Abstract
Rho-associated coiled-coil kinase (ROCK) inhibitor Y-27632 has been shown to increase proliferative capacity and even immortalize primary keratinocytes. Here, we demonstrate that rabbit primary limbal epithelial cells (LECs) treated with Y-27632 also exhibited improved colony-forming efficiency by enhancing the expansion of the stem/progenitor cells. Moreover, Y-27632 treatment improved the rapid adherence of limbal stem/progenitor cells in the initial inoculation of primary cells. In addition, Y-27632 treatment elevated the intracellular glutathione level and decreased cellular reactive oxygen species (ROS) accumulation during the expansion of LECs. Therefore, ROCK inhibitor Y-27632 increased the cloning efficiency of rabbit limbal stem/progenitor cells by improving their adherence and ROS scavenging capacity.
Introduction
Autologous or allogeneic limbal tissue transplantation is the traditional therapy for limbal stem cell deficiency (LSCD),1 a severe corneal surface disorder characterized by the loss of corneal epithelial stem cells that is caused by genetic diseases and chemical and thermal burns. However, it may cause a risk of LSCD in the donor eye used for autologous transplantation2 or require the long-term use of immunosuppressive drugs for the possible immunological rejection after allograft transplantation.3 Recently, the transplantation of ex vivo expanded limbal epithelial cells (LECs) or oral mucosal epithelial cells has become an alternative therapy for the structural and functional reconstruction of the corneal surface in LSCD.4–8 However, this therapy depends on the number and survival of the limbal stem cells with clonogenic capacity in the transplanted LEC sheet.8 Therefore, obtaining a sufficient number of limbal stem/progenitor cells in vitro is critical for LSCD therapy using cultured limbal epithelial transplantation.
In most current protocols, 3T3 feeder cells, cholera toxin, exogenous growth factors, hormones, and fetal bovine serum are necessary for the expansion of LECs in vitro.4,8 Although the use of clinical-grade 3T3-J2 cells has no adverse effects and has been approved in the United States, Japan, Italy, and South Korea during the past 30 years,9,10 the development of a xenofree defined limbal culture system is important for clinical applications because of the possible xenocontamination by bovine serum, mouse cells, or recombinant growth factors.11 Small molecules, particularly those with well-defined structures and target genes, have been confirmed as powerful tools for manipulating the fate, state, and function of various stem cells.12 Recently, there has been a significant progress in using small molecules to sustain pluripotency or induce the differentiation of embryonic stem cells, to replace transcription factors, and to enhance efficiency during somatic cell reprogramming.13,14 In a previous study, we found that pluripotin significantly promoted proliferation and colony-forming efficiency and prevented senescence in rabbit primary LECs.15
Y-27632, a selective inhibitor of p160-Rho-associated coiled-coil kinase, has recently been shown to increase greatly the cloning efficiency of human embryonic stem cells, keratinocytes, and corneal endothelial cells, to diminish dissociation-induced apoptosis, and even to enable cells to bypass senescence and become immortal in the presence of feeder layers.16–26 In the current study, we demonstrate that the treatment of primary LECs with Y-27632 significantly increased their colony-forming efficiency by enhancing the expansion of the limbal stem/progenitor cells. Moreover, Y-27632 improved the rapid adherence of limbal stem/progenitor cells in the initial inoculation. In addition, we found an elevated intracellular glutathione (GSH) level and decreased intracellular reactive oxygen species (ROS) accumulation during clonal expansion.
Materials and Methods
Isolation and treatment of primary LECs
All of the animal experiments were conducted in accordance with the Animal Care and Use Committee guidelines of the Shandong Eye Institute and the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The limbal tissues from New Zealand rabbits (n=20) were treated with 2.4 U/mL Dispase (Roche, Indianapolis, IN) in the Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) for 2 h at 37°C. The limbal epithelium was removed under a dissecting microscope and treated with 0.25% trypsin/0.02% EDTA for 15 min at 37°C. The acquired primary LECs were suspended in the DMEM/F-12 medium supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY), insulin-transferrin-selenium (Invitrogen), 1% nonessential amino acids (Invitrogen), 0.1 nM cholera toxin (Sigma, St. Louis, MO), 2 nM 3,3′5-triiodo-l-thyronine sodium salt (Sigma), 0.4 ng/mL hydrocortisone succinate (Wako, Osaka, Japan), 2 mM l-glutamine (Invitrogen), penicillin–streptomycin (Hyclone, Logan, UT), and 10 ng/mL recombinant human EGF (R&D Systems, Minneapolis, MN). The primary LECs were inoculated on an uncoated plate surface, treated with or without 10 μM Y-27632 (Sigma), and evaluated using a cell adherence assay, the colony-forming efficiency, immunofluorescent staining, the intracellular GSH level, and the ROS accumulation. Normally cultured cells were used as the control.
