Abstract
The usefulness of oral naltrexone has been limited by compliance. Sub-cutaneous implants would seem to offer a solution to this problem and improve long-term outcomes. The aim of the present study was to compare levels of blood serum naltrexone of patients who had received a naltrexone implant after detoxification to a number of dependent variables of interest. These dependent variables included drug use including urine screens of each patient, any adverse response to the implant, subjective evaluation of self-esteem, quality of relationships, and changes in social functioning. Sixty six patients received an implant and were surveyed; urine and blood samples were taken at about 1, 3, and 6 months after implantation. Naltrexone levels were on average above 1 ng/mL at 6 months after insertion and patients showed significant improvements on all dependent variables. The preliminary evidence indicates that implants can improve compliance rates and outcomes.
Keywords: naltrexone, implant, social-support, compliance, and opiate addiction
Introduction
Naltrexone, a potent opiate antagonist, has been shown to have valuable properties for the treatment of addiction to opiates such as heroin and methadone. The most important property is its ability to completely block the effects of heroin and methadone when taken orally at recommended doses,1 making relapse to regular opiate use almost impossible during the period of compliance. Research has shown that a dose of 50–100 mg of oral naltrexone provides effective protection against heroin for 2–3 days, and with chronic dosing, no accumulation of naltrexone or its metabolites have been observed.2 Naltrexone is claimed to be non-toxic.2,3 However, the manufacturers warn against use of the medication among patients who have renal impairment and state that it is contraindicated in patients who have acute Hepatitis C or liver failure as doses at five times the recommended dose of 50 mg/day over five to eight weeks may cause elevations in liver enzyme levels. Further, caution should be exercised when taking other medication and non-prescribed drugs and when the patient is pregnant or lactating.4 Contrary to these warnings, recent studies have indicated that naltrexone does not cause hepatotoxicity or exacerbate pre-existing serious liver disease and there are no indications of naltrexone interacting harmfully with other medications5,6 and produces no clinically important side-effects, including dysphoria and depression.2,3,7–9 Before the introduction of implants, the main factor restricting naltrexones’ widespread use in opiate dependency treatment was non-compliance rates.10–14
The ability to resist and ignore drug-misusing cues is not easy. Indeed 50% of clients who left a 3-week in-patient opiate detoxification program had misused opiates within several days.15 This early relapse undermines any chance of success as it does not allow the user the chance to implement new opiate-free behaviors and thoughts. Naltrexone use offers no (immediate) reinforcement and the discontinuation of naltrexone produces no adverse effects hence this makes it easy to cease taking it. This contrasts against heroin use, which offers strong reinforcement immediately after use and adverse withdrawal effects upon cessation. Additionally, for persons stabilized on methadone, methadone may give mild reinforcement upon ingestion and prevent sometimes severe and prolonged opiate withdrawal symptoms.6 Non-compliance to naltrexone-based treatment is of concern as after a period of abstinence from opiate use, tolerance is reduced and as such patients who relapse are at an increased risk of overdose and death.16 There does not seem to be any evidence that there is any increased risk compared to anyone who has been abstinent for a period, such as those leaving prison or a residential facility.17
Poor outcomes in the treatment of opiate dependency using naltrexone relates to the shortened time in treatment; time in treatment has been related to better long-term outcomes.18,19 Moreover, with no after-care counselling, compliance strategy or social support in place, studies have shown predictably poor long-term outcomes.12,20,21 However, when naltrexone is combined with an effective after-care program and social support to enhance compliance, results have been promising.22,23 This view has been supported empirically for other drug addiction treatment services.24,25
The current strategy to overcome the issue of non-compliance to naltrexone has been the development and use for some 10 years of sub-cutaneous naltrexone implants. The implant subject to most scrutiny is that of Go Medical Industries (the O’Neill implant). These implants enable slow release into the body at a rate of 8–10 mg/day26–28 and have been shown to effectively block the effects of opiates for between 180 and 240 days thus allowing an extended drug free period to deal with social and psychological problems that would otherwise lead to early relapse and risk of overdose.27,29 This frees the patient of the mental battle they face when trying to remain compliant to oral naltrexone use, and the need to sustain a support person relationship as part of a compliance strategy. Several studies have indicated the excellent bio-availability of naltrexone in subcutaneous form.6,8
