Abstract
The purpose of this study was to (1) evaluate joint pain and function in knee osteoarthritis (OA) patients treated with a joint-sparing, extracapsular implant and (2) identify patient characteristics that influenced clinical outcomes. This study included 99 patients with symptomatic medial knee OA refractory to conservative care who were treated with the KineSpring Knee Implant System and followed for a mean of 17 months (range, 1.5 to 48 months). All devices were successfully implanted and activated with no intraoperative complications. Statistically significant mean improvements of 56%, 50%, and 38% were observed for Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain, Function, and Stiffness scales, respectively (all P < 0.001). Regardless of gender, age group, body mass index classification, or disease severity, all WOMAC domain scores significantly improved during the postoperative follow-up period. WOMAC clinical success rates were 77.8% for Pain, 77.8% for Function, and 68.7% for Stiffness. Neither gender, age group, body mass index classification, nor disease severity predicted clinical success in any WOMAC domain. In conclusion, the KineSpring System yields clinically meaningful improvements in joint pain and function in patients with medial knee OA. Additionally, unlike joint-altering procedures such as knee arthroplasty or high tibial osteotomy, patient characteristics had little association with postoperative clinical outcomes.
Keywords: implant, KineSpring, knee, osteoarthritis
Introduction
Osteoarthritis (OA) is characterized by progressive destruction and the subsequent failure to repair damaged cartilage, bone, and synovial tissue. OA most commonly affects the knee, with 27 million American adults diagnosed with knee OA.1 The primary risk factor for knee OA development is chronic, excessive and/or aberrant joint loading.2 Approximately 50% of patients with radiographic evidence of OA are symptomatic,3 with joint pain and stiffness presenting as the cardinal signs of disease. The prevalence of symptomatic knee OA has increased substantially over the last several decades,4 and this trend is expected to continue for decades to come.5
Current treatments do not prevent or cure knee OA. Despite the widespread utilization of non surgical therapies to ameliorate knee OA symptoms, disease progression is unaffected by these treatments.6 In fact, conservative therapies may actually hasten OA progression by encouraging greater mechanical loading at the medial compartment.7–9 The typical disease course ultimately entails inconsistent, but reliable, progression after initial onset. Patients with mild or moderate disease commonly linger in the “treatment gap,” a period defined as the time from exhaustion of nonoperative treatment to surgical intervention and characterized by a period of years and often decades in which the patient experiences debilitating pain, reduced quality of life, and a significant financial burden.6 For patients with end-stage knee OA, total knee or unicompartmental arthroplasty is a cost effective surgical treatment.10,11 However, patients are extremely reluctant to undergo this invasive surgical procedure,12–14 and clinical outcomes frequently fall short of patient expectations.15
Many studies have attempted to identify patient-related factors that influence outcomes following knee arthroplasty. Young age and obesity are often considered relative contraindications to knee arthroplasty due to inferior outcomes reported in some studies in these patients.16,17 Effective and acceptable alternatives to knee arthroplasty are desperately needed, particularly in patients at high risk of perioperative complications or prosthesis failure.
Implantable medical devices have been utilized across many therapeutic areas with the goal of safely and effectively treating a condition while eliminating or reducing the complications associated with open surgery due to a less invasive procedure. The ideal characteristics of such a device for knee OA would include excellent patient safety, clinically meaningful improvements in joint pain and function, and minimal soft tissue and osseous disruption regardless of patient characteristics. Chronic excessive and/or abnormal joint loading is a major risk factor for knee OA.18,19 Conversely, joint unloading has been postulated to encourage cartilage healing.2,20 The purpose of this study was to evaluate joint pain and function in knee OA patients treated with a joint-sparing, extracapsular implant. A secondary goal of this study was to identify baseline patient characteristics that influenced clinical outcomes.
Methods
Ethics
This paper describes the collective clinical experience with the KineSpring System (Moximed, Inc., Hayward, CA, USA) across 3 clinical trials. The OASYS and OAKS clinical trials were registered at the Australian New Zealand Clinical Trials Register (ANZCTR) as ACTRN12608000451303 and ACTRN12609001068257, respectively. The COAST clinical trial was registered at International Standard Randomized Controlled Trial Number Register as ISRCTN63048529. Each clinical trial was approved by local hospital ethics committees and all subjects gave written informed consent to participate in accordance with the principles set forth by the Declaration of Helsinki.
Patients
Study entry criteria were similar among the clinical trials. The primary common inclusion criteria included age 25 years and older and symptomatic, radiographically confirmed medial knee OA resistant to nonoperative care. The main common exclusion criteria included symptomatic lateral compartment or patellofemoral OA, varus alignment > 10 degrees, inflammatory joint disease, prior traumatic knee injury, moderate to severe osteoporosis, previous surgery at the target knee, symptomatic instability, current smoking, active infection, and clinically significant comorbidity (eg, uncontrolled diabetes mellitus).