Cell adherent evaluation
For the evaluation of cell adherence, freshly isolated LECs were allowed to attach to the uncoated surface of cell culture plates at 37°C for 2 h. The LECs attached after 2 h were designated as rapid adherent cells, and the unattached cells were collected as slow adherent cells. The two cell populations were subjected to immunofluorescence staining.
Colony-forming assay
NIH-3T3 cells were treated with 4 mg/mL mitomycin C (Haizheng, Taizhou, China) for 2 h and reinoculated at a density of 4×104 cells/cm2 as feeder layers. Primary LECs were seeded at a density of 1000 cells per well and incubated for 8 days. The colonies were fixed with ice-cold methanol and stained with 2% Rhodamine B (Sigma), and the colony number was counted and evaluated using ImageJ software.
Immunofluorescence staining
For the identification of the limbal stem/progenitor cells, the rabbit cells were fixed with 4% paraformaldehyde, blocked with 1% normal goat serum, and incubated overnight at 4°C with the following primary antibodies: anti-ΔNP63 (1:100; Invitrogen), anti-telomerase (1:100; Santa Cruz), anti-β1-integrin (1:100; Biolegend, San Diego, CA) and anti-involucrin (1:150; Sigma). After washing with PBS, the cells were incubated for 1 h with rhodamine- or FITC-conjugated goat immunoglobulin G secondary antibodies (1:100; Santa Cruz). The nucleus was counterstained with DAPI, and the staining was viewed under an Eclipse TE2000-U microscope (Nikon, Tokyo, Japan).
Detection of ROS
For the detection of intracellular ROS accumulation, the 3T3 feeder cells were removed by transient 0.05% trypsin–EDTA treatment, followed by three washes with PBS.27,28 The colonies were then loaded with 10 μM peroxide-sensitive fluorescence probe 2,7-dichlorodihydrofluorescein diacetate–acetyl ester (H2-DCFDA; Molecular Probes, Eugene, OR) for 30 min at 37°C. The images were observed using Nikon confocal microscopy at 488/520 nm.
Measurement of intracellular GSH levels
The total cellular GSH content was measured using the GSH Assay Kit (Beyotime, Haimen, China) according to the manufacturer's instructions. Briefly, the cells in colonies were collected after the removal of the feeder cells and treated with three volumes of protein removal reagent. The suspension was freeze-thawed three times using liquid nitrogen and a 37°C water bath. The supernatant was collected and mixed with the kit-provided working buffer and NADPH. The content of total GSH was quantified by the comparison with known GSH standards. In some experiments, the colonies were incubated in a serum-free medium containing 50 μM monochlorobimane (MCB; Sigma) for 30 min. The staining of the intracellular GSH was observed using fluorescence microscopy.