Trials of slow-release naltrexone have shown very promising outcomes, although more studies appear warranted. Our paper published in 2005 comparing 42 and 41 patients either taking oral naltrexone or having a naltrexone implant (Go Medical Industries) respectively, showed much better outcomes for the latter group.27 Follow-up showed that 19 of the 42 individuals taking oral naltrexone (45%) relapsed to opiate use or were non-contactable at six months, while only eight out of 41 individuals (19%) were using opiates (or non-contactable) after receiving an implant at six months. This advantage was maintained for the implant group at twelve months with relapse rates at 61% and 40% respectively. That is, at twelve months 61% of the implant group were abstinent, while 40% were abstinent in the oral group. Since then a number of randomized controlled trails have been published that also demonstrate the efficacy of this implant. In a Norwegian study, 56 abstinence-oriented patients after detoxification were randomly and openly assigned to receive either a 6-month naltrexone implant or their usual aftercare. The results showed that patients who received a naltrexone implant had on average 45 days less heroin use and 60 days less opioid use than controls in the 180-day period (both P < 0.05) and naltrexone serum blood levels stayed above 1 ng/mL for the duration of the 6 months. They concluded that naltrexone implant treatment was safe and significantly reduced opioid use in a motivated population of patients.30 In the second study, 70 patients (35 in each group) were randomized to active a naltrexone implant (2.3 g of naltrexone) and placebo naltrexone tablets or placebo implant, and 50 mg oral naltrexone each day. At 6 month follow up, more implant than oral patients had levels above 2 ng/mL (P < 0.001); more oral patients returned to regular heroin use at 6 months (P < 0.003) and at an earlier stage (115 vs. 158 days). They concluded that the naltrexone implant effectively reduced relapse to regular heroin use compared with oral naltrexone and was not associated with major adverse events.29
More recently studies have shown similar results. In 2011, Krupitsky and colleagues31 published results of a RCT trial of a monthly injectable formulation of naltrexone approved by the U.S. Food and Drug Administration for preventing relapse to opioid dependence in 2010. The percentage of opioid-free weeks was significantly higher in the injectable naltrexone group than the placebo group (P = 0.0002). Total abstinence was reported in 36% of patients in the former group compared with 23% in the placebo group (P = 0.0224).
In summary, clinical studies of patients recovering from opiate addiction indicate that patients who have received a naltrexone implant have better outcomes than those who receive placebo naltrexone or oral naltrexone. The issue of compliance compared to oral naltrexone has been largely resolved with the use of naltrexone implants.29 There are still unanswered questions and these mainly concern the reliability of the implant, particularly consistency of release rates and long-term outcomes. It has been established that serum blood levels above 1 ng/mL are sufficient to block a normal street dose of heroin and to protect against overdose,17,26,30,32 although higher doses tend to be only partially blocked and patients report some sensation they associate with opiate use.
The aim of the study was to investigate the reliability of release of an effective dose of naltrexone over the life of the implant and to investigate adverse responses. It was hypothesized that the blood serum levels of naltrexone implants on average remain above 1 ng/mL for a period of 6 months. As a consequence it was also hypothesized that there would be commensurate improvements in drug use and social functioning among the group receiving the implant.
Method
Participants
As part of an open-label trickle-inclusion study, 66 patients were included, each patient receiving a 6 month naltrexone implant. All participants had completed detoxification and underwent a naloxone challenge prior to implantation. The initial data collection coincided with them having the implant. All participants had signed and had witnessed consent forms to participate in the study and each completed medical checks including liver function, thyroid and full blood counts. All patients had a urine drug screen and then completed a questionnaire. Patients were asked to return to the clinic to complete follow up questionnaires, do Urine Drug Screens (UDSs) and provide blood to be analyzed for naltrexone levels at one month, three months and six months after receiving the naltrexone implant.
Implant
Implants produced by Civil Life Scientific Company in Shenzhen, China were used. Each implant was 3.47 g total mass and designed to contain approximately 1.85 grams naltrexone base that had an in vitro release rate ranging from 0.2%–0.8% of its residual mass per day. The naltrexone was encapsulated in poly-DL-lactide (a polymer similar to that used in dissolvable surgical sutures and screws) microspheres compressed into pellets. Each implant consisted of 10 pellets. Subjects were given a single (10 pellets; 185 mg naltrexone) implant, which was surgically inserted into the subcutaneous tissues on the right or left side of the lower abdomen, in the fat tissue below the waist line. The length of time the implant was expected to release therapeutic doses of naltrexone was 6 months (approx. 180 days).
Procedure
Prior to detoxification, all patients underwent a psychosocial assessment to determine whether or not they were suitable for the program. Suitability was determined by the client’s motivation to be opiate free, their level of social support, any serious psychiatric diagnoses of mental illness, and any medical issues that were considered to be contraindications that might compromise safety.