Pretreatment procedures
Baseline assessments included inclusion/exclusion criteria evaluation, a complete clinical and orthopedic examination, and medical history. Imaging studies included standing X-rays (anteroposterior, lateral, and sunrise views) and magnetic resonance imaging. Disease severity was classified using the Kellgren-Lawrence (K-L) grading scale21 where 0 = normal; 1 = possible osteophyte, no joint space narrowing; 2 = definite osteophyte, possible joint space narrowing; 3 = multiple osteophytes, definite joint space narrowing, sclerosis, and possible deformity of bone ends; and 4 = large osteophytes, marked joint space narrowing, severe sclerosis, and definite deformity of bone ends. Patient-reported outcomes were measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) version 3.1.22 The WOMAC was designed to measure pain and dysfunction associated with OA of the lower extremities by assessing 5 pain-related activities, 17 functional activities, and 2 stiffness categories. Each item is based on recall over the previous 48 hours and is scored on a 0 to 4 scale where 0 represents none and 4 represents extreme. The scores are then normalized to a 0 to 100 scale, where a higher score represents a worse outcome.
Device details
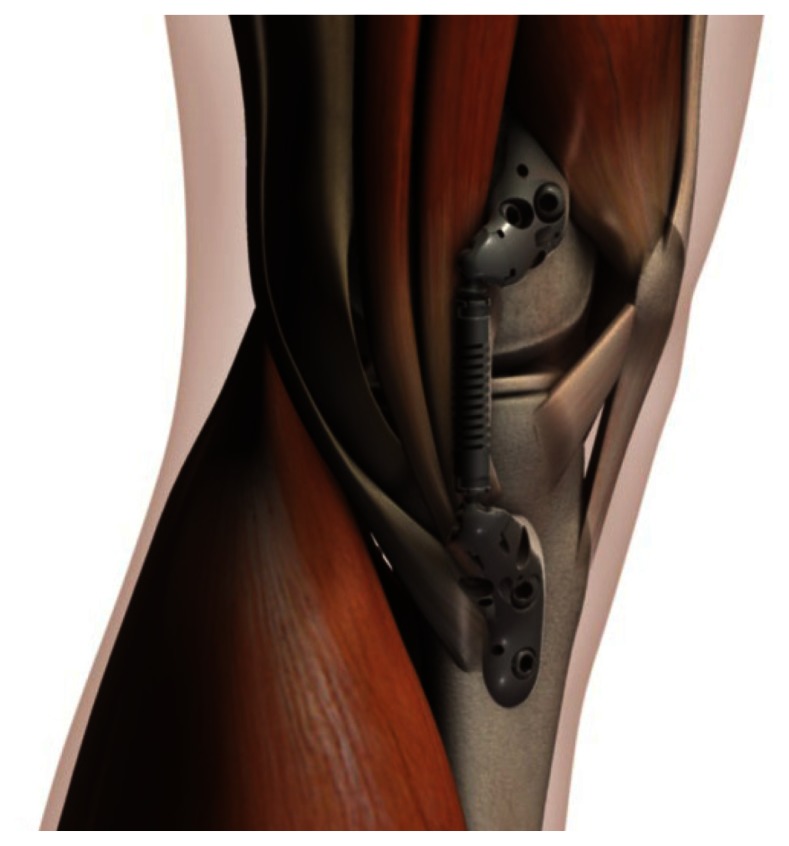
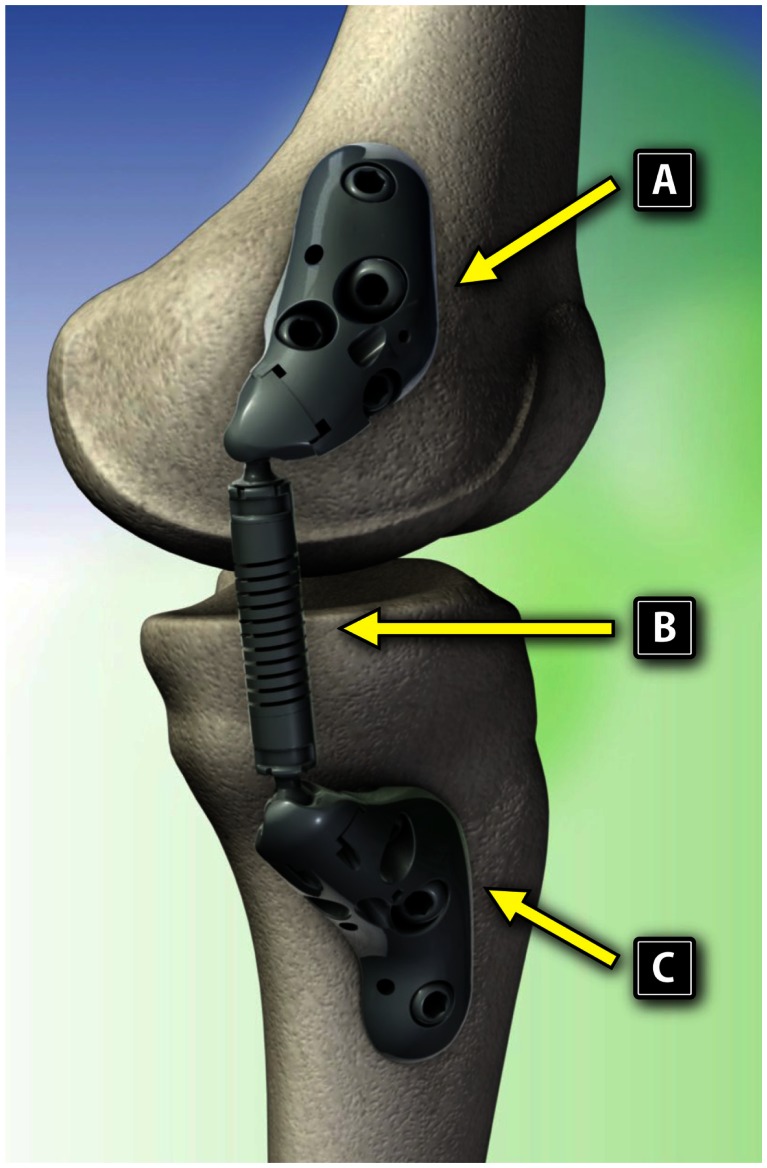
The KineSpring System (Fig. 1) is a minimally invasive knee implant that is specifically designed to fill the therapeutic gap between nonoperative care and invasive surgical interventions. The KineSpring System consists of titanium alloy femoral and tibial bases and a cobalt/cobalt chrome alloy absorber that reduces the load carried by the diseased medial compartment of the knee joint during the stance phase of gait (Fig. 2).