Statistical analysis
The data were presented as the means±SD. The differences between the control and experimental groups were tested with Student's t-test. A p-value of<0.05 was considered to be statistically significant.
Results
Y-27632 promoted the cloning expansion of limbal stem/progenitor cells
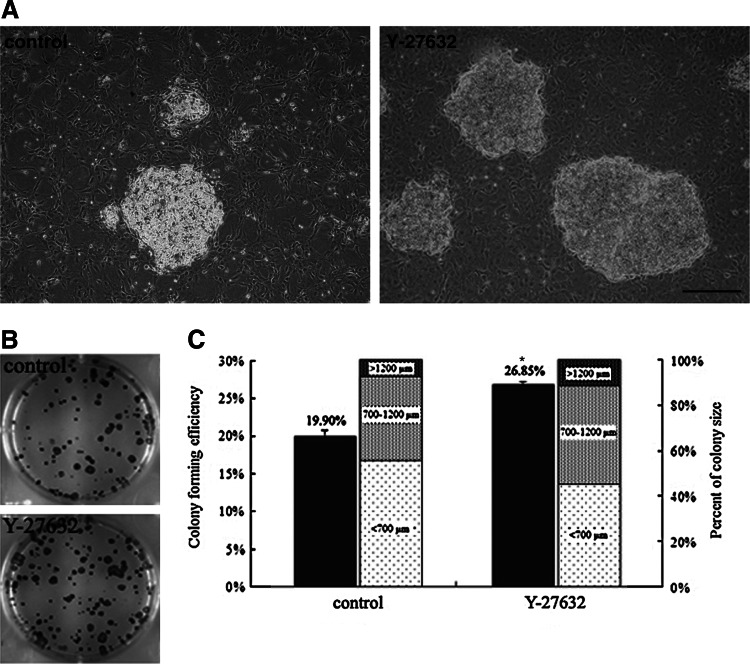
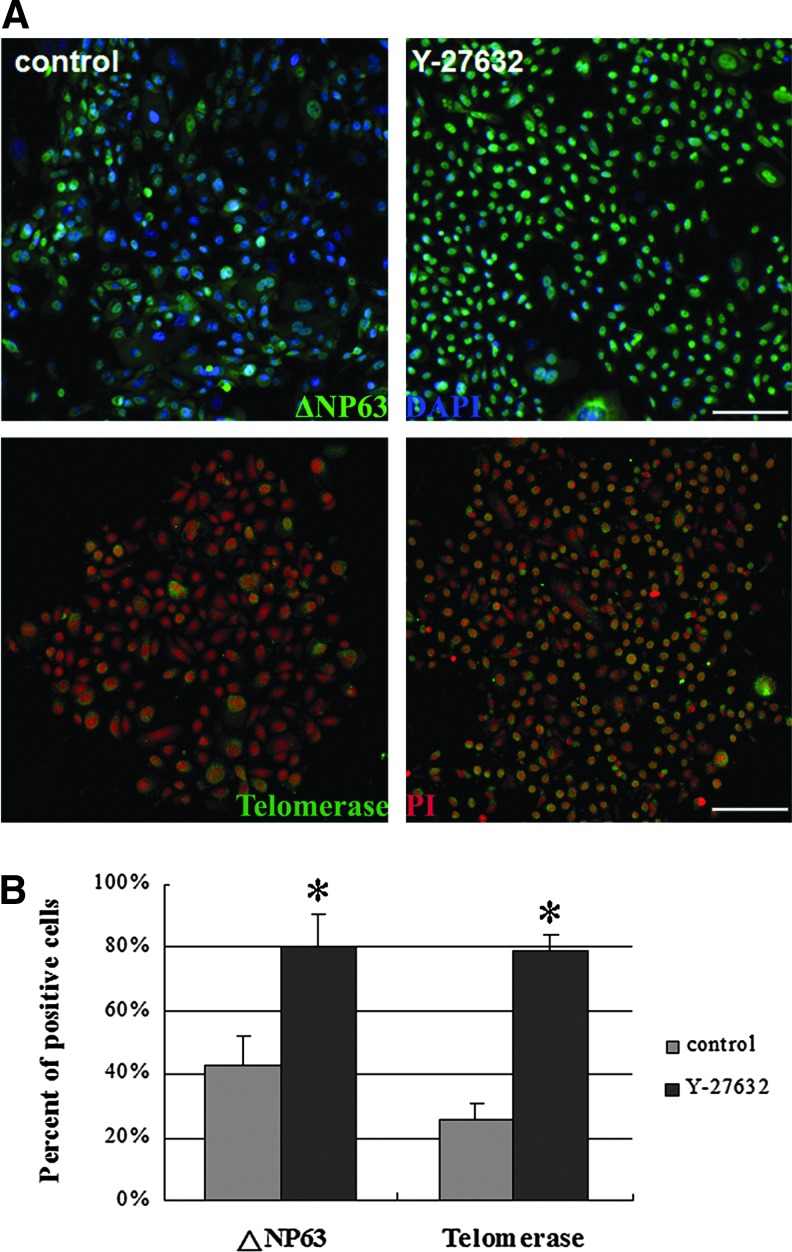
To examine the effects of Y-27632 on the cloning efficiency of limbal stem/progenitor cells, the rabbit cells were inoculated on mitomycin C-inactivated 3T3 feeder cells and incubated with 10 μM Y-27632 for 8 days. Although the primary LECs formed typical cell colonies on the 3T3 feeder cells, the Y-27632-treated cells grew into more dense and larger colonies than those without Y-27632 treatment (Fig. 1A, B). The colony-forming efficiencies ranged from 19.90%±8.49% for the control cells to 26.85%±3.54% for the Y-27632-treated cells, with significant differences between them (Fig. 1C). From the quantitative analysis of the colony diameter, it was found that the number of large-sized colonies (>1200 μm) and moderate-sized colonies (700–1200 μm) increased significantly in the presence of Y-27632 (11.73%±6.36% and 43.18%±3.65%) compared with the untreated normal cells (7.53%±3.16% and 36.79%±3.89%). In contrast, the number of small-sized colonies (<700 μm) decreased from 55.68%±6.79% for the control cells to 45.09%±4.28% for the Y-27632-treated cells (Fig. 1C). In addition, the immunofluorescence staining revealed that more cells treated with Y-27632 exhibited positive staining of a putative limbal stem/progenitor cell marker ΔNP63 and telomerase compared with the cells grown in the control medium (Fig. 2A). The quantitative analysis based on the counting of positive-staining cells showed that the difference between the two groups was significant (Fig. 2B).
FIG. 1.
Y-27632 promoted the colony-forming efficiency of limbal epithelial cells. Primary rabbit limbal epithelial cells were inoculated on mitomycin C-inactivated 3T3 feeder cells and incubated with or without Y-27632 for 8 days. The Y-27632-treated cells grew into more dense and larger colonies than the control cells, as shown by their morphology (A) and Rhodamine B staining (B). A quantitative analysis of the colony density and colony size showed that Y-27632 significantly increased the clonal growth of limbal stem/progenitor cells with larger sizes compared with the untreated cells (C). The experiment (n=5) was repeated three times, and representative photos are shown. *p<0.05. Scale bar: 500 μm.
