Abstract
Functional β-cell mass deficiency in diabetes results from imbalanced β-cell death and replication, and decreased PAK1 protein levels in human islets from donors with type 2 diabetes implicates a possible role for PAK1 in maintaining β-cell mass. Here, we aim to address the linkage between PAK1 and Survivin, a protein essential for β-cell replication. PAK1 knockout (KO) mouse islets exhibited decreased expression of Survivin protein. MIN6 β-cells with siRNA-mediated suppression of PAK1 also had decreased Survivin protein and exhibited an increased level of ubiquitinated-Survivin. However, no significant changes in Survivin mRNA were found in islets from PAK1 KO mice and PAK1-depleted MIN6 β-cells. The decreased Survivin level in MIN6 cells subjected to hyperglycemic stress was prevented by expression of exogenous PAK1. Moreover, overexpressing Survivin restored proliferation of β-cells that was impaired by the loss of PAK1. These data implicate a role for PAK1 in regulating Survivin protein stability in the β-cell and suggest PAK1 as a potential molecular target for the restoration of β-cell mass.
Keywords: MIN6, PAK1, Survivin, Ubiquitination, mouse islet, pancreatic β-cell, replication
Introduction
Deficiencies in β-cell mass occur in both type 1 and type 2 diabetes (T1D and T2D, respectively). Accumulated evidence suggests that ~50% of islets from patients with T2D are in a state of dysfunction or outright failure at the time of diagnosis.1-3 The homeostatic maintenance of adult β-cell mass largely relies on the replication of differentiated β-cells; therefore, understanding the physiological and molecular mechanisms that control β-cell replication is of critical importance. The pool of pre-existing β-cells has been shown to be one of the major sources of new β-cells in adult mice under normal physiological conditions or following a pancreatectomy.4-7
Survivin is a multifunctional protein that intersects cell death, cell division and cellular adaptation.8,9 Multiple studies highlight the importance of Survivin-dependent replication of pre-existing β-cells.10-12 The p21-activated kinase 1 (PAK1) is a ubiquitously expressed serine/threonine kinase implicated in the promotion of cell survival in neuronal and cardiac cells.13-15 PAK1 has also been reported to play a critical role in cell proliferation.16,17 Our previous work has indicated that PAK1 is a positive regulator of β-cell function, and a paucity of PAK1 protein in islets is correlated with human T2D.18-20 Most recently, we have demonstrated that PAK1 is essential for β-cell survival via limiting Bad expression.21 However, the role of PAK1 in β-cell proliferation has never been assessed.
Here, using PAK1 knockout (KO) mouse islets and PAK1-depleted MIN6 β-cells, we addressed the linkage between PAK1 and Survivin, as well as the potential role of PAK1 in β-cell proliferation. We observed that Survivin protein expression is decreased in PAK1 KO islets. In pancreatic MIN6 cells with siRNA-mediated depletion of PAK1, increased ubiquitinated-Survivin was observed. Impaired proliferation of β-cells induced by the loss of PAK1 was restored by overexpressing Survivin. In addition, decreased Survivin expression in MIN6 β-cells induced by chronic exposure to hyperglycemic conditions was protected in the presence of exogenous PAK1. Our findings reveal a novel role for PAK1 in maintaining Survivin protein expression levels in islet β-cells, suggesting that attenuated PAK1 abundance could hinder islet β-cell proliferation.
Results
PAK1 regulates β-cell proliferation
To determine the requirement for PAK1 in β-cell replication, DNA synthesis rates in pancreatic MIN6 β-cells with or without PAK1 depletion were determined by measuring the incorporation of [3H]methyl-thymidine into genomic DNA. A decrease in [3H]thymidine incorporation was observed in MIN6 cells with PAK1 depletion relative to control MIN6 cells (Fig. 1). These data indicate that Pak1 regulates β-cell replication.
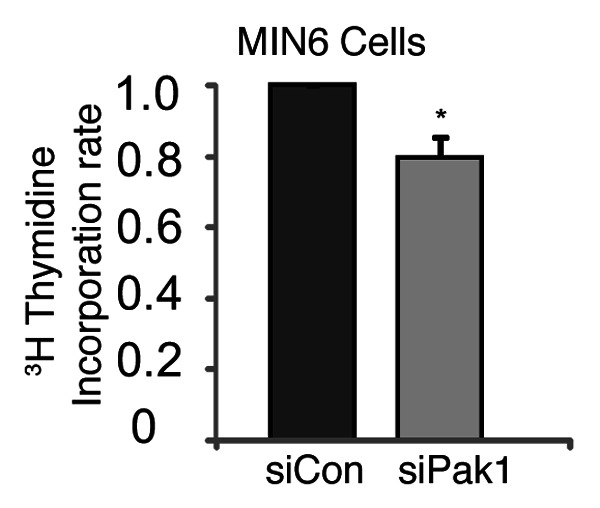
Figure 1. Decreased [3H]thymidine incorporation in PAK1-depleted MIN6 cells. Thirty-six hours after PAK1 depletion with siRNA, [3H]Methyl thymidine was added as described in methods. The amount of [3H]thymidine incorporated into DNA was measured by liquid scintillation counting and normalized to total cellular protein. Data represent the average ± SE for 3 independent experiments. *p < 0.05, vs. siCon.
