Abstract
Background
AdGV.EGR.TNF.11D (TNFerade™ Biologic) is a replication-deficient adenoviral vector expressing human tumor necrosis factor alpha (TNF-α) under the control of the chemoradiation-inducible EGR-1 promoter. TNF-α has been shown to function as a radiation sensitizer. We conducted a phase I dose escalation study to determine the maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of TNFerade™ Biologic, when added to chemoradiotherapy in poor prognosis patients with recurrent, previously irradiated head and neck cancer (HNC).
Methods
TNFerade™ Biologic was injected intratumorally on day 1 of each 14-day cycle and dose-escalated in log increments from 4 × 109 to 4 × 1011 PU. Daily radiation, infusional 5-fluorouracil (5-FU), and hydroxyurea were given on days 1–5 for seven cycles (FHX). Tumor biopsies were obtained before, during, and after treatment.
Results
Fourteen patients were treated. DLT was reached at a dose level of 3 (4 × 1011 PU) with three thrombotic events. The response rate was 83.3%. The median survival was 9.6 months. One patient (7.1%) remained alive 3 years after treatment. Biopsies were obtained in 90% of patients. Nearly all tumors expressed adenovirus receptors, TNF-α, and TNF-α receptors. Adenoviral DNA was detected in three biopsies from one patient.
Conclusions
TNFerade™ Biologic can be safely integrated with FHX chemoradiotherapy at an MTD of 4 × 1010 PU. Monitoring for thrombotic events is indicated.
Keywords: chemoradiation, gene therapy, head and neck cancer, recurrent disease, translational research
introduction
Head and neck cancer (HNC) is the sixth most common cancer worldwide with an annual incidence of >600 000 cases [1]. The majority (>90%) of cases are head and neck squamous cell carcinomas (HNSCC). Mortality remains substantial with 30%–40% of HNSCC patients dying from their disease [1]. Locoregional treatment failure remains a major cause of tumor recurrence and death. Once the cancer recurs treatment options are limited. Low-volume, resectable disease may be treated surgically; however, the majority of patients even with localized disease have unresectable disease, or are medically not fit for surgical resection [2]. Doublet chemotherapy with cetuximab results in a median survival of 10 months but is palliative in intent [3]. Several phase II trials suggest that multimodality treatment including reirradiation can achieve long-term survival (>3 years). Typically a subset of patients with confined locoregional recurrence can achieve long-term survival rates of 10%–40% [4, 5]. Nevertheless, radiation resistance remains a major obstacle. Little progress has been made in the past decade, as various drugs have been unable to provide incremental benefit. Novel approaches to enhance tumor cell kill and overcome treatment resistance are of great interest.
Our group at the University of Chicago completed several clinical chemotherapy-reirradiation trials using the FHX platform, consisting of infusional 5-fluorouracil (5-FU), hydroxyurea (H), and concomitant radiation (X), to which a novel drug could be added. Multiple trials from our group [2, 6–12], the Institut Gustave-Roussy, the University of Alabama-Birmingh am, and the Radiation Therapy Oncology Group clearly show the feasibility and safety of reirradiation. Nevertheless, this approach remains limited to the clinical trial setting [4, 13–16]. One randomized trial investigating FHX reirradiation in comparison to observation following salvage surgery indicated improved disease-free survival but not overall survival (OS) [17]. Analysis of data from subjects treated on reirradiation protocols determined that the use of surgery, multiagent chemotherapy, and higher radiation doses were independent prognostic factors for survival [2].
TNFerade™ Biologic (Ad GV.EGR.TNF.11D) is a second generation replication-defective adenoviral vector that carries a human TNF-α gene linked to a radiation-inducible promoter (EGR-1) [18–22]. TNF-α is a known chemotherapy and radiation sensitizer [19, 21]. A systemic administration of TNF-α is associated with severe toxic effects, whereas local administration is well tolerated with few systemic side–effects [19, 21]. TNFerade™ Biologic is injected into the tumor followed by radiation with the goal of induction of regional TNF-α production and radiation enhancement without associated systemic side-effects [18, 22–25] as well as immune system activation [26, 27]. For example in this context, using Ad GV.EGR.TNF.11D Meng et al. demonstrated extensive molecular and host changes induced by small amounts of TNF-α [28], which resulted in tumor-specific T-cell immunity and suppression of lymph node metastasis.
In this phase I trial, we combined chemoradiation using the FHX chemoradiotherapy platform [29–31] with TNFerade™ Biologic administration in a population of poor prognosis HNC patients with regionally recurrent, previously radiated disease including patients with larger, unresectable tumors, and patients with limited distant metastatic disease, who had dominant local symptoms.