Figure 1.
The KineSpring System, medial view. Reproduced with permission from Moximed.
Figure 2.
Components of the KineSpring System. (A) Femoral base, (B) absorber, and (C) tibial base. Reproduced with permission from Moximed.
The KineSpring System is both extra-articular and extracapsular. Implantation of the device is achieved without resection of bone, muscle, or ligaments, and without violation of the joint capsule. The load absorber resides in the subcutaneous tissue on the medial aspect of the knee and is positioned superficial to the medial collateral ligament. Device implantation without joint invasion means that the option of future device explant remains, if needed, thereby leaving the joint in its pretreatment state.
The kinematics of this novel device accommodate the natural motions of the knee joint by utilizing two ball-and-socket joints. The device accommodates the wide range of normal physiological knee motion with the capability for unlimited internal-external rotation, 50° varus-valgus angulation (35° in the OASYS study), and 155° flexion-extension movement.
The KineSpring System absorbs a maximum load of 30 pounds during full knee extension, corresponding to the stance phase of gait, which is comparable to lower knee adduction moments across a wide range of body weights and reduces chronic medial compartment loading without significant increases in lateral compartment loading. This magnitude of unloading is comparable to the amount of body weight loss shown to improve function and alleviate knee pain in OA patients.23
Procedural details
A detailed description of the surgical procedure for KineSpring System implant has been reported elsewhere.24 Briefly, the patient was placed supine with the operative leg elevated on a foot roll to allow a clear lateral radiographic view free from overlay of the contralateral limb. After surgical preparation and draping, a fluoroscopic C-arm was positioned to obtain a true lateral radiograph of the distal femur. The distal femur was exposed via a small incision and standard subvastus approach, taking care to ensure hemostasis from the superior medial geniculate vessels. The femoral center of rotation about which the implant will rotate was established under fluoroscopic guidance. Femoral base templates were trialed to obtain best anatomical fit. Correct rotational alignment of the femoral base was confirmed by a radiopaque marker at the distal end of the femoral base, which confirmed appropriate positioning of the absorber unit to the tibia. The chosen femoral base was then applied to the femur with cancellous compression and locking screws.
A second small incision was then made over the proximal subcutaneous surface of the tibia. The periosteum was elevated from the tibia and, posteriorly, the sartorious fascia was optionally elevated a few millimeters. The tibial base was seated on bone anterior to the pes anserine insertion. A subcutaneous tunnel was then prepared using blunt dissection between the two incisions.
On a back table, the tibial base and absorber unit were coupled for insertion. The tibial base/absorber assembly was then delivered from the distal wound through the subcutaneous pathway with the knee in slight flexion. To assist with coupling of the device, the knee was then flexed to approximately 45°. The absorber unit was then docked and locked to the femoral base. With the knee extended once more, alignment to the tibia was confirmed. The joint space was closed by applying a varus force to the knee at the point of maximal tibial wear (usually <10° of flexion). With the knee in this position, the tibial base was secured to the tibia. The load absorber was then activated, allowing elongation of the spring to resting length. Joint motion was assessed in both the anteroposterior and lateral planes before normal closure.
Patients were physically capable of bearing full body weight immediately following surgery. Braces and splints were not required; physical therapy and crutches were recommended at the discretion of the surgeon. Patients were advised to limit the amount of activity for the first 2 weeks to allow the surgical incision sites to heal properly.
Follow-up
Patients were followed through hospital discharge and returned for visits at 2 weeks, 6 weeks, 3 months, 6 months, and annually thereafter. Each visit included a complete clinical and orthopedic examination. Standing X-rays (anteroposterior, lateral, and sunrise views) were performed at discharge, 6 months, and annually thereafter. Magnetic resonance imaging was performed at 1 and 2 years in the OASYS and OAKS trials. The WOMAC questionnaire was administered at 6 weeks and at all subsequent follow-up visits. Patient follow-up is ongoing through 5 years.