PAK1-depletion results in decreased Survivin protein expression in islet β-cells
Survivin is a multifunctional protein that intersects cell death and division.8,9 Survivin is critical for β-cell replication during the embryonic and neonatal periods or during conditions of stress in adult islets.10-12 PAK1 has been shown to be able to promote survival by modulating the expression of Survivin in osteoclasts.22 To determine if a similar phenomenon exists in β-cells (i.e., does PAK1 control Survivin expression?), we evaluated Survivin levels in PAK1 KO mouse islets by Western analysis. Survivin expression has been previously reported to be restricted to β-cells 3 weeks after birth in mouse islet examined by immunofluorescent staining of pancreatic tissue,23 and its expression significantly declined after birth.10 To overcome the obstacle of detection sensitivity, we performed Western analysis on pooled islets (~200) directly lysed in sample loading buffer. As reported in Figure 2, PAK1 KO mouse islets contained ~50% less Survivin protein when compared with PAK1 wild-type (WT) islets.
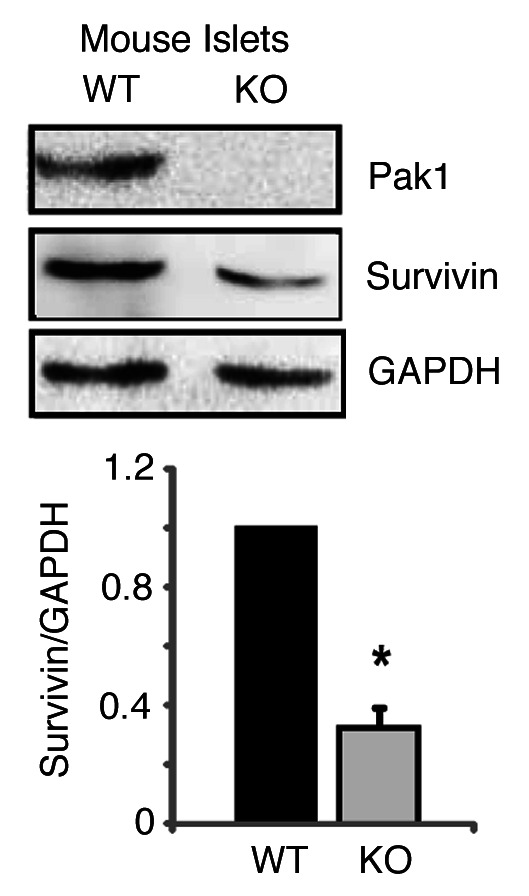
Figure 2. Survivin expression is decreased in PAK1−/− KO islets. Islets were isolated from PAK1−/− KO and wild type (WT) littermate mice and homogenized. Proteins were resolved by 12% SDS-PAGE for immunoblotting with antibodies as indicated. Data represent the average ± SE for 3 pairs of mice; *p < 0.05, vs. WT.
As ~20% of the cells in a rodent islet are non-β cells, we confirmed that this decrease in Survivin occurred in β-cells by evaluating PAK1-depleted clonal MIN6 pancreatic β-cells. As we routinely observed,18,19 PAK1 siRNA oligonucleotides successfully depleted PAK1 protein by more than 50% after 48 h of transfection (Fig. 3). Depletion of PAK1 significantly decreased Survivin protein levels as well, whereas restoration of PAK1 protein level by co-transfection with exogenous PAK1 correspondingly normalized the aberrant decrease in Survivin protein induced by PAK1-depletion (Fig. 3). These data suggest that the abundance of PAK1 specifically in the β-cells of the islet is important for maintaining Survivin expression levels.
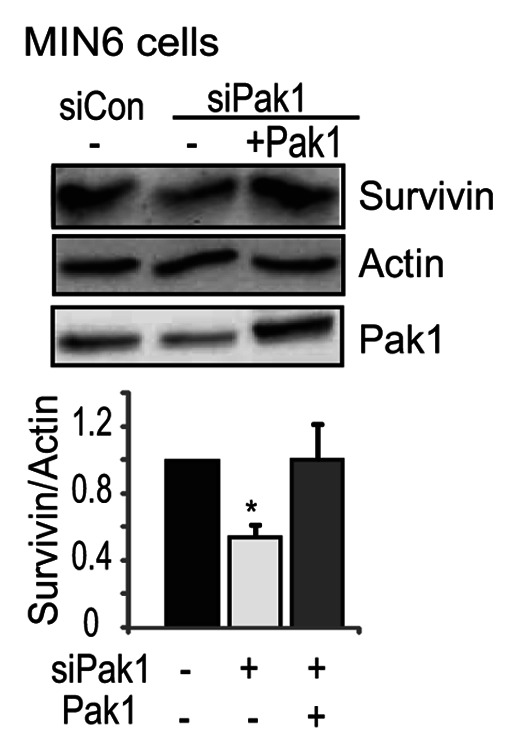
Figure 3. Depletion of PAK1 decreased Survivin expression in pancreatic MIN6 cells. MIN6 cells were transfected with PAK1 siRNA (siPAK1) or control (siCon) oligonucleotides with pCMV6 vector or pCMV6-myc-PAK1 plasmid. After 48 h incubation, cells were washed twice and incubated with glucose-free MKRBB for 2 h before harvesting. Whole cell detergent lysates were prepared and subjected to 12% SDS-PAGE for immunoblotting with antibodies indicated. Data represent the average ± SE for 3 independent experiments; *p < 0.05, vs. siCon
Depleted of PAK1 does not alter Survivin transcription, but increases Survivin ubiquitination in the β-cells
To address how PAK1 regulates Survivin expression, we first examined the level of Survivin mRNA in islets from PAK1 KO mice. Quantitative real-time PCR revealed no significant change of Survivin mRNA in PAK1 KO islets compared with WT islets (Fig. 4A). Similar results were observed in MIN6 cells when PAK1 was depleted by siRNA (Fig. 4B). These results suggest that, in the islet, PAK1 regulates Survivin protein expression via a post-transcriptional step.