The postulated benefit of this approach was that TNF-α production would be induced locally by administration of radiotherapy and tumors thus exposed to radiation in the presence of two different radiosensitizers, chemotherapy and TNF-α.
patients and methods
patient population and inclusion criteria
Patients were aged ≥18 years with previously irradiated, unresectable, recurrent HNC (squamous cell carcinomas or other HNC histologies). Patients with distant metastases of low volume with a need for locoregional palliation were also eligible. Prior radiotherapy had to be completed ≥4 months and/or chemotherapy ≥1 month ago and patients had to have recovered from previous side-effects to grade ≤1. Patients needed to have tumor amenable to injection as assessed by an otolaryngologist. Expected life expectancy of >12 weeks, Eastern Cooperative Oncology Group (ECOG) ≤1, and normal organ function were required. Patients with significant infection, immune deficiency, involvement of major vessels, and history of a bleeding diathesis/thrombosis were excluded. The institutional review board approved the protocol and consent was obtained from all patients.
treatment and trial design
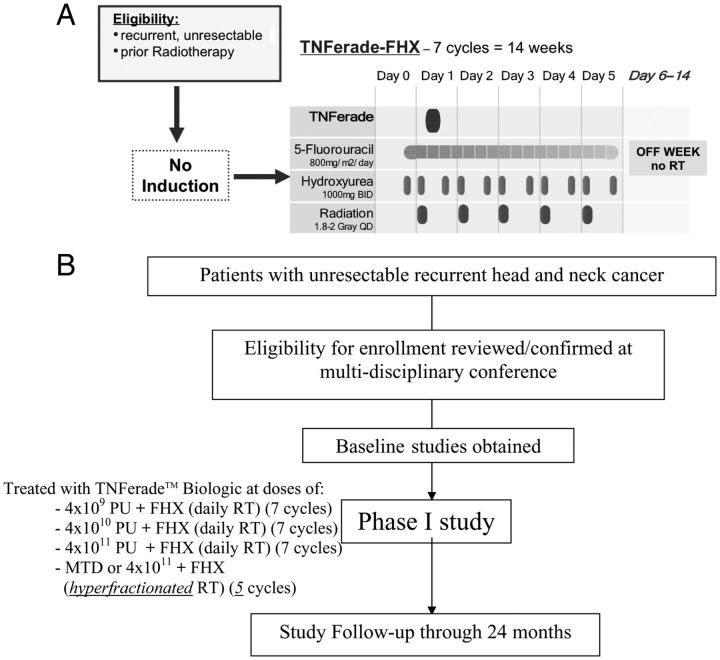
Three dose levels of TNFerade™ Biologic 4 × 109, 4 × 1010, and 4 × 1011 PU concomitant with once-daily radiation and chemotherapy were to be evaluated and the maximum tolerated dose (MTD) defined. If no MTD was reached at 4 × 1011 PU, this dose would be evaluated with hyperfractionated radiation.
A modified ‘3 + 3’ dose escalation (‘Design A’ by Storer) [32] was used. If none of the first three patients experienced a dose-limiting toxicity (DLT) by the end of treatment, accrual to the next dose level was started. If one of the first three patients experienced DLT, three additional patients were enrolled; the dose level was advanced after all six patients completed treatment if ≤1 DLT was observed. If ≥2 of the first three patients or ≥2 of six patients experienced DLT, the phase I portion stopped and the previous dose level was recommended as MTD (provided at least six patients had been treated at that dose level).
National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) 3.0 were employed. DLTs were defined as grade ≥3 toxicities considered to be at least possibly related to TNFerade™ Biologic seen during cycles one or two. Typical FHX chemoradiation-related toxicity (mucositis) documented in prior experience [33] was not considered dose limiting. Based on the prior observation of thromboembolic events [20, 23], patients were closely monitored for thrombotic events and grade ≥3 events counted as DLT. The MTD was defined as the highest dose levels in which the observed rate of DLT was <33% (≤1 of six patients).
The treatment schema, dose levels, and administration schedule are shown in Figure 1. Patients received treatment during seven 2-week cycles. One treatment cycle consisted of a 5-day treatment period followed by 9 days of recovery (‘week-on/week-off’ schedule).
Figure 1.
(A) Trial schema, (B) trial flow chart. TNFerade™ Biologic dose levels were (i) 4 × 109, (ii) 4 × 1010, and (iii) 4 × 1011 PU. Tumor biopsies: pretreatment, cycle 2 (2 days after injection), and after treatment.
All patients underwent CT-based radiotherapy planning using either 3D conformal or intensity modulated radiation treatment. Radiation was given on days 1–5 per cycle, in 1.8–2 Gy (daily RT) fractions, up to a total dose of 63–70 Gy (6 to 7 cycles). Radiation doses to gross disease were 63–70 Gy, 33–50 Gy to the supraclavicular fossa for high-risk microscopic disease, and boosted with a field reduction to 66–70 Gy for gross macroscopic disease. Doses to the posterior neck were 45–60 Gy and 66–70 Gy for gross macroscopic disease. The anterior neck doses were 50–60 Gy. Doses to the spinal cord were limited to <45 Gy
Hydroxyurea was given at 1000 mg by mouth every 12 h for 11 doses and a 5-day continuous infusion of 5-FU was given at 800 mg/m2/day.
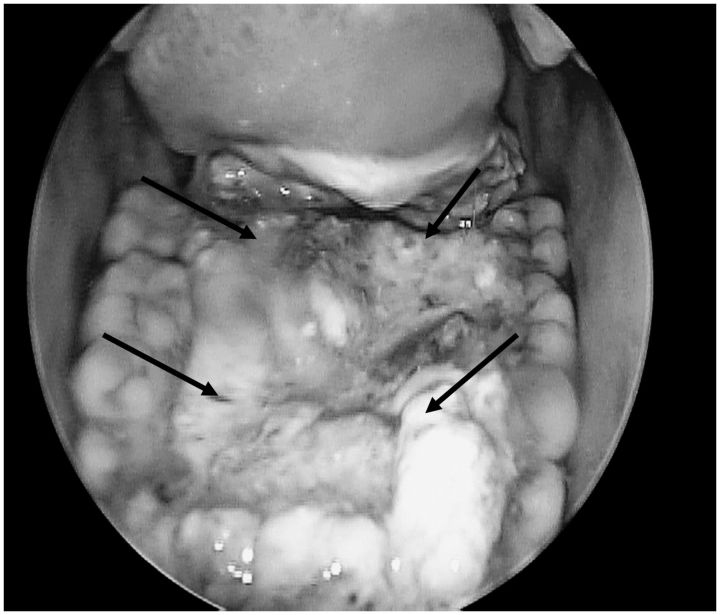
TNFerade™ Biologic was administered on day 1 or 2 of each cycle by the surgeon via direct, ultrasound, and/or endoscopic visualization. Up to four lesions within the radiation field could be injected; and with each subsequent injection, differing areas of the tumor were targeted (see example of TNFerade™ Biologic administration in Figure 2).