FIG. 2.
Y-27632 increased the number of cells expressing putative limbal stem/progenitor cell markers. Compared with the control limbal epithelial cells, more cells treated with Y-27632 showed positive staining of the putative limbal stem/progenitor cell markers ΔNP63 and telomerase (A). The difference was significant based on the count of positive-staining cells (B). The experiment (n=4) was repeated three times, and representative photos are shown. *p<0.05. Scale bar: 50 μm. Color images available online at www.liebertpub.com/tec
Y-27632 promoted the adherence of LECs
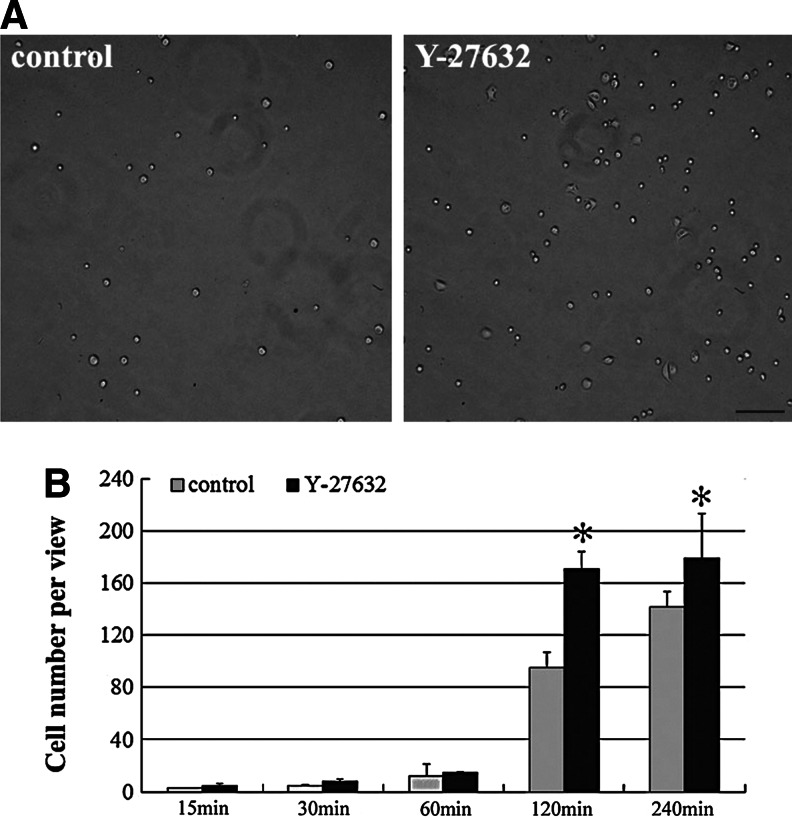
The adherence of rabbit LECs was evaluated by incubating cells on an uncoated plate surface for different lengths of time from 15, 30, 60, and 120 to 240 min. As shown in the images captured, the presence of Y-27632 significantly increased the number of adherent cells in comparison to the control cells without Y-27632 at 120 min (Fig. 3A). According to the counting and quantitative analysis, the number of adherent cells increased from 95±12 to 171±13/view field and from 142±11 to 179±34/view field for the control cells and Y-27632-treated cells at 120 and 240 min, respectively, whereas the cells maintained a stable level before 60-min or after 240-min incubation (Fig. 3B).
FIG. 3.
Y-27632 promoted the adherence of limbal epithelial cells. Rabbit limbal epithelial cells were inoculated on an uncoated plate surface for different durations, and the number of adhered cells per view field was counted and analyzed. Y-27632 significantly increased the number of adherent cells in comparison to the control cells at 120 min (A). A quantitative analysis revealed that the number of adherent cells significantly increased with the Y-27632 treatment after 120 and 240 min of incubation (B). The experiment (n=4) was repeated three times, and representative photos are shown. *p<0.05. Scale bar: 100 μm.