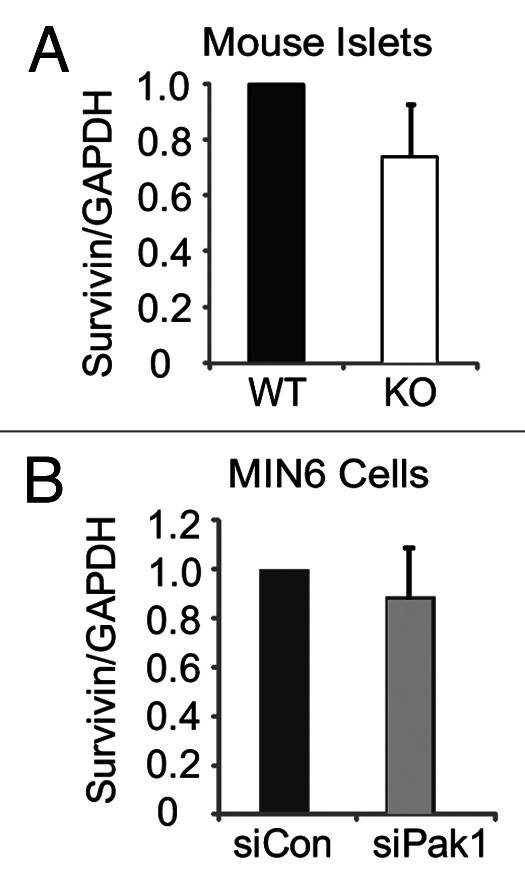
Figure 4. Survivin mRNA expression in islets from PAK1−/− KO mice and PAK1-depleted MIN6 cells. (A) Islets were isolated from PAK1−/− KO and littermate PAK1+/+ WT mice for use in Q-PCR analysis as described in methods. Data were quantified relative to GAPDH from three batches of islets, *p < 0.05 vs. WT. (B) MIN6 cells were transfected with PAK1 siRNA (siPAK1) or control (siCon) oligonucleotides. After 48 h incubation, cells were harvested for Q-PCR analysis as described in methods. Data were quantified relative to GAPDH from three independent experiments, *p < 0.05 vs. siCon.
Survivin can be degraded via the ubiquitin-proteasome pathway and turns over rapidly with a t1/2 of about 30 min.24 To determine the role of PAK1 in protease-mediated Survivin degradation, PAK1-depleted clonal MIN6 β-cells were treated with the proteasome inhibitor MG132 for 6 h before harvesting. As shown in Figure 5, the degradation of Survivin was blocked by pretreatment with the proteasomal inhibitor MG132, and increased levels of ubiquitinated-Survivin were observed via immunoblotting (Fig. 5A) and immunoprecipitation (Fig. 5B). These results indicate that PAK1 is involved in proteasome-mediated Survivin degradation in pancreatic β-cells.
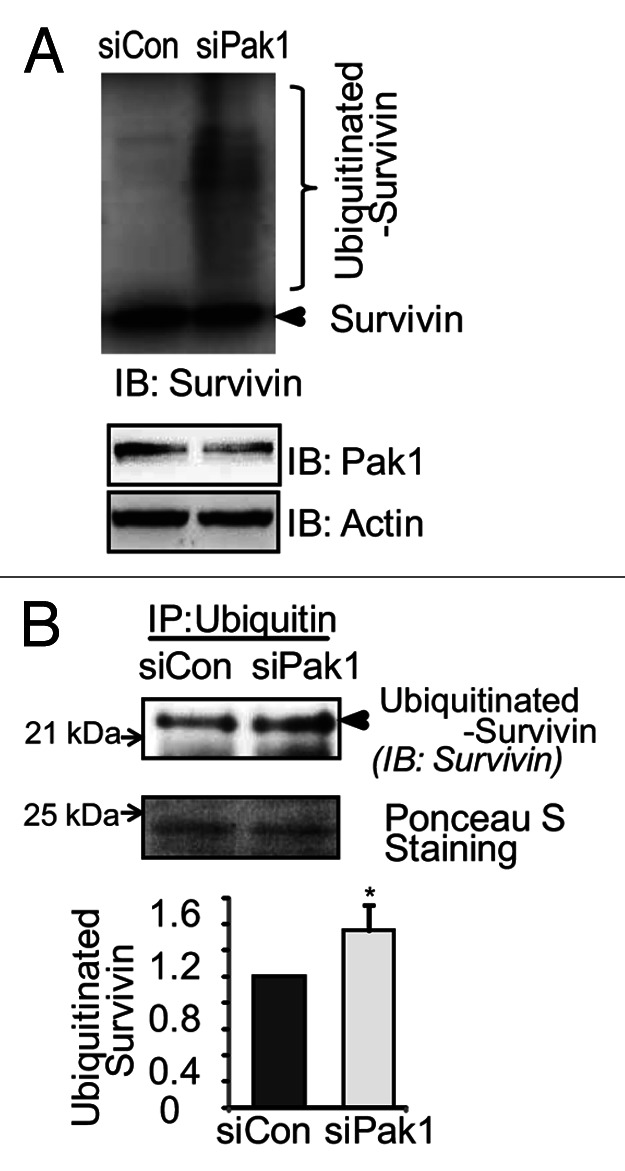
Figure 5. Depletion of PAK1 enhanced Survivin-ubiquitination in pancreatic β-cells. (A) MIN6 cells were transiently transfected with siPAK1 or siCon Oligos. Thirty-six hours after transfection, cells were cultured with proteasome inhibitor MG132 (10 µM) for 6 h. Whole cell detergent lysates were prepared and subjected to 12% SDS-PAGE for immunoblotting with antibodies indicated. Data represent the one of the two independent experiments yielding similar results. (B) MIN6 cells were treated as in A. One mg cleared detergent lysates were combined with 1 µg of rabbit anti-Ubiquitin antibody as described in methods. The immunoprecipitates were subjected to immunoblotting with anti-survivin antibody. Data represent the one of the three independent experiments yielding similar results.