Figure 2.
TNFerade™ Biologic administration procedure (example). TNFerade™ Biologic is given in up to four divided doses into different quadrants or sites of the tumor. The site and depth of the injection are recorded at each treatment. Different sites and depths are injected during the course of treatment to increase the delivery of the TNFerade™ into the entire volume of the tumor. Multiple passes through the same injection site were carried out to cover a larger volume.
assessments
Antitumor efficacy was assessed beginning 4–6 weeks after completion of therapy using radiographic response (CT scan), clinical response (clinical exam), and pathologic response (biopsy), if required.
In addition to the pretreatment biopsy, a repeat tumor biopsy from TNFerade™ Biologic-treated sites was carried out during the second cycle of treatment and ≥4 weeks after completion of treatment for TNFerade™ Biologic correlative studies.
Before treatment, all patients underwent dental consultation, speech-swallow evaluation, and triple endoscopy.
objectives
The primary objectives were to determine the recommended phase II dose and DLT. The secondary end-points were survival and response rate. After completion of chemoradiotherapy, imaging was analyzed based on the modified RECIST criteria (no confirmatory scans) [34].
OS was defined as the time from the date of first study treatment until death from any cause. OS was calculated using the Kaplan–Meier estimator. The cause of death was determined to be due to disease or other causes, and the cause-specific cumulative incidence estimates were used to assess the probability of failure due to each cause [35].
correlatives
Immunohistochemistry (IHC) for the following markers was completed using a standard IHC methodology: adenovirus receptors [CXADR: coxsackie virus and adenovirus receptor (Santa Cruz H-300), MSR1: macrophage scavenger receptor (Abcam AB93290)], TNF-α (Abcam AB78289), TNFRSF1A (TNF Receptor 1) (Santa Cruz H-271), and TNFRSF1B (TNF receptor 2) (Santa Cruz L-20).
TNFerade™ Biologic specific vector DNA was detected by qPCR (see Supplementary Methods, available at Annals of Oncology online) from tumor tissues. We measured the expression of adenovirus receptors (CXADR: coxsackie virus and adenovirus receptor), ITGAV: integrin-α V beta (transcript variants 1 and 3, and transcript variant 2), MSR1 [macrophage scavenger receptor (transcript variants 1 and 2)], as well as TNF-α (tumor necrosis factor alpha), TNFRSF1A (TNF receptor 1), TNFRSF1B (TNF receptor 2), and the house-keeping genes TBP (TATA box binding protein), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
results
patient characteristics
Between 1 January 2007 and 9 September 2009, 14 patients were enrolled. Patient baseline characteristics are reported in Table 1. Three patients (21%) had distant metastatic disease [lung (N = 3), liver (N = 1)] with dominant locoregional, macroscopic disease (amenable to injection). Thirteen patients had HNSCC, one patient had a radiation-induced sarcoma of the head and neck area. Patients were in need of palliation for dominant local symptoms and no effective standard of care treatment options were available. All patients had received prior radiation and the median previous radiation dose was 69.4 Gy (range 60–76 Gy).
Table 1.
Patient and tumor characteristics
| All patients N = 14 (%) | |
|---|---|
| Gender | |
| Female | 5 (35.7) |
| Male | 9 (64.3) |
| Age | |
| Mean | 59.1 |
| Range | 36–71 |
| ECOG performance status | |
| 0 | 2 (14.3) |
| 1 | 10 (71.4) |
| 2 | 2 (14.3) |
| Distant metastasis | |
| M1 | 3 (21.4) |
| Treatment history | |
| Prior radiotherapy to HN | 14 (100) |
| Histologic subtype | |
| Squamous cell carcinoma | 13 (92.9) |
| Head and neck sarcoma | 1 (7.1) |
| Tumor sites | |
| Oropharynx | 3 (21.4) |
| Base of tongue | 2 (14.3) |
| Tonsil | 1 (7.1) |
| Oral cavity | 9 (64.3) |
| Oral tongue | 4 (28.6) |
| Buccal mucosa | 1 (7.1) |
| Floor of mouth | 3 (21.4) |
| Alveolar ridge | 1 (2.3) |
| Larynx/piriform sinus | 2 (14.3) |
| Tobacco use | |
| Current or prior | 13 (92.9) |
| Alcohol use | |
| Current or prior | 7 (50) |
adverse events
Severe adverse events (SAE) are listed in Table 2A. At dose level 1, a sinus and jugular vein thrombosis was seen in one patient and the cohort was expanded to six patients. No additional DLTs were identified in dose levels 1 and 2. At dose level 3, one, then two additional thrombotic events in the expansion cohort occurred (two grade 3 deep vein thromboses and one grade 4 pulmonary embolism). These were assessed as dose limiting and dose level 2 (4 × 1010 PU) was identified as recommended phase II dose. All thrombotic events were managed with anticoagulation without further complications.