Data analysis
Patients from each clinical trial with a minimum of 6-week postoperative data were included in this analysis. The final available follow-up data point was used for analysis purposes. Patients were stratified by gender, age (<50 years, 50–59 years, and ≥60 years), body mass index (BMI) (<30 kg/m2 [non-obese] and ≥30 kg/m2 [obese]), and K-L grade (I or II, III, and IV). Continuous data were reported as mean ± standard deviation (SD) and categorical data were reported as frequencies and percentages. Longitudinal changes in clinical outcomes were assessed with repeated measures analysis of variance. Clinical success for each WOMAC domain was defined as a ≥20% improvement from baseline.25 Univariate and multivariate logistic regression models were used to identify baseline predictors of WOMAC success. Independent variables that loaded into the model at P < 0.1 were included in the multivariate model. A P value < 0.05 was considered statistically significant. Data were analyzed using Predictive Analytics Software (v. 18, SPSS, Inc., Chicago, IL).
Results
Patients
A total of 99 patients met the inclusion criteria for this study. Mean follow-up was 17 months (range, 1.5 to 48 months). Baseline patient characteristics are presented in Table 1. Patients were predominantly (75%) male, middle-aged (mean, 52 years; range, 31 to 75 years), with a mean BMI of 30 kg/m2 who, despite exhausting conservative treatments, suffered from moderate knee OA pain and dysfunction with a severity comparable to patients undergoing total knee arthroplasty (TKA).15,26
Table 1.
Baseline subject characteristics.
| Variable | Mean ± SD or n (%) (n = 99) |
|---|---|
| Gender, n (%) | |
| Male | 74 (75) |
| Female | 25 (25) |
| Age, yr | 52 ± 9 |
| <50 | 33 (33) |
| 50–59 | 46 (47) |
| ≥60 | 20 (20) |
| Body mass index, kg/m2 | 30 ± 5 |
| <30 | 48 (48) |
| ≥30 | 51 (52) |
| Kellgren-Lawrence grade* | 3.0 ± 0.7 |
| I | 2 (3) |
| II | 10 (15) |
| III | 37 (57) |
| IV | 16 (25) |
| WOMAC subscores | |
| Pain | 45 ± 17 |
| Function | 44 ± 18 |
| Stiffness | 52 ± 21 |
Note:
n = 65.
Clinical outcomes
Technical success was 100%; all devices were successfully implanted and activated with no intraoperative complications. Mean operative time was 67 ± 17 minutes, median blood loss was 0 cc (range, 0 to 500 cc), and median hospital stay was 1 day (range, 1 to 13 days). Statistically significant improvements were observed in all domains of the WOMAC questionnaire following implant with the KineSpring System. The mean WOMAC Pain score decreased from 45 ± 17 at pretreatment to 20 ± 18 at final follow-up, representing a 56% overall improvement (P < 0.001). WOMAC Function scores improved from 44 ± 18 at pretreatment to 22 ± 18, a 50% improvement (P < 0.001). WOMAC Stiffness scores improved from 52 ± 21 at pretreatment to 32 ± 24 at final follow-up, a 38% improvement (P < 0.001).
Influence of baseline characteristics on WOMAC scores
The influence of baseline characteristics on changes in WOMAC scores is detailed in Tables 2–4. Within each gender, age group, BMI classification, and K-L grade, all WOMAC domain scores significantly improved during the postoperative follow-up period. Neither gender, age group, nor K-L grade influenced changes in WOMAC scores. However, obese patients experienced significantly greater improvements in all WOMAC domains in comparison with nonobese patients. Specifically, WOMAC improvements in obese versus nonobese patients were 60% versus 48% for WOMAC Pain, 58% versus 39% for WOMAC Function, and 47% versus 24% for WOMAC Stiffness (all time-by-group P values < 0.01).
Table 2.
Changes in WOMAC pain scores by baseline characteristic.
| Variable | WOMAC pain | P value | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Pre | Final | Mean Absolute Decrease | Mean % Decrease | Time | Time-by-group | |
| Gender, n (%) | ||||||
| Male (n = 74) | 44 ± 18 | 20 ± 18 | 24 | 55 | <0.001 | 0.98 |
| Female (n = 25) | 47 ± 12 | 20 ± 16 | 24 | 51 | <0.001 | |
| Age, yr | ||||||
| <50 (n = 33) | 43 ± 16 | 17 ± 17 | 26 | 60 | <0.001 | 0.75 |
| 50–59 (n = 46) | 46 ± 18 | 23 ± 17 | 23 | 50 | <0.001 | |
| ≥60 (n = 20) | 45 ± 15 | 20 ± 18 | 25 | 56 | <0.001 | |
| Body mass index, kg/m2 | ||||||
| <30 (n = 48) | 40 ± 16 | 21 ± 19 | 19 | 48 | <0.001 | 0.004 |
| ≥30 (n = 51) | 50 ± 16 | 20 ± 17 | 30 | 60 | <0.001 | |
| Kellgren-Lawrence grade* | ||||||
| I or II (n = 12) | 40 ± 23 | 11 ± 19 | 28 | 70 | 0.001 | 0.47 |
| III (n = 37) | 43 ± 17 | 20 ± 18 | 23 | 53 | <0.001 | |
| IV (n = 16) | 46 ± 17 | 27 ± 18 | 19 | 41 | 0.002 | |
Note:
n = 65.