PAK1 restores hyperglycemia/glucotoxic stress-induced decreases of Survivin protein expression in mouse pancreatic islets
Elevated fasting blood glucose is a hallmark characteristic of individuals with frank T2D.3,25,26 Short-term low levels of glucose induce Survivin expression after serum starvation.27 Yet, whereas hyperglycemia is presumed to ultimately provoke β-cell apoptosis and impair proliferation,28 how hyperglycemia/glucotoxic stress alters Survivin protein expression has never been addressed. In MIN6 cells, we observed that glucotoxicity was able to substantially decrease Survivin protein levels; however, the decrease of Survivin induced by high-glucose was completely rescued by exogenous expression of PAK1 (Fig. 6).
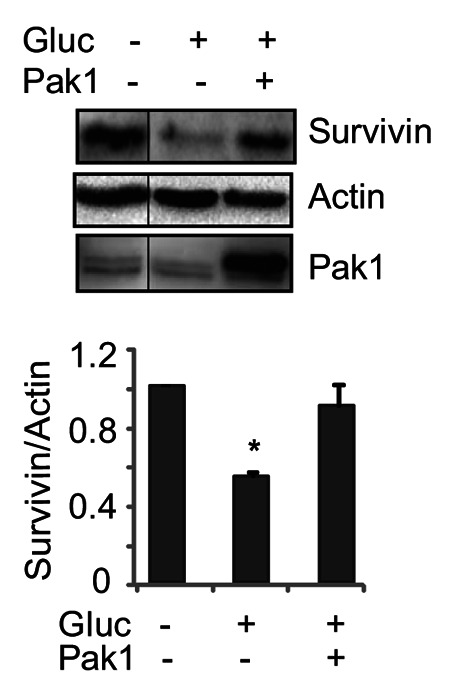
Figure 6. Glucotoxic/hyperglycemic stress reduced Survivin protein levels in pancreatic β-cells. MIN6 cells were transfected with pCMV6 vector or pCMV6-myc-PAK1 plasmid. After 48 h incubation, cells were washed twice with and incubated for 2 h in freshly prepared modified Krebs-Ringer bicarbonate buffer. Cells were stimulated with 20 mM D-glucose for 20 min. Cells were then washed twice before harvesting, and whole cell lysates were subjected to immunoblotting with antibodies indicated. Data represent the average ± SE for 3 independent experiments; *p < 0.05 vs. basal.
Overexpressing Survivin restored proliferation of β-cells that was impaired by loss of PAK1
Having established the notion that PAK1 regulates pancreatic MIN6 β-cell replication and that Survivin is regulated by PAK1, we then asked if the impaired cell replication due to PAK1 depletion in MIN6 clonal cell lines can be recapitulated in islets, and if overexpressing Survivin is able to restore this defect. To address the first question, we examined the proliferation rate of islets from our PAK+/− heterozygous mouse because these mice most closely simulate the ~50–80% loss of PAK1 protein that occurs in T2D human islets.19 Indeed, a significantly lower [3H]thymidine incorporation was observed in islets from PAK1+/− mice compared with islets from PAK1+/+ mice (Fig. 7A). To elucidate whether Survivin indeed functions downstream of PAK1 in β-cell proliferation, exogenous Survivin was “added-back” to PAK1-depleted MIN6 β-cell to compensate for the lack of endogenous Survivin. As shown in Figure 7B, significantly reduced cell-proliferation in PAK1-depleted MIN6 cells (co-transfected with the vector) was largely rescued by co-transfection with Survivin.