Table 2.
(A) Severe adverse events (SAEs) that were deemed to be at least possibly TNFerade treatment related (Grades 3–5). (B) Severe toxicities deemed unrelated to TNFerade (related to chemoradiation or unrelated complications)
| (A) | |||
| Dose level 1: N = 6 (4 × 109) | Grade 3 | Grade 4 | Grade 5 |
| Thrombosis | 1 | 0 | 0 |
| Dose level 2: N = 3 (4 × 1010) | |||
| none | |||
| Dose level 3: N = 5 (4 × 1011) | |||
| Thrombosis | 2 | 0 | 0 |
| Pulmonary Embolism | 0 | 1 | 0 |
| Thrombocytopenia | 0 | 1 | 0 |
| (B) | |||
| Dose level 1: N = 6 (4 × 109) | |||
| Hyponatremia | 0 | 1 | 0 |
| Pneumothorax (spontaneous) | 0 | 1 | 0 |
| Constipation | 2 | 0 | 0 |
| Altered mental status | 1 | 0 | 0 |
| Hypoxic encephalopathy | 0 | 0 | 1 |
| Fever | 1 | 0 | 0 |
| Cellulitis | 1 | 0 | 0 |
| Respiratory alkalosis | 0 | 1 | 0 |
| Pneumonia | 5 | 0 | 0 |
| Mucositis | 5 | 0 | 0 |
| Dermatitis | 3 | 0 | 0 |
| Anemia | 2 | 0 | 0 |
| Hand foot syndrome | 1 | 0 | 0 |
| Neutropenia | 1 | 0 | 0 |
| Neuropathy | 1 | 0 | 0 |
| Low glucose | 1 | 0 | 0 |
| Elevated glucose | 1 | 0 | 0 |
| Sentinal bleed | 0 | 1 | 0 |
| Dose level 2: N = 3 (4 × 1010) | |||
| Bacteremia | 1 | 0 | 0 |
| Abscess | 1 | 0 | 0 |
| Pneumonia | 1 | 0 | 0 |
| Elevated liver enzymes | 3 | 0 | 0 |
| Mucositis | 3 | 0 | 0 |
| Dermatitis | 1 | 0 | 0 |
| Anemia | 0 | 0 | 0 |
| Hand foot syndrome | 1 | 0 | 0 |
| Neutropenia | 0 | 1 | 0 |
| Neuropathy | 1 | 0 | 0 |
| Elevated glucose | 1 | 0 | 0 |
| Dose level 3: N = 5 (4 × 1011) | |||
| Neutropenia | 0 | 1 | 0 |
| Pneumonia | 2 | 0 | 0 |
| Hypoxia | 1 | 0 | 0 |
| Acute renal failure | 1 | 0 | 0 |
| Cellulitis | 1 | 0 | 0 |
| Hyperkalemia | 1 | 0 | 0 |
| Fever | 3 | 0 | 0 |
| Altered mental status | 1 | 0 | 0 |
| Thrombocytopenia | 1 | 0 | 0 |
| Mucositis | 3 | 0 | 0 |
| Dermatitis | 1 | 0 | 0 |
| Hand foot syndrome | 1 | 0 | 0 |
| Cough | 1 | 0 | 0 |
TNFerade™ Biologic-unrelated grade ≥3 toxicities are listed in Table 2B and are consistent with the side-effect profile of FHX-based reirradiation therapy [32]. Transient pain (grade 1/2) at the injection site was common. One sentinel bleed event occurred from tumor invasion into the carotid artery during treatment and was controlled by carotid artery stenting (Table 2B, cohort 1).
Three patients had a declining performance status and declined to complete the entire course of therapy. Their respective radiation doses were (54 Gy, 60 Gy, and 38 Gy). Three patients had chemotherapy dose reductions for chemotherapy-related neutropenia and growth factor support was used.
survival and treatment response
The overall locoregional response rate was 83.3%: five patients achieved a complete response (CR), five patients a partial response (PR), two patients had stable disease (SD), and two patients were not assessable after TNFerade™ Biologic based reirradiation (Table 3).
Table 3.
Overall responses at the end of treatment
| Dose level 1 | Dose level 2 | Dose level 3 | All patients | |
|---|---|---|---|---|
| Assessable patientsa | N = 6 | N = 3 | N = 3 | N = 12 |
| CR | 3c | 1 | 1 | 5 |
| PR | 3 | 1 | 1 | 5 |
| SD | 0 | 1 | 1 | 2 |
| PD | 1c | 1d | 1d | 3 |
| Response rate | 100% | 66% | 66% | 83.3% |
| Unassessable# | N = 0 | N = 0 | N = 2 | N = 2 |
| Surgical alvageb | 1 | 1 | 0 | 0 |
| No evaluationc | 0 | 0 | 0 | 0 |
The overall response rate was 83.3%.
Response assessment is based on the pathologic confirmation or if not available imaging and clinical evaluation using the modified RECIST (no confirmatory scans).
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
aPatients with quantifiable disease after treatment
bPatients underwent surgery after chemoradiotherapy
cCR within the field, but PD for known distant metastatic disease
dSD within the field, but PD outside of the field
The response assessment is based on the modified RECIST criteria (no confirmatory scans) or where available––pathologic confirmation.