Table 4.
Changes in WOMAC Stiffness scores by baseline characteristic.
| Variable | WOMAC stiffness | P value | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Pre | Final | Mean Absolute Decrease | Mean % Decrease | Time | Time-by-group | |
| Gender, n (%) | ||||||
| Male (n = 74) | 49 ± 21 | 32 ± 24 | 18 | 37 | <0.001 | 0.27 |
| Female (n = 25) | 58 ± 19 | 33 ± 24 | 25 | 43 | 0.001 | |
| Age, yr | ||||||
| <50 (n = 33) | 48 ± 23 | 28 ± 26 | 19 | 40 | <0.001 | 0.46 |
| 50–59 (n = 46) | 54 ± 21 | 37 ± 23 | 17 | 31 | <0.001 | |
| ≥60 (n = 20) | 53 ± 17 | 26 ± 24 | 26 | 49 | <0.001 | |
| Body mass index, kg/m2 | ||||||
| <30 (n = 48) | 42 ± 19 | 32 ± 28 | 10 | 24 | 0.02 | 0.002 |
| ≥30 (n = 51) | 60 ± 19 | 32 ± 21 | 28 | 47 | <0.001 | |
| Kellgren-Lawrence grade* | ||||||
| I or II (n = 12) | 49 ± 25 | 20 ± 20 | 29 | 59 | 0.002 | 0.26 |
| III (n = 37) | 51 ± 21 | 34 ± 29 | 17 | 33 | 0.004 | |
| IV (n = 16) | 49 ± 18 | 38 ± 18 | 11 | 22 | 0.04 | |
Note:
n = 65.
WOMAC success rates
WOMAC clinical success rates were 77.8% for Pain, 77.8% for Function, and 68.7% for Stiffness. The distribution of WOMAC clinical success rates according to baseline characteristic classification is presented in Table 5, and univariate predictors of WOMAC clinical success are presented in Table 6. For WOMAC Pain and Function clinical success, only one variable loaded into each model between a P value of 0.05 and 0.10. Therefore, multivariate analysis was not performed. For WOMAC Stiffness clinical success, BMI and pretreatment WOMAC Stiffness each loaded into the model at P < 0.1. In multivariate analysis, only pretreatment WOMAC Stiffness was predictive of WOMAC Stiffness clinical success (odds ratio [OR] 1.04, 95% confidence interval [CI], 1.02–1.07). A second multivariate analysis that additionally included K-L grade as in independent variable was performed, yielding a sample size of 65. K-L grade did not load into the model at P < 0.1 for any WOMAC clinical success subscore. Therefore, this analysis is not presented, and the model without K-L grade (n = 99) serves as the sole multivariate analysis.
Table 5.
WOMAC clinical success rates (≥20% improvement): overall and by baseline characteristic.
| Variable | WOMAC domain | ||
|---|---|---|---|
|
|
|||
| Pain | Function | Stiffness | |
| All subjects (n = 99) | 77.8 | 77.8 | 68.7 |
| Gender, n (%) | |||
| Male (n = 74) | 75.7 | 75.7 | 67.6 |
| Female (n = 25) | 84.0 | 84.0 | 72.0 |
| Age, yr | |||
| <50 (n = 33) | 84.9 | 78.8 | 69.7 |
| 50–59 (n = 46) | 76.1 | 80.4 | 63.0 |
| ≥60 (n = 20) | 70.0 | 70.0 | 80.0 |
| Body mass index, kg/m2 | |||
| <30 (n = 48) | 70.8 | 70.8 | 60.4 |
| ≥30 (n = 51) | 84.3 | 84.3 | 76.5 |
| Kellgren-Lawrence grade* | |||
| I or II (n = 12) | 83.3 | 83.3 | 83.3 |
| III (n = 37) | 75.7 | 75.7 | 70.3 |
| IV (n = 16) | 68.8 | 68.8 | 56.3 |
Note:
n = 65.
Table 6.
Univariate baseline predictors of WOMAC success (≥20% improvement) at final postoperative follow-up.
| Pain | Function | Stiffness |
|---|---|---|
| 0.09 Age | 0.08 Body mass index | <0.001 WOMAC stiffness |
| 0.17 Body mass index | 0.11 WOMAC function | 0.08 Body mass index |
| 0.20 WOMAC function | 0.30 Age | 0.68 Gender |
| 0.26 WOMAC stiffness | 0.39 Gender | 0.70 WOMAC function |
| 0.29 WOMAC pain | 0.41 WOMAC stiffness | 0.76 WOMAC pain |
| 0.39 Gender | 0.63 WOMAC pain | 0.90 Age |
Notes: Cell values are P values. Model includes gender, age, body mass index, and pretreatment WOMAC pain, function, and stiffness scores.