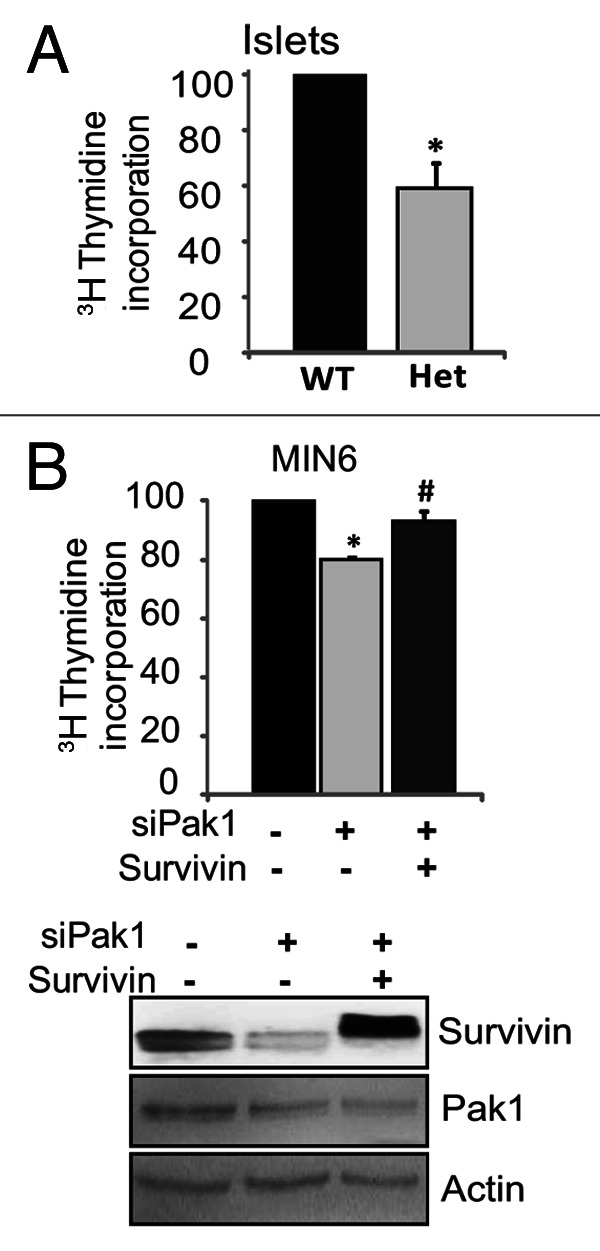
Figure 7. (A) Decreased [3H]methyl-thymidine incorporation in islets from PAK1+/− Het mice. [3H]Methyl-thymidine was added to pools of about 200 islets for 18 h. The islets were washed three times with cold medium. The amount of [3H]thymidine incorporated into DNA was measured by liquid scintillation counting and normalized to total cellular protein. Data represent the average ± SE for 3 independent experiments. *p < 0.05, vs. WT. (B) Overexpressing Survivin restored impaired-proliferation of MIN6 cells induced by loss of PAK1 in pancreatic MIN6 β-cells. MIN6 cells were transfected with PAK1 siRNA (siPAK1) or control (siCon) oligonucleotides with MIEG3 vector or Survivin plasmid as described in the Materials and Methods. Thirty-six hours after transfection, [3H]methyl-thymidine was added for 2 h, and the amount of [3H]thymidine incorporated into DNA was measured as described in the Materials and Methods. Data are the average ± SE from 3 independent experiments. *p < 0.05 vs. siCon; # p < 0.05 vs. siPAK1.
Discussion
In this study, we demonstrated that PAK1 is required for pancreatic β-cell replication, and that overexpressing Survivin restores impaired proliferation of β-cells due to loss of PAK1. We also showed that either hyperglycemic conditions or a loss of PAK1 is sufficient to trigger the downregulation of Survivin protein in islet β-cells, whereas downregulation of Survivin protein level induced by glucotoxic stress is prevented in the presence of exogenous PAK1. These observations suggest that PAK1 plays a role in regulating Survivin protein abundance in islet β-cells and could confer a replication advantage under high-glucose stress. Toward this end, our data here suggest that PAK1 is an important regulator for β-cell replication.
Although initially recognized as a cancer gene and implicated in the control of cell division and the regulation of apoptosis in cancer cells, Survivin is also required for the replication of most normal cells including hepatocytes, bone marrow stem cells, neural progenitor cells and highly proliferative adult tissues.29-32 Survivin has been promoted as an attractive candidate for regulating β-cell replication and survival.23,27 In pancreases from patients with longstanding T1D, Survivin is only detected in the islets with insulin-positive cells, but not in insulin-deficient islets, suggesting that Survivin is critical for maintenance of β-cell population.33 Because PAK1 is required to prevent Survivin from ubiquitin-mediated Survivin degradation in the β-cell, PAK1 may be important for regulating β-cell expansion by stabilizing Survivin protein.
We have made the unique observation that PAK1 inhibits ubiquitin-mediated Survivin degradation in pancreatic β-cells. We also have reported that glucose rapidly decreases Survivin protein expression; given that Survivin has a very short half-life of about 30 min,24 this result is not unexpected. However, it remains puzzling why exogenous PAK1 increases Survivin level that was downregulated by glucose exposure when no change in PAK1 levels were observed in glucotoxic conditions. Glucose has been shown to be associated with degradation of multiple proteins by ubiquitin-dependent mechanisms34-36; whether a ubiquitination-dependent mechanism is involved in high-glucose-induced downregulation of Survivin awaits further investigation. Bearing these in mind, a possible explanation is that increasing PAK1 may override glucose-induced Survivin ubiquitination. Interestingly, regardless of Survivin expression, short-time glucose-stimulated insulin secretion is not affected (data not shown). This indicates that Survivin is distal to insulin secretion, and the downregulation of Survivin by high-glucose may have long-term effects (such as replication) on β-cells.
Our recent work indicates that, in pancreatic β-cells, PAK1 limits Bad expression by transcriptional and post-transcriptional regulation.21 The data shown here suggest that Survivin is regulated by PAK1 via a post-transcriptional step involving ubiquitination, not a transcriptional step. Yet how PAK1 signals to the protein ubiquitination machinery remains unclear. It has been shown that ERK signaling is required for Survivin ubiquitination in pancreatic β-cells,27 and we have demonstrated that PAK1 is upstream of ERK activation in β-cells.19,20 Whether the axis of PAK1-ERK-Survivin (ubiquitination) signaling pathway is present in β-cells and how ERK is involved in PAK1-regulated Survivin-ubiquitination in β-cells are currently under investigation.
We previously demonstrated that PAK1 KO mice exhibit normal β-cell mass and insulin content in islets,19 which has been recently confirmed by another group.37 The lack of changes in β-cell mass with PAK1 deficiency may be due to the fact that the original β-cell mass determinations were made in younger mice (~10 weeks of age) without any metabolic challenges or appreciable declines in β-cell mass. Thus compensatory replication was not necessary or was yet to be initiated. To demonstrate a compensatory role for PAK1 in regulating functional β-cell mass, studies of β-cell mass and replication of the PAK1−/− and PAK+/− mouse models with inclusion of diet-induced stress are currently undergoing.