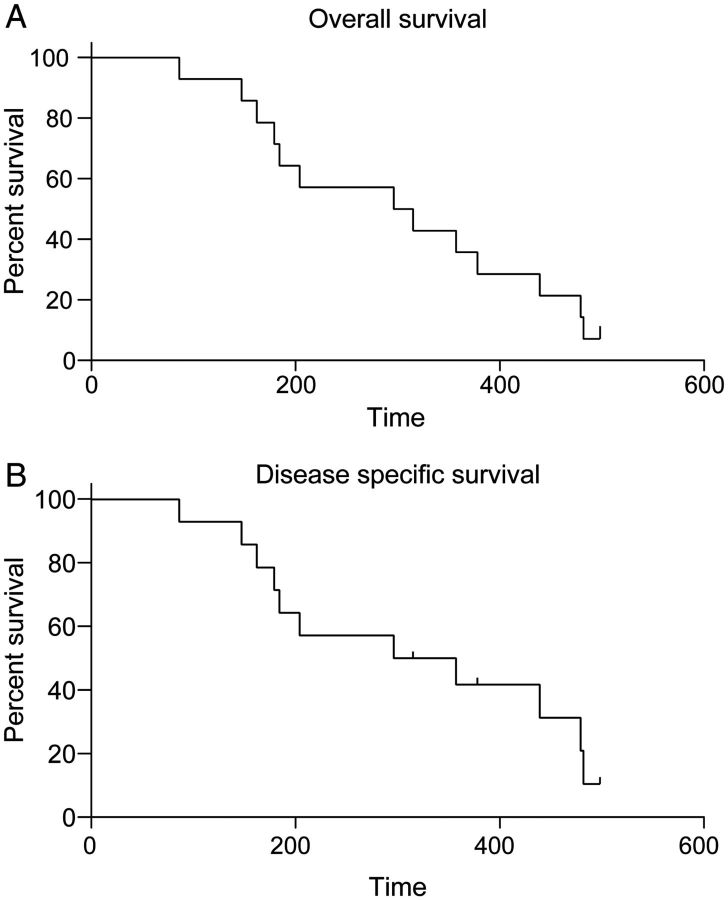
The median OS was 9.6months (Figure 3A). The 1- and 2-year survival rates were 35.7% and 7.1%, respectively. The disease-specific median survival was also 9.6 months (Figure 3B).
Figure 3.
Survival. (A) Overall survival (OS) and (B) disease-specific survival. Time is provided in days. The median OS was 9.6 months.
One patient (7.1%) remained alive and cancer free 3.5 years after completion of therapy. Eleven patients died from or with active cancer (78%), and two patients died from complications/comorbidities after therapy. The three patients with distant metastatic disease all progressed distantly, and two patients received palliative chemotherapy. Patients died 2 months, 1 month, and 13 months after completion of chemoradiotherapy.
Two patients died from causes deemed unrelated to HNC (cancer-free based on the most recent exam/imaging) or cancer therapy: one patient was found dead 6 months after completion of therapy (cause of death unknown). One patient died from aspiration pneumonia 9 months after completion of therapy.
correlative laboratory studies
TNFerade™ Biologic relevant adenovirus receptors [Coxsackie virus and adenovirus receptor (CXADR), integrin α V beta (ITGAV), and macrophage scavenger receptor variants 1 and 2 (MSR1)] were present in tumor biopsies of all patients (based on DNA and/or protein expression, supplementary Tables S1–S3, available at Annals of Oncology online). Also TNF-α and TNF receptors 1 and 2 (TNF, TNFRSF1A/B) were detected in the majority of samples. The housekeeping genes TBP and GAPDH, which were used as positive controls, were positive in all samples.
The results of PCR-based detection from OCT frozen and paraffin-embedded tissues, and IHC results were consistent among patients. There was a trend towards lower detection rates in the samples obtained during treatment and after treatment (compared with pretreatment biopsies).
Of note, TNFerade™ Biologic-derived TNF-α DNA was detected in 3 samples from one patient at two different time points (during treatment and one month post treatment).
discussion
Gene therapy approaches provide the potential opportunity to change the biology of cancer and/or the patient's immune system by intratumoral insertion of custom designed genetic material. The occurrence of unexpected side-effects [36], as well as the challenge of vector delivery to the cancer locoregionally and systemically, has slowed progress. Nevertheless, recently a gene therapy approach achieved apparent eradication of chronic lymphocytic leukemia cells in patients who were otherwise refractory to therapy [37], and gene therapy has also been able to cure immunodeficiency syndromes as well as hemophilia [38–43].
TNFerade™ Biologic is a replication-deficient adenoviral vector expressing human TNF-α under the control of the chemoradiation-inducible EGR-1 promoter intended to lead to intratumoral TNF-α production when the tumor is radiated [18–22]. In this phase I trial, we determined the recommended phase II dose of TNFerade™ Biologic in combination with chemoradiation in patients with poor prognosis, previously radiated HNC. Preclinical evidence suggests that TNF-α is a potent radiosensitizer that may overcome radioresistance and lead to tumor necrosis with tumor blood vessel thrombosis [19–25, 44, 45].
