. Journal of Affective Disorders. 2000;60(2):87–95. doi: 10.1016/s0165-0327(99)00160-3. doi:10.1016/S0165-0327(99)00160-3. [DOI] [PubMed] [Google Scholar]
- Wells K. B., Stewart A., Hays R. D., Burnam M. A., Rogers W., Daniels M., et al. The functioning and well-being of depressed patients. Results from the medical outcomes study. Journal of the American Medical Association. 1989;262(7):914–919. doi:10.1001/jama.262.7.914. [PubMed] [Google Scholar]