Comparison of WOMAC successes and failures
Overall, baseline characteristics were not different between WOMAC clinical successes and failures. Subjects with clinical success in WOMAC Function had higher baseline WOMAC Function scores, while subjects with clinical success in WOMAC Stiffness had higher baseline WOMAC Stiffness scores. No other statistical differences were detected in baseline characteristics between WOMAC successes and failures.
Discussion
The collective outcomes from 3 prospective clinical trials demonstrate that the KineSpring System offers clinically meaningful improvements in pain, function, and stiffness in patients with medial knee OA. Additionally, gender, age, BMI, and disease severity had little association with clinical outcomes, a finding in sharp contrast to many studies of knee arthroplasty and high tibial osteotomy.
Osteoarthritis symptom severity in patients treated with the KineSpring System was comparable to that of patients undergoing unicompartmental or total knee arthroplasty or high tibial osteotomy. The magnitude of clinical improvement at a mean of 17 months post-treatment with the KineSpring System was comparable to outcomes of knee arthroplasty. In the current study, pretreatment WOMAC scores ranged from 44 to 52, and WOMAC improvements following KineSpring implant ranged from 38% for Stiffness to 56% for Pain. Several clinical studies of patients who underwent unicompartmental or total knee arthroplasty reported mean WOMAC improvements ranging from 43% to 73%, depending on subscore and surgery type.26,27 Similar WOMAC improvements have been observed following open and closed wedge tibial osteotomy.28
Obese patients experienced greater clinical improvements in all WOMAC scores compared with nonobese patients. However, over 7 in 10 nonobese patients achieved WOMAC Pain or Function clinical success, a finding that underscores the utility of the KineSpring System regardless of BMI. While the reasons for the slightly better outcomes in obese patients are unclear, we speculate that the etiology of knee OA in the obese is strongly related to chronic, excessive loading at the knee joint whereas disease etiology in the nonobese patient is related to other factors such as aberrant loading patterns, intense physical activity, and/or previous trauma. Support for this hypothesis includes the exponential increase in knee OA risk at and above a BMI of 30 kg/m2,29 as well as higher rates of previous injury and menisectomy in normal weight versus obese knee OA patients.30 Others have similarly concluded that previous sports-related injury partially explains the recent exponential utilization in TKA, independent of obesity.31 Unfortunately, a comprehensive medical and physical activity history was unavailable for the patients in the current study, and, therefore, we could not further explore this hypothesis. Regardless of disease etiology, results from this study support the use of the KineSpring System in obese and nonobese patients alike.
In contrast to the findings of the current study, knee arthroplasty in obese patients is associated with inferior outcomes. A recent meta-analysis by Kerkhoffs et al32 that included over 15,000 TKA patients concluded that obesity is associated with higher rates of infection (OR = 1.9), deep infection (OR = 2.4), and revision surgery (OR = 1.3). Obese patients also have significantly higher postsurgical WOMAC scores versus the nonobese.33 Additionally, age and gender are known influencers of acute postoperative pain, clinical outcomes, functional limitations, length of hospital stay, and mortality following knee arthroplasty.34–36 With the exception of age and physical activity status, the indications for high tibial osteotomy are similar to those for knee arthroplasty. As such, high tibial osteotomy is generally contraindicated in the obese patient37 since obesity is an independent risk factor for major surgical complications.38 This suggests that clinical outcomes with the KineSpring System are favorable in patients who may be denied knee arthroplasty or high tibial osteotomy due to relative contraindications including obesity, advanced age, and/or female gender.
In conclusion, the KineSpring System yields clinically meaningful improvements in joint pain and function in patients with medial knee OA. Additionally, patient characteristics had little association with postoperative clinical outcomes.
Table 3.
Changes in WOMAC function scores by baseline characteristic.
| Variable | WOMAC function | P value | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Pre | Final | Mean Absolute Decrease | Mean % Decrease | Time | Time-by-group | |
| Gender, n (%) | ||||||
| Male (n = 74) | 43 ± 19 | 21 ± 18 | 22 | 51 | <0.001 | 0.91 |
| Female (n = 25) | 46 ± 13 | 23 ± 19 | 23 | 50 | <0.001 | |
| Age, yr | ||||||
| <50 (n = 33) | 41 ± 19 | 20 ± 17 | 21 | 51 | <0.001 | |
| 50–59 (n = 46) | 47 ± 18 | 23 ± 19 | 24 | 51 | <0.001 | 0.85 |
| ≥60 (n = 20) | 42 ± 14 | 20 ± 20 | 21 | 50 | <0.001 | |
| Body mass index, kg/m2 | ||||||
| <30 (n = 48) | 38 ± 16 | 22 ± 20 | 15 | 39 | <0.001 | 0.001 |
| ≥30 (n = 51) | 50 ± 18 | 21 ± 17 | 29 | 58 | <0.001 | |
| Kellgren-Lawrence grade* | ||||||
| I or II (n = 12) | 40 ± 22 | 13 ± 17 | 27 | 68 | 0.003 | 0.57 |
| III (n = 37) | 42 ± 17 | 22 ± 21 | 20 | 48 | <0.001 | |
| IV (n = 16) | 45 ± 16 | 27 ± 17 | 19 | 42 | 0.003 | |
Note:
n = 65.
Table 7.