Our results demonstrate that TNFerade™ Biologic can be integrated at a dose of 4 × 1010 PU every 2 weeks with FHX chemoradiotherapy. The DLT in this study was thromboembolism with 4 out of 14 patients developing either deep vein thrombosis or pulmonary embolism (28.6%). Given earlier reports of fatal pulmonary embolism in a cohort of esophageal cancer patients treated with TNFerade™ Biologic thrombosis was considered a potential DLT and possibly related to TNFerade™ Biologic despite very low systemic exposure to the agent. HNC patients frequently experience thromboembolic events and a rate of up to 30% is commonly reported [33]. Further investigation in a randomized study will be required to determine the significance of thromboembolic events in this setting. Overall, TNFerade™ Biologic administration was well tolerated with mild-to-moderate, transient injection site pain. Overall treatment was well tolerated and there were no synergistic side-effects with FHX chemoradiotherapy. No typical TNF-α related side-effects were observed, suggesting that no clinically significant systemic leakage of TNF-α into the bloodstream occurred. The rate of severe side-effects whether related or unrelated to therapy appears similar to observations in other studies in this high-risk population [2, 5–8, 10–13, 32].
This phase I study was not designed for efficacy assessment. The observed response rate of 83.1% is encouraging in this poor prognosis patient population, although OS continues to be unsatisfactory. The observed activity would support an additional, preferably randomized phase II study to assess the efficacy in a better prognosis group of patients.
Preclinical radiobiology experiments lend strong support to the addition of TNF-α to radiation. The results of these studies suggest the ability to overcome radioresistance, arguably the most significant survival limiting factor in the treatment of previously radiated, recurrent HNC patients [19–25, 44–47]. It should be noted that TNF-α has also been associated with pro-inflammatory and growth-promoting/cancerogenic effects, albeit chronic TNF-α exposure is usually required [48]. Given the locoregional delivery of TNF-α in this study, limitations of the samples we obtained, and the observed strong antitumor activity in a setting where radioresistance is a major barrier to good clinical outcome, we did not investigate the broader effects of TNF-α further. Nevertheless, additional systematic studies are indicated to further clarify the effect of TNF-α on the tumor microenvironment.
One potential challenge in locoregional therapy is the delivery of the agent to sufficient/all tumor-involved areas. Inter-patient and inter-operator differences are common, and standardization and operator training are essential. In this context, further refinement of the therapeutic strategy and potential administration methods of TNFerade™ Biologic for HNC should be determined before use in a larger phase II study.
TNF-α complementary DNA encoded by TNFerade™ Biologic contains a unique 3′UTR polymorphism that distinguishes it from endogenous, host-derived TNF-α DNA. This polymorphism is detectable by PCR and permits the qualitative/quantitative assessment of TNFerade™ Biologic in post-treatment biopsy specimens. A preclinical assessment of TNFerade™ Biologic-derived TNF-α was detected in A549 (lung), PC3 (prostate), and U87 (glioblastoma) hind-limb xenograft tumors in athymic nude mice when injected with 2.2 × 109 PU of AD.EGR.TNF and irradiated with 2 Gy for 5 days (data not shown).
In this study, TNFerade™ Biologic-specific DNA was detected in three samples derived from one patient, obtained at different time points. This includes a specimen obtained 4 weeks after treatment indicating that adenoviral TNFerade™ Biologic-specific DNA can persist for a prolonged period. Whether the radiation-inducible EGR-1 promoter remains active at this time point, and could be re-employed is unclear. We were unable to detect TNFerade™ Biologic-derived TNF-α in additional samples: there are several possible explanations for this. Based on preclinical modeling in animals we know that needle biopsies have low detection sensitivity due to variation in the area of injection versus sampling. In addition, the occurrence of necrotic tissue in general can lead to rapid TNF-α degradation. Indeed, IHC results show evidence of necrosis in multiple samples. Finally, efficient eradication of TNFerade™ Biologic by the patients' immune system might occur via endogenous anti-adenoviral antibodies. No pre-or post-observational assessment of anti-viral antibody titers was done in this study. It is possible that induction of TNF-α production was simply not achieved or determined at the threshold level of detection of the assays employed herein. The presence of adenovirus receptors in HNC is an important factor to be determined in the clinical efficacy of adenoviral vectors in the treatment of head and neck tumors and should be investigated as an independent risk factor. The detection of TNF-α and respective TNF receptors supports a clinically significant role for TNF-α in this disease setting/therapeutic approach.
In conclusion, we have completed a phase I study combining TNFerade™ Biologic with FHX-based chemoradiotherapy. The recommended phase II dose for TNFerade™ Biologic is 4 × 1010 PU. Monitoring for thromboembolic events is warranted based on the DLT observed, although routine management appeared to be sufficient in this study. Optimization of the route of administration as well as consistent TNFerade™ Biologic-derived TNF-α detection will be important in the design of future studies with TNFerade™ Biologic.
funding
This study was supported by the NCI R01 CA113662 grant (RRW), an ASCO Translational Professorship (EEV), The Francis L. Lederer Foundation (EEV), as well as GenVec, Inc. Gaithersburg, MD.
disclosure
RRW is a stockholder and consultant to GenVec, Inc. All other authors have declared no conflicts of interest.
acknowledgements
We would like to acknowledge the Gleason family, for their kind support of Head and Neck Cancer Research.