Part of the psychosocial assessment also entailed the completion of a questionnaire. This included questions relating to any adverse effects of the implant, subjective level of craving for opiates, legal and health history, days of using heroin in the previous month, and use of illicit and licit drugs. All participants were asked to rate their self-esteem and the quality of their primary relationships on a 0–10 Likert scale both before and after treatment. Participants were also required to provide blood samples scheduled at 1, 3, and 6 months post-implantation to be analyzed to determine serum levels of naltrexone and its major metabolite, 6-beta-naltrexol, and a urine sample to indicate the presence of opiates and other illicit drugs, including amphetamines, methamphetamines, cocaine, cannabis, and benzodiazepines
All patients were told prior to receiving the implant that there were other forms of treatment available including agonist replacement therapy, as well as the costs and benefits of the naltrexone implants. Each signed informed consent forms prior to inserting the implant in accordance with the Helsinki Declaration of 1975. One of the consent forms included permission to release the data collected for research purposes and included other information relating to the nature and risks attached to use of naltrexone. Use of the implant was authorized under the Special Access Scheme of the Therapeutic Goods Administration. The trial had received approval from the Ultimo Rehabilitation Practice Ethics Committee that conformed with the National Regulations on the Ethical Conduct of Human Research and the Therapeutic Goods Administration approved the trial under the Clinical Trial Notification provisions (CTN 2010/0510, Protocol No. A10) in accordance with Item 3 of Schedule 5A of the Therapeutic Goods Regulations.
Analysis
The survey data, blood samples, and urine was collected over a period of 20 months. Blood serum levels of naltrexone were analyzed by the Royal Prince Alfred Hospital Blood Analysis Laboratory and the Western Australian Chemistry Centre. Other data was compared for significant differences using two-tailed t-tests with alpha level set at 0.05.
Results
The characteristics of the patients were recorded at their first interview, prior to having the implant. Table 1 shows the means and standard deviations on a number of characteristics, including gender, age, total time they had been using opiates (heroin), the amount of heroin being used at the time of the interview (any methadone users had relapsed to heroin before entering the program), the year they left school, whether they were employed, and whether they used other drugs.
Table 1.
Characteristics of subjects prior to detoxification from opiates.
| Patient characteristics | Means |
|---|---|
| Male (%) | 59 (92%) |
| Age (standard deviation) | 29.56 (8.07) |
| Mean years using opiates | 6.29 (SD 5.88) |
| Mean years of schooling | 10.6 |
| Employed (%) | 37 (58%) |
| Mean heroin | 0.41 g (SD 0.24) |
| Mean counselling sessions | 8.5 (SD 2.7) (2 months) |
| Drug related convictions | 38 (60%) |
| Poly drug use | 77.4% |
T tests were conducted to determine if the differences over time were statistically significant. Subjective reports using a Likert scale (0, Disastrous to 10, Excellent) showed that ratings of self-esteem improved over the 6 months of the trial. While ratings of relationship quality was not significant, improvements were observed in this area. At 6 months, the differences since detoxification was significant for self-esteem with an alpha level of 0.05 (P = 0.018), while the relationship ratings approached significance (P = 0.085). The lack of significance was due in part to a number of participants rating their relationship highly (10) despite their drug problem at the first interview. The differences between the two areas of social function that were measured when ratings were compared in the period from 1 to 6 months were all non-significant, indicating that improvements in self-esteem and relationships tended to be maintained.
Self-ratings of craving, before detoxification, while detoxing, as well as at around 1, 3, and 6 months were recorded. Participants were also asked to indicate if they had used heroin in the previous month and how many days they had used in that month. UDSs were used to verify these reports, although they could not determine if a person had used some days before testing in the previous month or how often they may have used. The presence of other drugs was tested for and it was found that self-report was consistent with the UDS results, although there was a tendency to under report stimulant use.
Altogether 108 urine samples were taken. Forty six samples indicated that people were using more than one drug, mostly stimulants, cannabis, and/or benzodiazepines. At month 1, of the 31 tests completed, 5 tested negative for all drugs, 19 tested positive for stimulants, 17 for benzodiazepines, 16 for cannabis, and 6 positive for opiates. Of the 48 that were taken at 6 months, 23 indicated no illicit drug use, 13 tested positive to cannabis, 15 tested positive for amphetamines or methamphetamines, 19 for benzodiazepines (most would have been prescribed), and six had positive results for opiates. Of the remaining samples taken between 1 and 6 months the pattern remained the same with high levels of poly drug use, including one positive test for cocaine, although 16 were negative to all drugs and only three tested positive to opiates over this time.
The most obvious result was the sharp decline in opiate use despite some providing urine samples well after the implant was due to run out. Other drug use also tended to decline over the 6 months.
The number of participants using no drugs rose over the 6 months to 44% of the sample compared to 16% at one month. Of those using 2 to 4 other drugs, the number fell from 77.4% pre-implant to 61% at month 1 to 33% at 6 months. The results show a trend toward less use of drugs quite apart from opiate use.