T2D occurs as a result of pancreatic β-cell failure in combination with insulin resistance in peripheral tissues. Around ~50% reduction in β-cell mass has been observed at the onset of clinical diagnosis.2,3 The fact that new β-cells continue to form in the adult animal4-7,38 indicates that understanding the details of how β-cells replicate themselves will be essential for the development of therapies that preserve β-cell mass and avoid the otherwise inevitable progression toward β-cell failure and T2D onset. Initially, we have shown that PAK1 is essential to whole-body glucose homeostasis given its unique role in both pancreatic β-cells and skeletal muscle19. Recent work by others also highlighted the importance of PAK1 in insulin secretion39 and insulin signaling.40 Consistent with a special requirement for PAK1 in insulin signaling, mRNA levels of PAK1 are significantly reduced in soleus and gastrocnemius skeletal muscle from obese diabetes-susceptible mice.41 Our work indicated that PAK1 is also involved in pancreatic β-cell survival21 and replication. These accumulated evidences indicate that PAK1 is a new hub of susceptibility for β-cell apoptosis, proliferation and function, and may play critical roles in the development of T2D. We speculate that PAK1 could be a potential therapeutic target for the treatment of diabetes, and efforts to minimize the loss of PAK1 protein/activation could help restore β-cell mass and function.
Materials and Methods
Materials
The rabbit anti-PAK1 antibody was purchased from Cell Signaling. The rabbit anti-Survivin antibody was purchased from R&D Systems. Rabbit anti-actin was obtained from Santa Cruz Biotechnology. Rabbit anti-Ubiquitin antibody was purchased from Cell Signaling. Goat anti-rabbit horseradish peroxidase and anti-mouse horseradish peroxidase secondary antibodies were acquired from Bio-Rad. Mouse PAK1 siRNA oligonucleotide duplexes and siRNA non-targeting control oligonucleotides were purchased from Ambion as described previously.18 Proteasome inhibitor MG132 was purchased from Millipore. Lipofectamine 2000, the SuperScript First Strand cDNA Synthesis Kit and Platinum SYBR Green qPCR SuperMix-UDG kit were purchased from Invitrogen. Enhanced chemiluminescence (ECL) reagent was obtained from Amersham Biosciences. d-glucose was obtained from Sigma. The pCMV-myc-Pak1 and pCMV vector plasmids were a gift from Dr. Lawrence Quilliam (Indiana University School of Medicine).
Methods
Mouse islets isolation
The PAK1 KO mouse is a classic whole-body gene-ablation model on the C57Bl6J strain background, generated as previously described.42 All islets were obtained using paired littermate mice as controls. Mouse pancreatic islets were isolated as previously described.18 Briefly, pancreata from 10–16 week old male mice were batch-digested with collagenase, purified using a Ficoll density gradient, and incubated in CRML at 37°C, 5% CO2 for further experiments. For western analysis of islets, 200 islets were lysated in 50 ul 2x SDS sample buffer (115mM TRIS-HCl (pH6.8), 4% SDS, 10% glycerol, 0.0025% Bromophenol blue, and 3% β-mercaptoethanol), followed by 12% SDS-PAGE separation. All studies involving mice followed the guidelines for the care and use of laboratory animals and the protocols were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Cell culture and transient transfection
MIN6 mouse pancreatic β-cells were cultured in DMEM (25 mM glucose) supplemented with 15% fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin, 292 µg/ml L-glutamine, and 50 µM β-mercaptoethanol as described previously.18 For PAK1 depletion, MIN6 β-cells were transiently transfected with PAK1 siRNA oligonucleotide duplexes along with pCMV6 vector plasmid or with pCMV6-myc-Pak1 plasmid as previously reported,18 or con-transfected along with MIEG3 vector or wild-type mouse Survivin.43 Cells were harvested in 1% Nonidet P-40 lysis buffer and lysates cleared by centrifugation at 14,000 × g for 10 min at 4°C for further experiments.
Islet RNA isolation and quantitative PCR (Q-PCR)
Total RNA from mouse islets or MIN6 β-cells was obtained using the RNeasy mini kit (Qiagen). RNA (0.5 mg) was reverse transcribed with the SuperScript First Strand cDNA Synthesis Kit (Invitrogen), and 10% of the product was used for Q-PCR. The primers used were as follows: Survivin primers, forward 5′-gat ctg gca gct gta cct ca-3′ and reverse 5′-aaa aca ctg ggc caa atc ag -3′; GAPDH primers, forward 5′- tgc ccc cat gtt tgt gat g-3′ and reverse 5′-tgt ggt cat gag ccc ttc c-3′. The Q-PCR conditions were as follows: 50°C for 2 min hold (UDG incubation), 95°C for 2 min hold, 40 cycles of 95°C for 15 sec, and 60°C for 30 sec.
Co-immunoprecipitation and immunoblotting
For immunoprecipitation, 1 mg cleared detergent lysates were combined with 1 µg of rabbit anti-Ubiquitin antibody and allowed to rotate overnight at 4°C. Protein G Plus agarose beads were added and reactions were rotated at 4°C for an additional 2 h. After three washes with lysis buffer, the resulting immunoprecipitates were subjected to 12% SDS-PAGE followed by transfer to PVDF membrane for immunoblotting. Membranes were incubated with primary anti-Survivin antibody at 4°C overnight, followed by incubation with secondary antibodies conjugated to horseradish peroxidase for 1 h at room temperature and visualized by enhanced chemiluminescence.