references
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Salama JK, Vokes EE, Chmura SJ, et al. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2006;64(2):382–391. doi: 10.1016/j.ijrobp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 4.Spencer SA, Harris J, Wheeler RH, et al. RTOG 96–10: reirradiation with concurrent hydroxyurea and 5-fluorouracil in patients with squamous cell cancer of the head and neck. Int J Radiat Oncol Biol Phys. 2001;51(5):1299–1304. doi: 10.1016/s0360-3016(01)01745-x. [DOI] [PubMed] [Google Scholar]
- 5.Stevens KR, Jr, Britsch A, Moss WT. High-dose reirradiation of head and neck cancer with curative intent. Int J Radiat Oncol Biol Phys. 1994;29(4):687–698. doi: 10.1016/0360-3016(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 6.Vokes EE, Panje WR, Schilsky RL, et al. Hydroxyurea, fluorouracil, and concomitant radiotherapy in poor-prognosis head and neck cancer: a phase I-II study. J Clin Oncol. 1989;7(6):761–768. doi: 10.1200/JCO.1989.7.6.761. [DOI] [PubMed] [Google Scholar]
- 7.Brockstein B, Haraf DJ, Stenson K, et al. A phase I–II study of concomitant chemoradiotherapy with paclitaxel (one-hour infusion), 5-fluorouracil and hydroxyurea with granulocyte colony stimulating factor support for patients with poor prognosis head and neck cancer. Ann Oncol. 2000;11(6):721–728. doi: 10.1023/a:1008324131519. [DOI] [PubMed] [Google Scholar]
- 8.Brockstein B, Haraf DJ, Stenson K, et al. Phase I study of concomitant chemoradiotherapy with paclitaxel, fluorouracil, and hydroxyurea with granulocyte colony-stimulating factor support for patients with poor-prognosis cancer of the head and neck. J Clin Oncol. 1998;16(2):735–744. doi: 10.1200/JCO.1998.16.2.735. [DOI] [PubMed] [Google Scholar]
- 9.Vokes EE, Haraf DJ, Mick R, et al. Intensified concomitant chemoradiotherapy with and without filgrastim for poor-prognosis head and neck cancer. J Clin Oncol. 1994;12(11):2351–2359. doi: 10.1200/JCO.1994.12.11.2351. [DOI] [PubMed] [Google Scholar]
- 10.Milano MT, Vokes EE, Salama JK, et al. Twice-daily reirradiation for recurrent and second primary head-and-neck cancer with gemcitabine, paclitaxel, and 5-fluorouracil chemotherapy. Int J Radiat Oncol Biol Phys. 2005;61(4):1096–1106. doi: 10.1016/j.ijrobp.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Haraf DJ, Weichselbaum RR, Vokes EE. Reirradiation with concomitant chemotherapy of unresectable recurrent head and neck cancer: a potentially curable disease. Ann Oncol. 1996;7(9):913–918. doi: 10.1093/oxfordjournals.annonc.a010793. [DOI] [PubMed] [Google Scholar]
- 12.Salama JK, Haraf DJ, Stenson K, et al. Phase I study of concomitant chemoradiotherapy with irinotecan, 5-FU, and hydroxyurea for patients with advanced and/or recurrent head and neck cancer. Cancer J. 2005;11(2):140–146. doi: 10.1097/00130404-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 13.De Crevoisier R, Bourhis J, Domenge C, et al. Full-dose reirradiation for unresectable head and neck carcinoma: experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol. 1998;16(11):3556–3562. doi: 10.1200/JCO.1998.16.11.3556. [DOI] [PubMed] [Google Scholar]
- 14.De Crevoisier R, Domenge C, Wibault P, et al. Full dose reirradiation combined with chemotherapy after salvage surgery in head and neck carcinoma. Cancer. 2001;91(11):2071–2076. doi: 10.1002/1097-0142(20010601)91:11<2071::aid-cncr1234>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Spencer SA, Wheeler RH, Peters GE, et al. Concomitant chemotherapy and reirradiation as management for recurrent cancer of the head and neck. Am J Clin Oncol. 1999;22(1):1–5. doi: 10.1097/00000421-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of radiation therapy oncology group protocol 9911. J Clin Oncol. 2007;25(30):4800–4805. doi: 10.1200/JCO.2006.07.9194. [DOI] [PubMed] [Google Scholar]
- 17.Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008;26(34):5518–23. doi: 10.1200/JCO.2007.15.0102. [DOI] [PubMed] [Google Scholar]
- 18.Hallahan DE, Mauceri HJ, Seung LP, et al. Spatial and temporal control of gene therapy using ionizing radiation. Nat Med. 1995;1(8):786–791. doi: 10.1038/nm0895-786. [DOI] [PubMed] [Google Scholar]
- 19.Mauceri HJ, Beckett MA, Liang H, et al. Translational strategies exploiting TNF-alpha that sensitize tumors to radiation therapy. Cancer Gene Ther. 2009;16(4):373–381. doi: 10.1038/cgt.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLoughlin JM, McCarty TM, Cunningham C, et al. TNFerade, an adenovector carrying the transgene for human tumor necrosis factor alpha, for patients with advanced solid tumors: surgical experience and long-term follow-up. Ann Surg Oncol. 2005;12(10):825–830. doi: 10.1245/ASO.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Kufe D, Weichselbaum R. Radiation therapy: activation for gene transcription and the development of genetic radiotherapy-therapeutic strategies in oncology. Cancer Biol Ther. 2003;2(4):326–329. doi: 10.4161/cbt.2.4.495. [DOI] [PubMed] [Google Scholar]