Y-27632 promoted the rapid adherence of limbal stem/progenitor cells
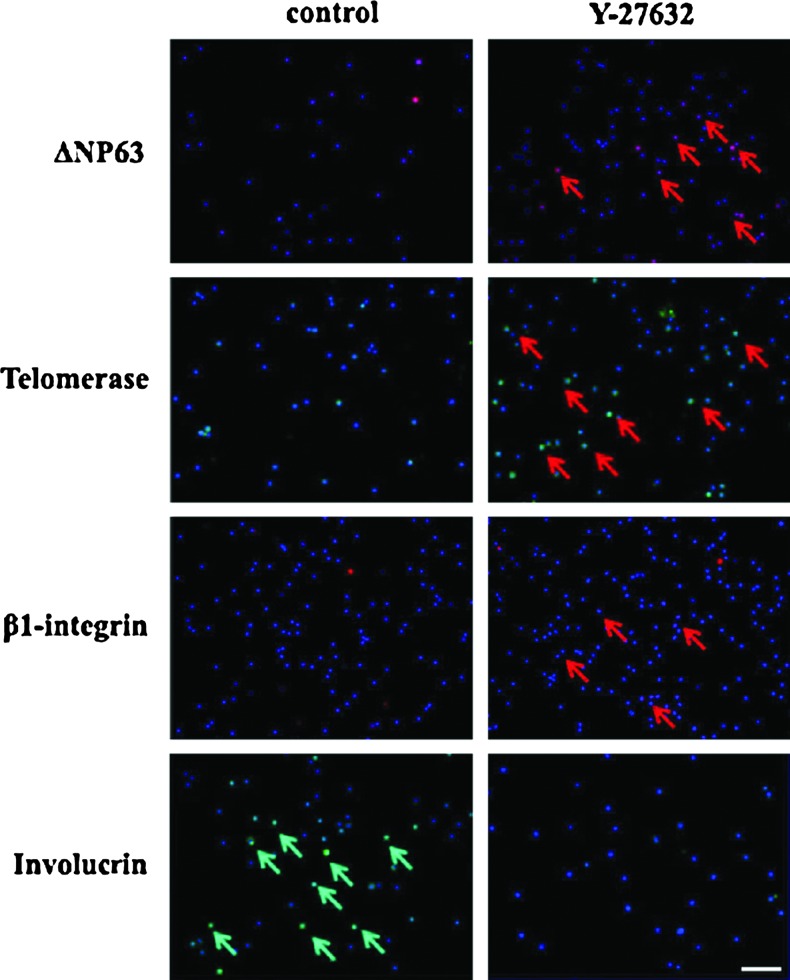
To evaluate the phenotype of rapid adherent cells increased by the Y-27632 treatment, the cells were collected, centrifuged on the slides using a cytospin, and prepared for immunofluorescence staining with antibodies against putative limbal stem/progenitor cell markers, including β1-integrin, ΔNP63, telomerase, and the differentiated corneal epithelial cell marker involucrin. As shown in Figure 4, the rapid adhered cell population treated with Y-27632 showed more positively staining cells for the putative limbal stem cell markers and fewer cells positive for the differentiated corneal epithelial cell marker. Thus, the results showed that Y-27632 promoted the rapid adherence of limbal stem/progenitor cells.
FIG. 4.
Y-27632 promoted the rapid adherence of limbal stem/progenitor cells. The rapid adhered cell populations after 120 min of incubation were collected and analyzed. The Y-27632 treatment resulted in more positively staining cells for the putative limbal stem cell markers ΔNP63, telomerase, and β1-integrin and less staining for the differentiated corneal epithelial cell marker involucrin. The experiment (n=3) was repeated three times, and representative photos are shown. Arrows show the positive-staining cells. Scale bar: 20 μm. Color images available online at www.liebertpub.com/tec
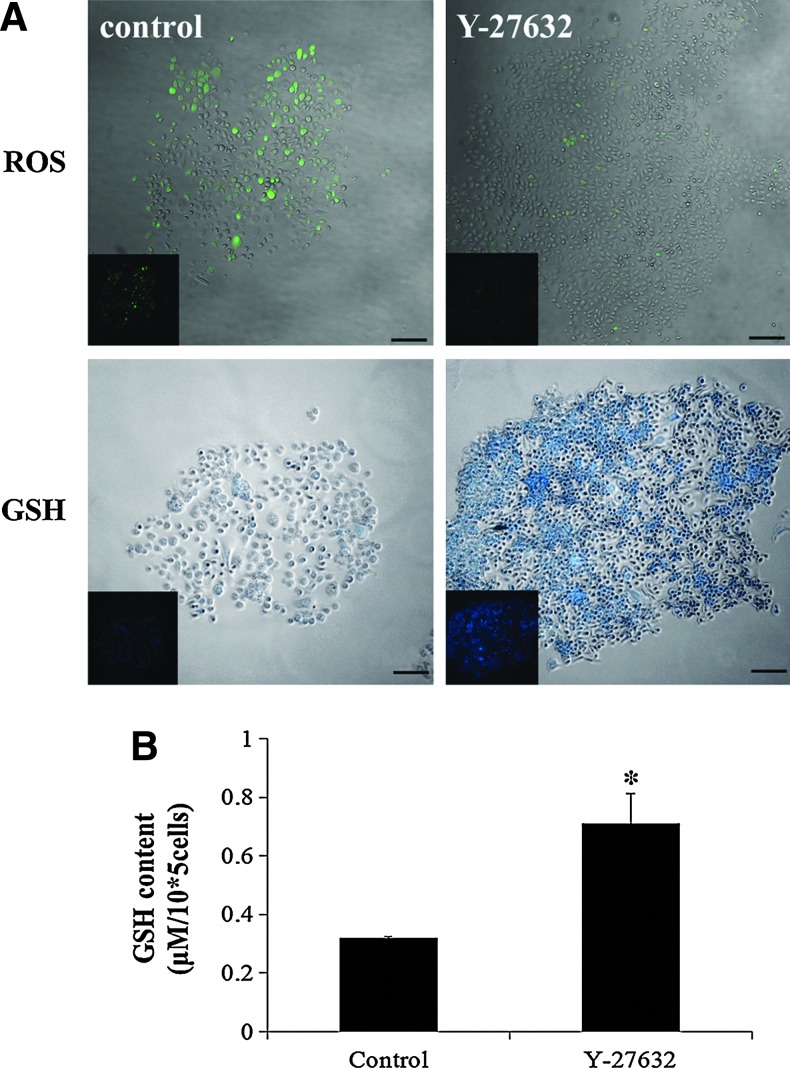
Y-27632 scavenged accumulated ROS and increased GSH level
To examine the accumulation of ROS and GSH, the 3T3 feeder cells were removed when the limbal stem cell colonies formed, and the cloned cells were loaded with H2-DCFDA for 30 min at 37°C. A significant amount of ROS was generated in the control cells that were cultured in the traditional medium, whereas the addition of Y-27632 significantly decreased the accumulation of ROS in the limbal stem/progenitor colonies. Moreover, Y-27632 increased the intracellular GSH level based on MCB staining (Fig. 5A and inset). According to the biochemical analysis of the total cellular GSH content, Y-27632 increased the intracellular GSH level more than 2-fold compared with the control cells (Fig. 5B).