Baseline subject characteristics of WOMAC clinical successes and failures.
| Variable | WOMAC domain | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Pain | Function | Stiffness | ||||
|
|
|
|
||||
| Success | Failure | Success | Failure | Success | Failure | |
| Male gender, % | 73 | 82 | 73 | 82 | 74 | 77 |
| Age, yr | 52 ± 9 | 55 ± 7 | 52 ± 9 | 54 ± 8 | 52 ± 9 | 53 ± 9 |
| Body mass index, kg/m2 | 30 ± 5 | 29 ± 4 | 31 ± 5 | 29 ± 4 | 31 ± 5 | 29 ± 5 |
| Kellgren-Lawrence grade* | 3.0 ± 0.7 | 3.1 ± 0.8 | 3.0 ± 0.7 | 3.1 ± 0.8 | 3.0 ± 0.7 | 3.2 ± 0.8 |
| WOMAC subscores | ||||||
| Pain | 46 ± 16 | 42 ± 18 | 45 ± 18 | 43 ± 11 | 45 ± 18 | 46 ± 13 |
| Function | 45 ± 18 | 40 ± 16 | 45 ± 19** | 39 ± 12 | 44 ± 19 | 43 ± 15 |
| Stiffness | 53 ± 22 | 47 ± 15 | 52 ± 22 | 48 ± 15 | 56 ± 20*** | 41 ± 18 |
Notes:
n = 65;
P < 0.05;
P < 0.001.
Acknowledgements
We thank Mr. Randy Asher for graphical assistance.
Footnotes
Author Contributions
Conceived and designed the experiments: NL, JS. Analyzed the data: LM. Wrote the first draft of the manuscript: LM. Contributed to the writing of the manuscript: NL, JS, JB. Agree with manuscript results and conclusions: NL, JS, LM, JB. Jointly developed the structure and arguments for the paper: NL, JS, LM, JB. Made critical revisions and approved final version: NL, JS, LM, JB. All authors reviewed and approved of the final manuscript.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
Competing Interests
NJL, LEM and JEB received financial support from Moximed, Inc. (Hayward, CA).
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waller C, Hayes D, Block JE, London NJ. Unload it: the key to the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1823–9. doi: 10.1007/s00167-011-1403-6. [DOI] [PubMed] [Google Scholar]
- 3.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155(11):725–32. doi: 10.1059/0003-4819-155-11-201112060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 6.London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses. 2011;76(6):887–92. doi: 10.1016/j.mehy.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 7.Briem K, Axe MJ, Snyder-Mackler L. Medial knee joint loading increases in those who respond to hyaluronan injection for medial knee osteoarthritis. J Orthop Res. 2009;27(11):1420–5. doi: 10.1002/jor.20899. [DOI] [PubMed] [Google Scholar]
- 8.Henriksen M, Simonsen EB, Alkjaer T, et al. Increased joint loads during walking—a consequence of pain relief in knee osteoarthritis. Knee. 2006;13(6):445–50. doi: 10.1016/j.knee.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Hurwitz DE, Sharma L, Andriacchi TP. Effect of knee pain on joint loading in patients with osteoarthritis. Curr Opin Rheumatol. 1999;11(5):422–6. doi: 10.1097/00002281-199909000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Daigle ME, Weinstein AM, Katz JN, Losina E. The cost-effectiveness of total joint arthroplasty: A systematic review of published literature. Best Pract Res Clin Rheumatol. 2012;26(5):649–58. doi: 10.1016/j.berh.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113–21. doi: 10.1001/archinternmed.2009.136. discussion 1121–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212–20. doi: 10.1002/art.22146. [DOI] [PubMed] [Google Scholar]
- 13.Hawker GA, Wright JG, Badley EM, Coyte PC. Perceptions of, and willingness to consider, total joint arthroplasty in a population-based cohort of individuals with disabling hip and knee arthritis. Arthritis Rheum. 2004;51(4):635–41. doi: 10.1002/art.20524. [DOI] [PubMed] [Google Scholar]
- 14.Hawker GA, Wright JG, Coyte PC, et al. Determining the need for hip and knee arthroplasty: the role of clinical severity and patients’ preferences. Med Care. 2001;39(3):206–16. doi: 10.1097/00005650-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Becker R, Doring C, Denecke A, Brosz M. Expectation, satisfaction and clinical outcome of patients after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1433–41. doi: 10.1007/s00167-011-1621-y. [DOI] [PubMed] [Google Scholar]
- 16.Mulhall KJ, Ghomrawi HM, Mihalko W, Cui Q, Saleh KJ. Adverse effects of increased body mass index and weight on survivorship of total knee arthroplasty and subsequent outcomes of revision TKA. J Knee Surg. 2007;20(3):199–204. doi: 10.1055/s-0030-1248043. [DOI] [PubMed] [Google Scholar]
- 17.Berend KR, Lombardi AV, Jr, Adams JB. Obesity, young age, patellofemoral disease, and anterior knee pain: identifying the unicondylar arthroplasty patient in the United States. Orthopedics. 2007;30(Suppl 5):19–23. [PubMed] [Google Scholar]