Subjective ratings of craving for opiates declined dramatically from the time before the implant was inserted compared to the period after insertion, and were sustained for the 6 months. Nevertheless, ten reported that they had tried using heroin in the first month and all reported that there was no subjective effect, and two started using heroin at 5 months, reporting little effect and some withdrawal. One has returned to being abstinent and continued counselling while the other continued to use on a daily basis. There were no other adverse events reported apart from five tissue reactions (sterile abscesses) and three implants extruded (7.8% and 4.6%, respectively) due to an inflammatory response. One subject relapsed to heavy cocaine use after one month, his implant extruded and he relapsed. Two others whose implant extruded after 3 months relapsed to heroin. Both had been using stimulants regularly. Two others also showed early signs of rejection and were treated with a steroid anti-inflammatory medication and they did not proceed to extrusion, but settled without further problems.
The main aim of the research was to determine the consistency of release of naltrexone and if, on average, levels of over 1 ng/mL were maintained over the claimed 6 month life of the implant. Tables 7–9 show serum blood levels approximately 1 (30.2 days), 3 (88.6 days) and 6 months (191.2 days). At around 1 month, 33 blood samples were taken over a range of 21 to 37 days from 35 subjects. By this time, 2 had refused to participate and one had been jailed.
Table 7.
Serum blood levels at 1 month.
| Days | Naltrexone (ng/mL) | Naltrexol (ng/mL) | |
|---|---|---|---|
| Mean | 30.2 | 5.2 | 9.1 |
| Standard deviation | 4.5 | 3.2 | 6.0 |
Table 9.
Serum blood levels at 6 months.
| Days | Naltrexone (ng/mL) | Naltrexol (ng/mL) | |
|---|---|---|---|
| Mean | 191.2 | 0.9 | 3.5 |
| Standard deviation | 40.0 | 3.2 | 3.14 |
A second batch of 51 blood samples, of which 8 were first samples, were taken over a range of 42 to 139 days from 43 subjects. By this time 2 had refused to participate, 3 had been jailed, 3 were non-contactable, 5 had gone overseas and 1 interstate, one had a MVA, and 2 implants extruded due to an allergic reaction.
The time over which a third batch of samples were taken ranged between 140 to 322 days and 53 blood samples were taken (5 of which were first samples) from 48 participants. Of the 66 who commenced the trial, 18 participants were eventually lost to follow up: 2 refused to take part, 3 were jailed, 3 extruded the implant due to an allergic response and relapsed to heroin, 1 had a MVA and had implant removed and was stable on methadone at 6 months, 6 travelled overseas or interstate and 3 were non-contactable.
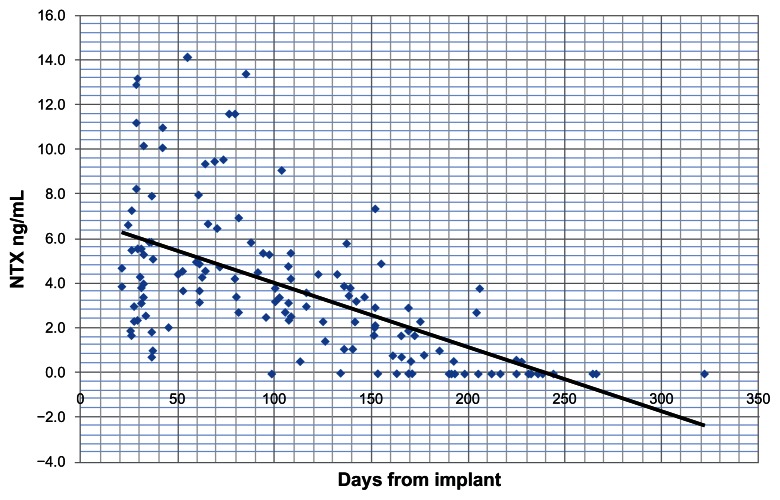
Figure 1 plots naltrexone levels in ng/mL over time. There were two samples at one month that indicated levels of naltrexone of 39.5 and 43.2 ng/mL respectively. These were considered to be outliers that would have distorted the trend shown in the graph and were omitted from calculations. Overall the graph indicates that mean levels of naltrexone, as shown by the trend line, stayed above 1 ng/mL for over 180 days. Figure 2 shows a similar trend for the major active metabolite of naltrexone, 6-beta-naltrexol. Again, outliers of 121.3 and 125.1 ng/mL were not included.
Figure 1.
Levels of naltrexone as measured in blood serum at 6 months in ng/mL.
Figure 2.
Levels of 6-beta-naltrexol as measured in blood serum at 6 months in ng/mL.
Examination of the results of individuals showed that there were 9 subjects who recorded a level of naltrexone that was undetectable within the 6 month period. The first occurred at 98 days, then at 134, 153, 165 (2), 170, 171, and 177. In all cases detectable levels of 6-beta-naltrexol above 2 ng/mL were recorded. There were no reported incidents of drug overdose during the trial period.