FIG. 5.
Y-27632 scavenged accumulated reactive oxygen species (ROS), and increased the glutathione (GSH) level. Rabbit limbal epithelial cells were cultured with or without Y-27632 on 3T3 feeder cells for 8 days. The cloned cells were removed from the feeder cells and loaded with H2-DCFDA or monochlorobimane to examine the accumulated intracellular ROS and GSH levels. The control cells showed a significant amount of ROS and a low GSH level, whereas the addition of Y-27632 significantly decreased the accumulation of ROS and increased the GSH level in the limbal stem/progenitor colonies (A and inset). The biochemical analysis revealed that Y-27632 increased the total intracellular glutathione content more than twofold over the control cells (B). The experiment (n=3) was repeated three times, and representative photos are shown. *p<0.05. Scale bar: 100 μm. Color images available online at www.liebertpub.com/tec
Discussion
Rho-associated coiled-coil kinase (ROCK) inhibitor Y-27632 has been shown to increase proliferative capacity and even immortalize primary keratinocytes in the presence of feeder cells.23,25,29 In the present study, we confirmed that the treatment of primary LECs with Y-27632 significantly increased their colony-forming efficiency by enhancing the expansion of the limbal stem/progenitor cells. Moreover, Y-27632 improved the rapid adherence of the limbal stem/progenitor cells in the initial inoculation of primary cells and elevated the intracellular GSH level and decreased the cellular ROS accumulation during clonal expansion. Therefore, ROCK inhibitor Y-27632 increased the cloning efficiency of limbal stem/progenitor cells, at least in part, by improving their rapid adherence and ROS-scavenging ability.
Previous studies have demonstrated that Y-27632 greatly increased the cloning efficiency of human embryonic or adult stem cells, diminished dissociation-induced apoptosis, and even enabled the cells to bypass senescence and become immortal in the presence of feeder layers.16–25 In the present study, we found that Y-27632 significantly increased the colony-forming efficiency of limbal stem/progenitor cells, particularly the cell population with the capacity of forming colonies of large diameter. Moreover, the cloned cells maintained the expression of the putative limbal stem cell markers ΔNP63 and telomerase, suggesting that Y-27632 could inhibit spontaneous differentiation or even reverse the senescence of limbal stem/progenitor cells in vitro, similar to that described for human keratinocytes.23 In addition, we also found that Y-27632 could promote the rapid adherence of LECs almost two-fold and that the rapid adhered cell population demonstrated upregulated expression of limbal stem/progenitor cell markers. The results also provide an alternative explanation for the Y-27632-mediated increasing in the colony-forming capacity. Moreover, we also confirmed the Y-27632-induced promotion of clonal expansion and selective adherence of human LECs and upregulated mRNA expression of putative limbal stem cell markers in vitro (data not shown). Accordingly, Y-27632 may be a good candidate molecule to enhance the in vitro expansion of limbal stem/progenitor cells, which may be used to improve the success of the clinical transplantation of cultured LEC sheets for ocular surface reconstruction.30
It has become increasingly clear that low levels of intracellular ROS may act as essential signaling molecules to mediate multiple cellular functions, including proliferation, apoptosis, migration, and stem cell maintenance and differentiation.31–34 However, the exacerbated production of ROS may directly cause cellular protein, lipid, and DNA damage, ultimately resulting in tissue aging and the development of various diseases.35–40 The detrimental effect of intracellular ROS in hematopoietic stem cell function is widely accepted, and it has been shown that antioxidant agents prolong the life of hematopoietic stem cells.41,42 Taken together, the production of appropriate levels of ROS is tightly controlled to promote the proliferation and survival of stem/progenitor cells.43 This is the first report that Y-27632 can scavenge accumulated ROS and increase the intracellular GSH content, a critical factor involved in the maintenance of cellular redox homeostasis, in the clonal expansion of limbal stem/progenitor cells. The results suggest that the Y-27632-mediated promotion of the expansion and survival of embryonic or adult stem cells may depend on the ROS-scavenging capacity.