- 18.Block JA, Shakoor N. The biomechanics of osteoarthritis: implications for therapy. Curr Rheumatol Rep. 2009;11(1):15–22. doi: 10.1007/s11926-009-0003-7. [DOI] [PubMed] [Google Scholar]
- 19.Radin EL. Who gets osteoarthritis and why? An update. J Rheumatol. 2005;32(6):1136–8. [PubMed] [Google Scholar]
- 20.Radin EL, Burr DB. Hypothesis: joints can heal. Semin Arthritis Rheum. 1984;13(3):293–302. doi: 10.1016/0049-0172(84)90031-3. [DOI] [PubMed] [Google Scholar]
- 21.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 23.Zhao D, Banks SA, Mitchell KH, D’Lima DD, Colwell CW, Jr, Fregly BJ. Correlation between the knee adduction torque and medial contact force for a variety of gait patterns. J Orthop Res. 2007;25(6):789–97. doi: 10.1002/jor.20379. [DOI] [PubMed] [Google Scholar]
- 24.Clifford A, O’Connell M, Gabriel S, Miller LE, Block JE. The KineSpring load absorber implant: rationale, design and biomechanical characterization. J Med Eng Technol. 2011;35(1):65–71. doi: 10.3109/03091902.2010.535592. [DOI] [PubMed] [Google Scholar]
- 25.Barr S, Bellamy N, Buchanan WW, et al. A comparative study of signal versus aggregate methods of outcome measurement based on the WOMAC Osteoarthritis Index. Western Ontario and McMaster Universities Osteoarthritis Index. J Rheumatol. 1994;21(11):2106–12. [PubMed] [Google Scholar]
- 26.Bachmeier CJ, March LM, Cross MJ, et al. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cartilage. 2001;9(2):137–46. doi: 10.1053/joca.2000.0369. [DOI] [PubMed] [Google Scholar]
- 27.Lyons MC, MacDonald SJ, Somerville LE, Naudie DD, McCalden RW. Unicompartmental versus total knee arthroplasty database analysis: is there a winner? Clin Orthop Relat Res. 2012;470(1):84–90. doi: 10.1007/s11999-011-2144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaasbeek RD, Nicolaas L, Rijnberg WJ, van Loon CJ, van Kampen A. Correction accuracy and collateral laxity in open versus closed wedge high tibial osteotomy. A one-year randomised controlled study. Int Orthop. 2010;34(2):201–7. doi: 10.1007/s00264-009-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee R, Kean WF. Obesity and knee osteoarthritis. Inflammopharmacology. 2012;20(2):53–8. doi: 10.1007/s10787-011-0118-0. [DOI] [PubMed] [Google Scholar]
- 30.Vrezas I, Elsner G, Bolm-Audorff U, Abolmaali N, Seidler A. Case-control study of knee osteoarthritis and lifestyle factors considering their interaction with physical workload. Int Arch Occup Environ Health. 2010;83(3):291–300. doi: 10.1007/s00420-009-0486-6. [DOI] [PubMed] [Google Scholar]
- 31.Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201–7. doi: 10.2106/JBJS.J.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerkhoffs GM, Servien E, Dunn W, Dahm D, Bramer JA, Haverkamp D. The influence of obesity on the complication rate and outcome of total knee arthroplasty: a meta-analysis and systematic literature review. J Bone Joint Surg Am. 2012;94(20):1839–44. doi: 10.2106/JBJS.K.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarvenpaa J, Kettunen J, Soininvaara T, Miettinen H, Kroger H. Obesity has a negative impact on clinical outcome after total knee arthroplasty. Scand J Surg. 2012;101(3):198–203. doi: 10.1177/145749691210100310. [DOI] [PubMed] [Google Scholar]
- 34.Kreder HJ, Grosso P, Williams JI, et al. Provider volume and other predictors of outcome after total knee arthroplasty: a population study in Ontario. Can J Surg. 2003;46(1):15–22. [PMC free article] [PubMed] [Google Scholar]
- 35.Singh JA, O’Byrne M, Harmsen S, Lewallen D. Predictors of moderate-severe functional limitation after primary Total Knee Arthroplasty (TKA): 4701 TKAs at 2-years and 2935 TKAs at 5-years. Osteoarthritis Cartilage. 2010;18(4):515–21. doi: 10.1016/j.joca.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth ML, Tripp DA, Harrison MH, Sullivan M, Carson P. Demographic and psychosocial predictors of acute perioperative pain for total knee arthroplasty. Pain Res Manag. 2007;12(3):185–94. doi: 10.1155/2007/394960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dettoni F, Bonasia DE, Castoldi F, Bruzzone M, Blonna D, Rossi R. High tibial osteotomy versus unicompartmental knee arthroplasty for medial compartment arthrosis of the knee: a review of the literature. Iowa Orthop J. 2010;30:131–40. [PMC free article] [PubMed] [Google Scholar]
- 38.Song EK, Seon JK, Park SJ, Jeong MS. The complications of high tibial osteotomy: closing- versus opening-wedge methods. J Bone Joint Surg Br. 2010;92(9):1245–52. doi: 10.1302/0301-620X.92B9.23660. [DOI] [PubMed] [Google Scholar]