Discussion
The most important data to come from this study appears in Figures 1 and 2, showing the sustained release of naltrexone and its active metabolite for the claimed period of blockade. It effectively prevented heroin use for the vast majority over the time of the study.
It was hypothesized that blood serum levels of naltrexone would on average be above 1 ng/mL at 180 days, which is considered an effective blocking dose.17 This was exceeded with the trend line crossing this point at approximately 200 days. There was a significant range of scores with one sample recording a non-detectable level of naltrexone at 98 days and 8 others at 134 to 177 days. A small number of the group starting using opiates late in the trial saying they could feel an effect that coincided with this early depletion of naltrexone after the analytic results were available some months later. There were no over-doses reported throughout the trial and it seems that the major active metabolite (6-beta-naltrexol) affords a degree of protection for some time after the naltrexone is not detectable, which accords with the observations of Brewer and Streel17 and reported by Meyer et al2 and more recently by Hulse et al.26
In summary, of the 66 who enrolled in the study, 42 were opiate free after 6 months (63.6%). This was confirmed by the results of the UDSs. All subjects showed a significant decrease in opiate use from daily use to no use or for some, infrequent use after 6 months. After 6 months, only 6 subjects (9%) were confirmed as having relapsed to regular heroin use. Of these, four returned for a 2nd implant. There were another 18 who were lost to follow up. It could not be confirmed if they were using regularly at 6 months.
Furthermore, the present study would seem to provide strong preliminary evidence that the use of implants is an effective solution to the problem of compliance and that the effect tends to last for some time after the antagonistic effects of the implant has worn off. It seems that the lack of positive reinforcement (no subjective effect), and the strong negative reinforcement (wasting money) associated with using opiates and lack of craving, whilst an implant is releasing naltrexone into the body, is sufficient to prevent use of the drug. This allows time for the development of more adaptive coping behaviors, and for the patient time to deal with the underlying psychological issues (mainly depression and PTSD), that so often compel people to use these drugs. It remains to be seen how many of these patients remain abstinent at longer follow-up intervals, although the trend seems to be that the longer time in treatment and the ability to effect change in lifestyle the more chance that long-term recovery will be sustained.
This benefit of having an implant, apart from the significant reduction in opiate use, was evident by improved ratings on two measures of social functioning. Participants rated their self-esteem and general relationship quality comparably low before their detoxification from opiates and having the implant. As hypothesized, they showed increases in these ratings after their detoxification. It is expected that this would be indicative of improved mental health, greater social cohesion and an improvement in functioning that coincided with the blocking effect of the implant.
Overall the study demonstrates the potential for naltrexone implants to improve compliance rates, increase time in treatment, reduce other drug use, and improve abstinence rates.
Limitations of study
The major limitation of the study was the inconsistency in obtaining data including blood and urine samples from the participants. Appointments to come to the clinic were often not kept even though people were booked ahead of the scheduled collection dates and many were coming for follow up counselling. To overcome this problem, we were required to go to the homes of the participants to obtain the required information, particularly to collect data, including urine and blood samples, in the last phase of the study. This added considerably to the cost and time taken. Samples were collected over extended time periods that often did not coincide with the 1, 3, and 6 months scheduled time-frame. On the other hand, this resulted in a broad spread of samples over the whole period of the study.
With regard to the other variables of interest, the study would have produced more robust results if subjects had been randomly allocated to different treatment conditions, whereas in this study patient groups were self-selected by personal choice to undergo home detoxification and to have a naltrexone implant. In other words, there was no control group. Although it should be noted that the predominant aim was to examine naltrexone serum levels. Perhaps the patients who chose to use naltrexone might have been more motivated, selecting a treatment method that they had considered for some time and having found other alternatives not to be effective or to suit their goals or lifestyle choices. Alternatively this group may have felt they wanted to take responsibility for their own recovery and not proceed with the ‘easy way’.
The study also comprised patients who were screened for serious psychiatric problems, levels of motivation, and social support. Most patients were depressed when they entered the study. This was seen to be a product of the pharmacological effect of the drug and the negatives associated with the lifestyle of a drug user. It has always been our contention that the use of naltrexone should be limited to those who have a reasonable chance of long-term recovery, although no longer term studies to quantify this have been undertaken as yet. Notwithstanding, it can also be seen that the patient group presents with a range of psychological problems, which must be attended to, and with a history of multiple detoxification attempts, criminal activity, and poly-drug use. None of these problems are considered to be a bar to inclusion in the program. As other researchers have pointed out, naltrexone should be targeted to those who can most benefit, and the benefits of research is to clarify the best way to utilize this medication. To obtain some indication of improvement in psychological wellbeing, we used subjective measures of self-esteem and quality of close relationships. Future studies might benefit from using standardized psychometric instruments to more accurately gauge change in this variable.