[3H]Thymidine Incorporation
DNA synthesis rates were measured by measuring the incorporation of [3H]methyl-thymidine into genomic DNA as described44,45. For MIN6 cells, 36 h after PAK1 depletion with siRNA, [3H]methyl-thymidine was added at a final concentration of 1 μCi/ml to MIN6 cells for 4 h. The cells were washed three times with cold medium. DNA was precipitated with two 500-μl aliquots of cold 10% trichloroacetic acid and solubilized by addition of 250 μl 0.3 N NaOH. The amount of [3H]thymidine incorporated into DNA was measured by liquid scintillation counting and normalized to total cellular protein. For mice islets, [3H]Methyl thymidine was added at a final concentration of 1 μCi/ml to pools of about 200 islets for 18 h. The islets were washed three times with cold medium. DNA was precipitated with two 500-μl aliquots of cold 10% trichloroacetic acid and solubilized by addition of 250 μl 0.3 n NaOH. Groups of 25 islets were picked in triplicate, washed, and centrifuged twice at 300 × g for 3 min at 4 C. DNA was precipitated with 500 μl cold 10% trichloroacetic acid and solubilized by addition of 80 μl 0.3 n NaOH. The amount of [3H]thymidine incorporated into DNA was measured by liquid scintillation counting and normalized to total cellular protein.
Statistical analysis
Student's t-test was used to evaluate statistical significance. Differences were considered significant when p < 0.05. Data are reported as means ± SEM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Y.C. contributed data for Figure 1 and Figure 7. P.F. helped interpret the data and edited the manuscript. Z.W. designed the project, contributed data for Figure 1–7, and wrote the manuscript. This study was supported by CTSI-KL2 RR025760 (to Z. W.). We are grateful to Dr Lawrence Quilliam (Indiana University School of Medicine) and Dr John Hutton (University of Colorado Health Sciences Center) for their gifts of the pCMV-myc-PAK1 plasmid and MIN6 cells, respectively. We thank Dr Stephanie Yoder for providing the mice, Dr Michael Kalwat for technique assistance, as well as Natalie Stull for assistance with islet isolation.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/24029
References
- 1.Wajchenberg BL. Clinical approaches to preserve beta-cell function in diabetes. Adv Exp Med Biol. 2010;654:515–35. doi: 10.1007/978-90-481-3271-3_23. [DOI] [PubMed] [Google Scholar]
- 2.Meier JJ, Breuer TG, Bonadonna RC, Tannapfel A, Uhl W, Schmidt WE, et al. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia. 2012;55:1346–54. doi: 10.1007/s00125-012-2466-8. [DOI] [PubMed] [Google Scholar]
- 3.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 4.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44:249–56. doi: 10.2337/diabetes.44.3.249. [DOI] [PubMed] [Google Scholar]
- 5.Ackermann AM, Gannon M. Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol. 2007;38:193–206. doi: 10.1677/JME-06-0053. [DOI] [PubMed] [Google Scholar]
- 6.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–6. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 7.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–26. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–9. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 9.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Wang L, Schroer S, Choi D, Chen P, Okada H, et al. Perinatal survivin is essential for the establishment of pancreatic beta cell mass in mice. Diabetologia. 2009;52:2130–41. doi: 10.1007/s00125-009-1469-6. [DOI] [PubMed] [Google Scholar]
- 11.Dohi T, Salz W, Costa M, Ariyan C, Basadonna GP, Altieri DC. Inhibition of apoptosis by survivin improves transplantation of pancreatic islets for treatment of diabetes in mice. EMBO Rep. 2006;7:438–43. doi: 10.1038/sj.embor.7400640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Zhang Q, Wang X, Zhu J, Xu K, Okada H, et al. Survivin is required for beta-cell mass expansion in the pancreatic duct-ligated mouse model. PLoS One. 2012;7:e41976. doi: 10.1371/journal.pone.0041976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson K, D’Mello SR. p21-Activated kinase-1 is necessary for depolarization-mediated neuronal survival. J Neurosci Res. 2005;79:809–15. doi: 10.1002/jnr.20415. [DOI] [PubMed] [Google Scholar]
- 14.Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, et al. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol. 2010;48:406–14. doi: 10.1016/j.yjmcc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egom EE, Mohamed TM, Mamas MA, Shi Y, Liu W, Chirico D, et al. Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am J Physiol Heart Circ Physiol. 2011;301:H1487–95. doi: 10.1152/ajpheart.01003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong CC, Jubb AM, Zhou W, Haverty PM, Harris AL, Belvin M, et al. p21-activated kinase 1: PAK’ed with potential. Oncotarget. 2011;2:491–6. doi: 10.18632/oncotarget.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qing H, Gong W, Che Y, Wang X, Peng L, Liang Y, et al. PAK1-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Tumour Biol. 2012;33:985–94. doi: 10.1007/s13277-012-0327-1. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem. 2007;282:9536–46. doi: 10.1074/jbc.M610553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Oh E, Clapp DW, Chernoff J, Thurmond DC. Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J Biol Chem. 2011;286:41359–67. doi: 10.1074/jbc.M111.291500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalwat MA, Yoder SM, Wang Z, Thurmond DC. A p21-activated kinase (PAK1) signaling cascade coordinately regulates F-actin remodeling and insulin granule exocytosis in pancreatic β cells. Biochem Pharmacol. 2013;85:808–16. doi: 10.1016/j.bcp.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Thurmond D. Pak1 regulates expression of the pro-apoptotic protein Bad in pancreatic islet β-cells. FEBS Open Bio. 2012;2:273–7. doi: 10.1016/j.fob.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley EW, Ruan MM, Oursler MJ. PAK1 is a novel MEK-independent raf target controlling expression of the IAP survivin in M-CSF-mediated osteoclast survival. J Cell Physiol. 2008;217:752–8. doi: 10.1002/jcp.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y, Nishimura W, Devor-Henneman D, Kusewitt D, Wang H, Holloway MP, et al. Postnatal expansion of the pancreatic beta-cell mass is dependent on survivin. Diabetes. 2008;57:2718–27. doi: 10.2337/db08-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci. 2000;113:4363–71. doi: 10.1242/jcs.113.23.4363. [DOI] [PubMed] [Google Scholar]