Conclusions
We have demonstrated that Y-27632 significantly increased the colony-forming efficiency by enhancing the expansion of limbal stem/progenitor cells in vitro. The promotion is partially dependent on an improvement of the rapid adherence of limbal stem/progenitor cells in the initial inoculation. Elevated intracellular GSH levels and decreased cellular ROS accumulation were observed during clonal expansion.
Acknowledgments
This work was partially supported by the National Basic Research Program of China (2013CB967004 and 2012CB722409), National Natural Science Foundation of China (81170816), and Shandong Natural Science Foundation (ZR2010HQ019). Qingjun Zhou is partially supported by the Taishan Scholar Program (20081148) and Shandong Provincial Excellent Innovation Team Program.
Disclosure Statement
No competing financial interests exist.
References
- 1.Cauchi P.A. Ang G.S. Azuara-Blanco A. Burr J.M. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am J Ophthalmol. 2008;146:251. doi: 10.1016/j.ajo.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins C. Tuft S. Liu C. Buckley R. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye (Lond) 1993;7(Pt 5):629. doi: 10.1038/eye.1993.145. [DOI] [PubMed] [Google Scholar]
- 3.Tsai R.J. Tseng S.C. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13:389. doi: 10.1097/00003226-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Pellegrini G. Traverso C.E. Franzi A.T. Zingirian M. Cancedda R. De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T. Kinoshita S. Ocular surface reconstruction using cultivated mucosal epithelial stem cells. Cornea. 2003;22:S75. doi: 10.1097/00003226-200310001-00011. [DOI] [PubMed] [Google Scholar]
- 6.Tsai R.J. Li L.M. Chen J.K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 7.Nishida K. Yamato M. Hayashida Y. Watanabe K. Yamamoto K. Adachi E. Nagai S. Kikuchi A. Maeda N. Watanabe H. Okano T. Tano Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 8.Rama P. Matuska S. Paganoni G. Spinelli A. De Luca M. Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 9.De Luca M. Pellegrini G. Green H. Regeneration of squamous epithelia from stem cells of cultured grafts. Regen Med. 2006;1:45. doi: 10.2217/17460751.1.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Green H. The birth of therapy with cultured cells. Bioessays. 2008;30:897. doi: 10.1002/bies.20797. [DOI] [PubMed] [Google Scholar]
- 11.Sangwan V.S. Basu S. Vemuganti G.K. Sejpal K. Subramaniam S.V. Bandyopadhyay S. Krishnaiah S. Gaddipati S. Tiwari S. Balasubramanian D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95:1525. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 12.Li W. Jiang K. Ding S. A chemical approach to controlling cell fate and function. Stem Cells. 2012;30:61. doi: 10.1002/stem.768. [DOI] [PubMed] [Google Scholar]
- 13.Lyssiotis C.A. Foreman R.K. Staerk J. Garcia M. Mathur D. Markoulaki S. Hanna J. Lairson L.L. Charette B.D. Bouchez L.C. Bollong M. Kunick C. Brinker A. Cho C.Y. Schultz P.G. Jaenisch R. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci U S A. 2009;106:8912. doi: 10.1073/pnas.0903860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichida J.K. Blanchard J. Lam K. Son E.Y. Chung J.E. Egli D. Loh K.M. Carter A.C. Di Giorgio F.P. Koszka K. Huangfu D. Akutsu H. Liu D.R. Rubin L.L. Eggan K. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan H. Wang Y. Yang L. Qu M. Wang Q. Shi W. Zhou Q. Pluripotin enhances the expansion of rabbit limbal epithelial stem/progenitor cells in vitro. Exp Eye Res. 2012;100:52. doi: 10.1016/j.exer.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Terunuma A. Limgala R.P. Park C.J. Choudhary I. Vogel J.C. Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Eng Part A. 2010;16:1363. doi: 10.1089/ten.tea.2009.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichikawa H. Nakata N. Abo Y. Shirasawa S. Yokoyama T. Yoshie S. Yue F. Tomotsune D. Sasaki K. Gene pathway analysis of the mechanism by which the Rho-associated kinase inhibitor Y-27632 inhibits apoptosis in isolated thawed human embryonic stem cells. Cryobiology. 2012;64:12. doi: 10.1016/j.cryobiol.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Emre N. Vidal J.G. Elia J. O'Connor E.D. Paramban R.I. Hefferan M.P. Navarro R. Goldberg D.S. Varki N.M. Marsala M. Carson C.T. The ROCK inhibitor Y-27632 improves recovery of human embryonic stem cells after fluorescence-activated cell sorting with multiple cell surface markers. PLoS One. 2010;5:e12148. doi: 10.1371/journal.pone.0012148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X. Meng G. Krawetz R. Liu S. Rancourt D.E. The ROCK inhibitor Y-27632 enhances the survival rate of human embryonic stem cells following cryopreservation. Stem Cells Dev. 2008;17:1079. doi: 10.1089/scd.2007.0247. [DOI] [PubMed] [Google Scholar]