The other prominent feature was the large numbers of people who were non-contactable for one reason or another, especially in the latter period where this figure represented more than a quarter of the participants, many of whom may have been abstinent, although we could not confirm this. Despite considerable effort it was impossible to follow up some of the participants, particularly those who went overseas or interstate and those who were incarcerated.
Future research
Future research should include random allocation of subjects to different treatment conditions, although matching on significant confounding variables may be warranted before random allocation. It is also important to maintain other strategies, which have been shown to enhance outcomes and maintain the safety of the patients. Not only is this in keeping with the research, but there is also a strong ethical argument to proceed in this manner and to ensure equal access to supportive counselling. Even with the provision of counselling there appears to be a group of patients who are not likely to benefit from use of naltrexone and for whom methadone or buprenorphine is the preferred treatment.
While this was not the aim of the present study, in order to properly evaluate the usefulness of naltrexone it is important that future research examines longer term outcomes. The time-frame for collection of data should be extended to a point some years beyond the termination point of the implants. It is believed that the longer a person is in treatment the better the outcomes, and certainly the use of implants facilitates this. However, it has yet to be shown that the use of naltrexone implants translates directly into long-term improved outcomes.
The present study indicates the potential of the use of these devices in the treatment of opiate dependency and further research seems to be warranted. Clinical trials which are properly constituted with ethics approval, which compare outcomes for those who are on substitute treatment (methadone or buprenorphine) or naltrexone implants, and which extend well beyond the blocking effect of the implant are necessary to confirm the results of this study.
Table 2.
Mean self-ratings of self-esteem and general relationship quality at pre-detox and at 1, 3, and 6 months post-detox (range).
| Self esteem (sig P < 0.05) | Relationships (non-sign) | |
|---|---|---|
| Pre-detox | 3.65 (2–5) | 5.5 (0–10) |
| One month | 7.1 (4–10) | 8.4 (7–10) |
| Three months | 7.66 (4–8) | 8.8 (6–10) |
| Six months | 7.6 (2–10) | 8.4 (6–10) |
Table 3.
Types of drug tested for and the numbers who tested positive at approximately 1, 3, and 6 months post implant.
| Drug | 1 month (n = 31) | 3 months (n = 29) | 6 months (n = 48) |
|---|---|---|---|
| Amphetamine | 11 (35%) | 9 (31%) | 7 (15%) |
| Methamphetamine | 8 (25%) | 9 (31%) | 8 (17%) |
| Opiates | 6 (19%) | 3 (10%) | 6 (12%) |
| Benzodioazepines | 17 (55%) | 11 (37%) | 19 (40%) |
| Cannabis | 16 (52%) | 8 (27%) | 13 (27%) |
| Cocaine | 0 (0%) | 1 (3%) | 0 (0%) |
Table 4.
Number of drugs used over the research period.
| No. of drugs | 1 month | 3 months | 6 months |
|---|---|---|---|
| No drug use | 5 (16%) | 8 (28%) | 21 (44%) |
| One | 5 (16%) | 8 (28%) | 11 (23%) |
| Two to four | 19 (61%) | 12 (41%) | 16 (33%) |
| Four or more | 2 (6%) | 1 (3%) | 0 (0%) |
Table 5.
Days using during the previous month at base-line, and approximately 1, 3, and 6 months (range).
| Days using—Pre-detox | Days using—1 month | Days using—3 months | Days using—6 months |
|---|---|---|---|
| 28.8 (24–30) | 0.2 (0–2) | 2.1 (0–30) | 5.34 (0–30) |
Table 6.
Mean self rating of craving while using, during detoxification and at approximately 1, 3, and 6 months post implant (range).
| Craving while using | Craving during detox | Craving—1 month post implant | Craving—3 month post implant | Craving—6 month post implant |
|---|---|---|---|---|
| 6.7 (3–10) | 7.36 (5–10) | 1.36 (0–8) | 1.4 (0–7) | 1.4 (0–7) |
Note: All sig P < 0.05.
Table 8.
Serum blood levels at 3 months.
| Days | Naltrexone (ng/mL) | Naltrexol (ng/mL) | |
|---|---|---|---|
| Mean | 88.6 | 5.4 | 10.7 |
| Standard deviation | 28.1 | 4.1 | 11.1 |
Acknowledgements
Dr Robert Batey (MBBS, NSW Dept of Health) and Dr Colin Brewer (MBBS) made comment on the draft research design and made suggestions for the implementation of the trial, including ethics committee approval; Geoff Whittaker, (Senior Hospital Scientist, Chromatography Unit RPAH Sydney), provided instructions on the collection and preparation of blood samples; Dr Naswrin Moin, (MBBS) and Eda Morgan, (RN) assisted in collection of blood and urine sample; Thomas Piotrowski (BPsyc), and Zhang Yan (M Ec), collated, recorded and analysed the data and constructed the tables and graphs; Dr Richard Gosden (PhD) made comment and suggested some changes to the final draft of the paper. RMC presented the preliminary results of the research to the 10th Stapleford Addictions Conference in Athens in early 2011 and presented the paper and results (“Naltrexone Blood Serum Levels for Chinese 6 month Naltrexone Implant”) to the APSAD Conference in Hobart in November 2011.