- 22.Weichselbaum RR, Kufe D. Translation of the radio- and chemo-inducible TNFerade vector to the treatment of human cancers. Cancer Gene Ther. 2009;16(8):609–619. doi: 10.1038/cgt.2009.37. [DOI] [PubMed] [Google Scholar]
- 23.Mundt AJ, Vijayakumar S, Nemunaitis J, et al. A Phase I trial of TNFerade biologic in patients with soft tissue sarcoma in the extremities. Clin Cancer Res. 2004;10(17):5747–5753. doi: 10.1158/1078-0432.CCR-04-0296. [DOI] [PubMed] [Google Scholar]
- 24.Senzer N, Mani S, Rosemurgy A, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. J Clin Oncol. 2004;22(4):592–601. doi: 10.1200/JCO.2004.01.227. [DOI] [PubMed] [Google Scholar]
- 25.Sharma A, Mani S, Hanna N, et al. Clinical protocol. An open-label, phase I, dose-escalation study of tumor necrosis factor-alpha (TNFerade Biologic) gene transfer with radiation therapy for locally advanced, recurrent, or metastatic solid tumors. Hum Gene Ther. 2001;12(9):1109–1131. doi: 10.1089/104303401750214320. [DOI] [PubMed] [Google Scholar]
- 26.Calzascia T, Pellegrini M, Hall H, et al. TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice. J Clin Invest. 2007;117(12):3833–3845. doi: 10.1172/JCI32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slattery ML, Lundgreen A, Bondurant KL, et al. Tumor necrosis factor-related genes and colon and rectal cancer. Int J Mol Epidemiol Genet. 2011;2(4):328–338. [PMC free article] [PubMed] [Google Scholar]
- 28.Meng Y, Mauceri HJ, Khodarev NN, et al. Ad.Egr-TNF and local ionizing radiation suppress metastases by interferon-beta-dependent activation of antigen-specific CD8+ T cells. Mol Ther. 2010;18(5):912–920. doi: 10.1038/mt.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vokes EE, Beckett M, Karrison T, et al. The interaction of 5-fluorouracil, hydroxyurea, and radiation in two human head and neck cancer cell lines. Oncology. 1992;49(6):454–460. doi: 10.1159/000227092. [DOI] [PubMed] [Google Scholar]
- 30.Vokes EE, Weichselbaum RR, Lippman SM, et al. Head and neck cancer. N Engl J Med. 1993;328(3):184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 31.Vokes EE, Weichselbaum RR. Concomitant chemoradiotherapy: rationale and clinical experience in patients with solid tumors. J Clin Oncol. 1990;8(5):911–934. doi: 10.1200/JCO.1990.8.5.911. [DOI] [PubMed] [Google Scholar]
- 32.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45(3):925–937. [PubMed] [Google Scholar]
- 33.Seiwert TY, Haraf DJ, Cohen EEW, et al. Phase I study of bevacizumab added to fluorouracil- and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. J Clin Oncol. 2008;26(10):1732–1741. doi: 10.1200/JCO.2007.13.1706. [DOI] [PubMed] [Google Scholar]
- 34.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 35.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 36.Jenks S. Gene therapy death––‘everyone has to share in the guilt. J Natl Cancer Inst. 2000;92(2):98–100. doi: 10.1093/jnci/92.2.98. [DOI] [PubMed] [Google Scholar]
- 37.2011. Gene therapy shown to destroy leukemia tumors, Reuters http://www.reuters.com/article/2011/08/10/us-leukemia-genetherapy-idUSTRE7795NT20110810. (10 October 2012, date last accessed).
- 38.Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 39.Hacein-Bey-Abina S, Hauer J, Lim A, et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363(4):355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathwani AC, Tuddenham EGD, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaspar HB, Cooray S, Gilmour KC, et al. Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci Transl Med. 2011;3(97):97ra80. doi: 10.1126/scitranslmed.3002716. [DOI] [PubMed] [Google Scholar]
- 42.Gaspar HB, Cooray S, Gilmour KC, et al. Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci Transl Med. 2011;3(97):97ra79. doi: 10.1126/scitranslmed.3002715. [DOI] [PubMed] [Google Scholar]
- 43.Shaw KL, Kohn DB. A tale of two SCIDs. Sci Transl Med. 2011;3(97):97ps36. doi: 10.1126/scitranslmed.3002594. [DOI] [PubMed] [Google Scholar]
- 44.Mezhir JJ, Smith KD, Posner MC, et al. Ionizing radiation: a genetic switch for cancer therapy. Cancer Gene Ther. 2006;13(1):1–6. doi: 10.1038/sj.cgt.7700879. [DOI] [PubMed] [Google Scholar]
- 45.Murugesan SR, King CR, Osborn R, et al. Combination of human tumor necrosis factor-alpha (hTNF-alpha) gene delivery with gemcitabine is effective in models of pancreatic cancer. Cancer Gene Ther. 2009;16(11):841–847. doi: 10.1038/cgt.2009.32. [DOI] [PubMed] [Google Scholar]
- 46.Hallahan DE, Beckett MA, Kufe D, et al. The interaction between recombinant human tumor necrosis factor and radiation in 13 human tumor cell lines. Int J Radiat Oncol Biol Phys. 1990;19:69–74. doi: 10.1016/0360-3016(90)90136-8. [DOI] [PubMed] [Google Scholar]
- 47.Meng Y, Beckett MA, Liang H, et al. Blockade of tumor necrosis factor signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010;70:1534–1543. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]