- 25.Leonardi O, Mints G, Hussain MA. Beta-cell apoptosis in the pathogenesis of human type 2 diabetes mellitus. Eur J Endocrinol. 2003;149:99–102. doi: 10.1530/eje.0.1490099. [DOI] [PubMed] [Google Scholar]
- 26.Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–24. doi: 10.2337/diabetes.53.2007.S119. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Gambosova K, Cooper ZA, Holloway MP, Kassai A, Izquierdo D, et al. EGF regulates survivin stability through the Raf-1/ERK pathway in insulin-secreting pancreatic β-cells. BMC Mol Biol. 2010;11:66. doi: 10.1186/1471-2199-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Liew CW, Handy DE, Zhang Y, Leopold JA, Hu J, et al. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and beta-cell apoptosis. FASEB J. 2010;24:1497–505. doi: 10.1096/fj.09-136572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conway EM, Pollefeyt S, Steiner-Mosonyi M, Luo W, Devriese A, Lupu F, et al. Deficiency of survivin in transgenic mice exacerbates Fas-induced apoptosis via mitochondrial pathways. Gastroenterology. 2002;123:619–31. doi: 10.1053/gast.2002.34753. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda S, Pelus LM. Regulation of the inhibitor-of-apoptosis family member survivin in normal cord blood and bone marrow CD34(+) cells by hematopoietic growth factors: implication of survivin expression in normal hematopoiesis. Blood. 2001;98:2091–100. doi: 10.1182/blood.V98.7.2091. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y, de Bruin A, Caldas H, Fangusaro J, Hayes J, Conway EM, et al. Essential role for survivin in early brain development. J Neurosci. 2005;25:6962–70. doi: 10.1523/JNEUROSCI.1446-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adida C, Crotty PL, McGrath J, Berrebi D, Diebold J, Altieri DC. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol. 1998;152:43–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Gianani R, Campbell-Thompson M, Sarkar SA, Wasserfall C, Pugliese A, Solis JM, et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia. 2010;53:690–8. doi: 10.1007/s00125-009-1642-y. [DOI] [PubMed] [Google Scholar]
- 34.Lee JO, Lee SK, Kim N, Kim JH, You GY, Moon JW, et al. E3 ubiquitin ligase, WWP1, interacts with AMPKalpha2 and downregulates its expression in skeletal muscle C2C12 cells. J Biol Chem. 2013;288:4673–80. doi: 10.1074/jbc.M112.406009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Livak MF, Bernier M, Muller DC, Carlson OD, Elahi D, et al. Ubiquitination is involved in glucose-mediated downregulation of GIP receptors in islets. Am J Physiol Endocrinol Metab. 2007;293:E538–47. doi: 10.1152/ajpendo.00070.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes R, Carvalho AL, Kumagai A, Seica R, Hosoya K, Terasaki T, et al. Downregulation of retinal GLUT1 in diabetes by ubiquitinylation. Mol Vis. 2004;10:618–28. [PubMed] [Google Scholar]
- 37.Chiang YA, Shao W, Xu XX, Chernoff J, Jin T. P21-activated protein kinase 1 (Pak1) mediates the cross talk between insulin and β-catenin on proglucagon gene expression and its ablation affects glucose homeostasis in male C57BL/6 mice. Endocrinology. 2013;154:77–88. doi: 10.1210/en.2012-1781. [DOI] [PubMed] [Google Scholar]
- 38.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114:963–8. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nie J, Sun C, Faruque O, Ye G, Li J, Liang Q, et al. Synapses of amphids defective (SAD-A) kinase promotes glucose-stimulated insulin secretion through activation of p21-activated kinase (PAK1) in pancreatic β-Cells. J Biol Chem. 2012;287:26435–44. doi: 10.1074/jbc.M112.378372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ijuin T, Takenawa T. Regulation of insulin signaling by the phosphatidylinositol 3,4,5-triphosphate phosphatase SKIP through the scaffolding function of Pak1. Mol Cell Biol. 2012;32:3570–84. doi: 10.1128/MCB.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, et al. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res. 2008;18:706–16. doi: 10.1101/gr.074914.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen JD, Jaffer ZM, Park SJ, Burgin S, Hofmann C, Sells MA, et al. p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood. 2009;113:2695–705. doi: 10.1182/blood-2008-06-160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Fukuda S, Pelus LM. Survivin regulates the p53 tumor suppressor gene family. Oncogene. 2004;23:8146–53. doi: 10.1038/sj.onc.1207992. [DOI] [PubMed] [Google Scholar]
- 44.Fueger PT, Schisler JC, Lu D, Babu DA, Mirmira RG, Newgard CB, et al. Trefoil factor 3 stimulates human and rodent pancreatic islet beta-cell replication with retention of function. Mol Endocrinol. 2008;22:1251–9. doi: 10.1210/me.2007-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fueger PT, Hernandez AM, Chen YC, Colvin ES. Assessing replication and beta cell function in adenovirally-transduced isolated rodent islets. J Vis Exp. 2012;64 doi: 10.3791/4080. [DOI] [PMC free article] [PubMed] [Google Scholar]