Footnotes
Author Contributions
RMC conceived and designed the experiments, wrote the first draft of the manuscript and made critical revisions and approved the final version.
Competing Interests
RMC was the principle of Addiction Treatment and Psychology Services and was responsible for over-seeing the treatment program. Addiction Treatment and Psychology Services received money from the Addiction Treatment Foundation Inc., to conduct the research including payment of fees to RMC.
Disclosures and Ethics
As a requirement of publication the author has provided signed confirmation of compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published else-where, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
The implants were supplied by Civil Life Scientific Co., Shenzhen, China. The company that provided the pharmaceutical products had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. Other costs associated with the study, including wages, telephone calls, consumables and overheads to collect, collate and analyze the data and write the paper, were incurred by Addiction Treatment and Psychology Services and included some funding from the Addiction Treatment Foundation Inc.
References
- 1.Tennant FS, Jr, Rawson RA, Cohen AJ, Mann A. Clinical experience with naltrexone in suburban opiate addicts. J Clin Psychiatry. 1984 Sep;45(9 Pt 2):42–5. [PubMed] [Google Scholar]
- 2.Meyer MC, Straughn AB, Lo MW, Schary WL, Whitney CC. Bioequivalence, dose-proportionality and pharmacokinetics of naltrexone after oral administration. J Clin Psychiatry. 1984 Sep;45(9 Pt 2):15–9. [PubMed] [Google Scholar]
- 3.Volavka J, Resnick RB, Kestenbaum RS, Freedman AM. Short-term effects of naltrexone in 155 heroin addicts. Biol Psychiatry. 1976 Dec;11(6):679–85. [PubMed] [Google Scholar]
- 4.Revia Product Guide: Approved Product Information. Orphan Australia; Melbourne: 2003. [Google Scholar]
- 5.Brewer C, Wong VS. Naltrexone: report of lack of hepotoxicity in acute viral hepatitis, with a review of the literature. Addict Biol. 2004 Mar;9(1):81–7. doi: 10.1080/13556210410001674130. [DOI] [PubMed] [Google Scholar]
- 6.Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology (Berl) 2002 Feb;159(4):351–60. doi: 10.1007/s002130100909. Epub Nov 1, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King AC, Volpicelli JR, Gunduz M, O’Brien CP, Kreek MJ. Naltrexone biotransformation and incidence of subjective side-effects: A preliminary study. Alcohol Clin Exp Res. 1997 Aug;21(5):906–9. [PubMed] [Google Scholar]
- 8.Perez-Reyes M, Wall ME. A comparative study of oral, intravenous and subcutaneous administration of H-naltrexone to normal male volunteers. NIDA Res Monogr. 1981;28:93–101. [PubMed] [Google Scholar]
- 9.Dean AJ, Saunders JB, Jones R, Young RM, Connor J, Lawford BR. Does naltrexone treatment lead to depression? Findings from a randomized controlled trial in subjects with opioid dependence. J Psychiatry Neurosci. 2006 Jan;31(1):38–45. [PMC free article] [PubMed] [Google Scholar]
- 10.Anton RF, Hogan I, Jalali B, Riordan CE, Kleber HD. Multiple family therapy and naltrexone in the treatment of opiate-dependence. Drug Alcohol Depend. 1981 Sep;8(2):157–68. doi: 10.1016/0376-8716(81)90110-1. [DOI] [PubMed] [Google Scholar]
- 11.Azatian A, Papiasvilli A, Joseph H. A study of the use of clonidine and naltrexone in the treatment of opioid addiction in the former USSR. J Addict Dis. 1994;13(1):35–52. doi: 10.1300/J069v13n01_04. [DOI] [PubMed] [Google Scholar]
- 12.Bell JR, Young MR, Masterman SC, Morris A, Mattick RP, Bammer G. A pilot study of naltrexone-accelerated detoxification in opioid dependence. Med J Aust. 1999 Jul 5;171(1):26–30. doi: 10.5694/j.1326-5377.1999.tb123493.x. [DOI] [PubMed] [Google Scholar]
- 13.Hulse GK, Basso MR. The association between naltrexone compliance and daily supervision. Drug Alcohol Rev. 2000;19(1):41–8. [Google Scholar]
- 14.Wodak A, Saunders JB, Mattick RP, Hall W. Rapid opiate detoxification and naltrexone treatment. Past, present and future. Drug Alcohol Rev. 2001;20(4):349–50. doi: 10.1080/09595239700186701. [DOI] [PubMed] [Google Scholar]