- 20.Takehara T. Teramura T. Onodera Y. Kakegawa R. Fukunaga N. Takenoshita M. Sagawa N. Fukuda K. Hosoi Y. Rho-associated kinase inhibitor Y-27632 promotes survival of cynomolgus monkey embryonic stem cells. Mol Hum Reprod. 2008;14:627. doi: 10.1093/molehr/gan061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe K. Ueno M. Kamiya D. Nishiyama A. Matsumura M. Wataya T. Takahashi J.B. Nishikawa S. Muguruma K. Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 22.Li X. Krawetz R. Liu S. Meng G. Rancourt D.E. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum Reprod. 2009;24:580. doi: 10.1093/humrep/den404. [DOI] [PubMed] [Google Scholar]
- 23.Liu X. Ory V. Chapman S. Yuan H. Albanese C. Kallakury B. Timofeeva O.A. Nealon C. Dakic A. Simic V. Haddad B.R. Rhim J.S. Dritschilo A. Riegel A. McBride A. Schlegel R. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L. Valdez J.M. Zhang B. Wei L. Chang J. Xin L. ROCK inhibitor Y-27632 suppresses dissociation-induced apoptosis of murine prostate stem/progenitor cells and increases their cloning efficiency. PLoS One. 2011;6:e18271. doi: 10.1371/journal.pone.0018271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman S. Liu X. Meyers C. Schlegel R. McBride A.A. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okumura N. Koizumi N. Ueno M. Sakamoto Y. Takahashi H. Tsuchiya H. Hamuro J. Kinoshita S. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol. 2012;181:268. doi: 10.1016/j.ajpath.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Li A. Simmons P.J. Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A. 1998;95:3902. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hybbinette S. Bostrom M. Lindberg K. Enzymatic dissociation of keratinocytes from human skin biopsies for in vitro cell propagation. Exp Dermatol. 1999;8:30. doi: 10.1111/j.1600-0625.1999.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 29.van den Bogaard E.H. Rodijk-Olthuis D. Jansen P.A. van Vlijmen-Willems I.M. van Erp P.E. Joosten I. Zeeuwen P.L. Schalkwijk J. Rho Kinase Inhibitor Y-27632 Prolongs the life span of adult human keratinocytes, enhances skin equivalent development, and facilitates lentiviral transduction. Tissue Eng Part A. 2012;18:1827. doi: 10.1089/ten.tea.2011.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rama P. Matuska S. Paganoni G. Spinelli A. De Luca M. Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 31.Valko M. Leibfritz D. Moncol J. Cronin M.T. Mazur M. Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Ushio-Fukai M. Urao N. Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal. 2009;11:2517. doi: 10.1089/ars.2009.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 34.Li T.S. Marban E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells. 2010;28:1178. doi: 10.1002/stem.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kregel K.C. Zhang H.J. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 36.Trachootham D. Lu W. Ogasawara M.A. Nilsa R.D. Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu R.M. Gaston Pravia K.A. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med. 2010;48:1. doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocha M. Apostolova N. Hernandez-Mijares A. Herance R. Victor V.M. Oxidative stress and endothelial dysfunction in cardiovascular disease: mitochondria-targeted therapeutics. Curr Med Chem. 2010;17:3827. doi: 10.2174/092986710793205444. [DOI] [PubMed] [Google Scholar]
- 39.Hirooka Y. Sagara Y. Kishi T. Sunagawa K. Oxidative stress and central cardiovascular regulation.—Pathogenesis of hypertension and therapeutic aspects. Circ J. 2010;74:827. doi: 10.1253/circj.cj-10-0153. [DOI] [PubMed] [Google Scholar]
- 40.Kao M.P. Ang D.S. Pall A. Struthers A.D. Oxidative stress in renal dysfunction: mechanisms, clinical sequelae and therapeutic options. J Hum Hypertens. 2010;24:1. doi: 10.1038/jhh.2009.70. [DOI] [PubMed] [Google Scholar]
- 41.Ito K. Hirao A. Arai F. Takubo K. Matsuoka S. Miyamoto K. Ohmura M. Naka K. Hosokawa K. Ikeda Y. Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 42.Yahata T. Takanashi T. Muguruma Y. Ibrahim A.A. Matsuzawa H. Uno T. Sheng Y. Onizuka M. Ito M. Kato S. Ando K. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi C.I. Suda T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J Cell Physiol. 2012;227:421. doi: 10.1002/jcp.22764. [DOI] [PubMed] [Google Scholar]