- 15.Gossop M, Green L, Phillips G, Bradley B. What happens to opiate addicts immediately after treatment: a prospective follow-up study. Br Med J (Clin Res Ed) 1987 May 30;294(6584):1377–80. doi: 10.1136/bmj.294.6584.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caplehorn JR, Dalton MS, Haldar F, Petrenas AM, Nisbet JG. Methadone maintenance and addicts’ risk of fatal heroin overdose. Subst Use Misuse. 1996 Jan;31(2):177–96. doi: 10.3109/10826089609045806. [DOI] [PubMed] [Google Scholar]
- 17.Brewer C, Streel E. Recent developments in altrexone implants and depot injections for opiod abuse: the new kid on the block is approaching adulthood. Adicciones. 2010;22(4):285–91. [PubMed] [Google Scholar]
- 18.Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs. 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000 Mar 8;283(10):1303–10. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- 19.Simpson DD. The relation of time spent in drug abuse treatment to post-treatment outcome. Am J Psychiatry. 1979 Nov;136(11):1449–53. doi: 10.1176/ajp.136.11.1449. [DOI] [PubMed] [Google Scholar]
- 20.Rawson RA, McCann MJ, Shoptaw SJ, et al. Naltrexone for opioid dependence: Evaluation of a manualised psychosocial protocol to enhance treatment response. Drug Alcohol Rev. 2001;20(1):67–78. [Google Scholar]
- 21.Strang J, Bearn J, Gossop M. Opiate detoxification under anaesthesia. BMJ. 1997 Nov 15;315(7118):1249–50. doi: 10.1136/bmj.315.7118.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shufman EN, Porat S, Witztum E, Gandacu D, Bar-Hamburger R, Ginath Y. The efficacy of Naltrexone in preventing re-abuse of heroin after detoxification. Biol Psychiatry. 1994 Jun 15;35(12):935–45. doi: 10.1016/0006-3223(94)91240-8. [DOI] [PubMed] [Google Scholar]
- 23.Colquhoun RM. New Horizons: Reducing Drug Harm in the New Millennium. Alcohol and Drug Foundation (Qld); Brisbane: 1999. Outcomes of a Naltrexone Treatment Program for Opiate Dependency. [Google Scholar]
- 24.Woody GE, Luborsky L, McLellan AT, et al. Psychotherapy for opiate addicts. Does it help? Arch Gen Psychiatry. 1983 Jun;40(6):639–45. doi: 10.1001/archpsyc.1983.04390010049006. [DOI] [PubMed] [Google Scholar]
- 25.Ziedonis DM, Kosten TR. Phramacotherapy improves treatment outcomes in depressed cocaine addicts. J Psychoactive Drugs. 1991 Oct-Dec;23(4):417–25. doi: 10.1080/02791072.1991.10471612. [DOI] [PubMed] [Google Scholar]
- 26.Hulse GK, Arnold-Reed DE, O’Neil G, Chan CT, Hansson RC. Achieving long-term continuous blood naltrexone and 6 beta-naltrexol coverage following sequential naltrexone implants. Addict Biol. 2004 Mar;9(1):67–72. doi: 10.1080/13556210410001674112. [DOI] [PubMed] [Google Scholar]
- 27.Colquhoun R, Tan DY, Hull S. Comparison of oral and implant naltrexone at 12 months. J Opioid Manag. 2005 Nov-Dec;1(5):249–56. doi: 10.5055/jom.2005.0054. [DOI] [PubMed] [Google Scholar]
- 28.Colquhoun RM. The Use of Naltrexone in the Treatment of Opiate Dependency. Lambert Academic; Saarbrucken, Germany: 2010. [Google Scholar]
- 29.Hulse GK, Morris N, Arnold-Reed D, Tait RJ. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry. 2009 Oct;66(10):1108–15. doi: 10.1001/archgenpsychiatry.2009.130. [DOI] [PubMed] [Google Scholar]
- 30.Kunøe N, Lobmaier P, Vederhus JK, et al. Naltrexone implants after in-patient treatment for opioid dependence: randomised controlled trial. Br J Psychiatry. 2009 Jun;194(6):541–6. doi: 10.1192/bjp.bp.108.055319. [DOI] [PubMed] [Google Scholar]
- 31.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011 Apr 30;377(9776):1506–13. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 32.Foster J, Brewer C. Naltrexone implants completely prevent early (one-month) relapse after opiate detoxification Paper presented at annual meeting. Society for the Study of Addiction; York, England. Nov 5, 1998; Abstract published in Addiction Biology; 1999. [DOI] [PubMed] [Google Scholar]