Abstract
Objective
Evaluate the efficacy & safety of a novel 0.02% mechlorethamine (MCH) gel in mycosis fungoides (MF).
Design
Multi-center, randomized, observer-blinded, active controlled trial, comparing 0.02% MCH gel with 0.02% MCH compounded ointment. MCH was applied once daily for up to 12 months. Tumor response and adverse events (AEs) were assessed every month (months 1–6) and every 2 months (months 7–12). Serum drug levels were drawn on a subset of subjects.
Setting
Academic medical and/or cancer centers
Patients
260 Stage IA-IIA MF patients who had not used topical MCH within 2 years and were naïve to prior use of topical carmustine therapy.
Main Outcome Measures
Response rates (RRs) of all subjects based on primary (composite assessment of index lesion severity - CAILS) and secondary (modified severity weighted assessment tool (mSWAT) and time to response analyses) clinical endpoints.
Results
RR for MCH gel and ointment were 59% vs. 48% by CAILS and 46.9% vs. 46.2% by mSWAT, respectively. The ratio of CAILS RR of MCH gel to ointment was 1.23 (CI: 0.97–1.55), which met the pre-specified criterion for non-inferiority. Time to response demonstrated superiority of MCH gel to ointment (p<0.012). No drug-related serious AEs were seen. Twenty percent (20%) of enrolled patients on gel and 17% on ointment withdrew due to drug-related skin irritation. No systemic absorption of the study medication was detected.
Conclusions
These results demonstrate the safety and efficacy of a novel 0.02% MCH gel in the treatment of MF patients.
Clinical Trial Registration
Introduction
Mechlorethamine (methyl – bis (2-chloroethyl) amine) hydrochloride, commonly known as nitrogen mustard, is an alkylating agent. First reported in 1946 to be active in lymphoid malignancies 1 mechlorethamine (MCH) was the first systemic chemotherapy agent to be approved in the United States for the treatment of Hodgkin’s disease, lymphosarcoma, chronic myelocytic or lymphocytic leukemia, polycythemia vera, bronchogenic carcinoma and mycosis fungoides (MF), the most common type of cutaneous T-cell lymphoma (CTCL) 2.
Successful use of MCH as a topical agent in the treatment of MF/CTCL was first reported in the1950s 3, and provided a rationale for skin-directed chemotherapy that minimized systemic toxicity. Initially, lyophilized MCH (Mustragen®) was dissolved into water and painted onto the skin 4. Application of aqueous solutions was complicated by high rates (up to 67%) of delayed type cutaneous hypersensitivity5, often limiting its prolonged use. Subsequently MCH was compounded into petrolatum-based ointments that resulted in lower and tolerable rates of delayed type cutaneous hypersensitivity (≤10%) 6.
Large, uncontrolled case series have documented the safety and efficacy of MCH formulations in the treatment of MF/CTCL with overall response rates of 63%–83% (in clinical stages I–III)6–16. Complete responses to topical MCH in early stage (IA) MF/CTCL are associated with lower risk of disease progression 6,17,18. As such, topical MCH is considered a primary treatment of MF/CTCL in treatment guidelines 19,20.
Despite the well-documented clinical experience with topical MCH treatment of MF/CTCL, there is no United States Food and Drug Administration (FDA) approved topical MCH formulation. This has become problematic for physicians and patients for several reasons. Pharmacy-compounded MCH formulations are not subject to rigorous quality assurance, such as evaluations of potency and stability. Further, most health insurance formularies rarely include compounded medicines or those without FDA approval. Additionally, petrolatum-based ointments may often be difficult to apply to the skin, are esthetically unappealing and can compromise patient compliance.
To address these needs, a randomized, controlled, multi-center, observer-blind, pivotal trial was conducted to evaluate the safety and efficacy of a novel 0.02% MCH gel versus a compounded 0.02% MCH ointment.
Patients and Methods
Patient Eligibility
Established MF/CTCL patients with persistent or recurrent stage IA, IB and IIA and no history of progression beyond T2N1M0B0 (stage IIA) with at least one prior treatment were eligible. Patients previously treated with carmustine were excluded as were patients treated with topical MCH within two years or radiation therapy (local or total body) within one year. A skin biopsy of a representative lesion, obtained within ninety days prior to study initiation and after a four week washout period of treatments directed at the disease, was deemed diagnostic of MF/CTCL utilizing the histologic criteria previously employed in clinical trials for MF 21 and also incorporating a diagnostic algorithm for defining early MF/CTCL 22. Histologic variants, folliculotropic/syringotropic MF and large cell transformation (LCT) were eligible. Participants were free of concurrent illness or infection, met laboratory criteria and were adequately washed out of MF/CTCL therapies for four weeks prior to the start of study. Male and female study subjects were required to use contraception during the trial and women could not be pregnant or breast-feeding. Institutional Review Board approval of the clinical trial was obtained at all study sites and all patients provided written informed consent prior to enrollment.
Study Design
This study was a randomized, controlled, observer-blinded, multi-center clinical trial involving thirteen medical and/or cancer centers in the United States and registered as a pivotal phase II trial (NCT00168064). Subjects were randomized to receive either a 0.02% MCH gel (provided by Ceptaris Therapeutics, Inc., 101 Lindenwood Drive, Malvern, PA 19355) or a compounded 0.02% MCH ointment (provided by Ceptaris Therapeutics, Inc. through a contracted compounding pharmacy). The study drug, 0.02% MCH gel, was manufactured under United States Food and Drug Administration approved good manufacturing practice guidelines and formulated to enhance ease of application to the skin. The comparator, 0.02% MCH ointment, was compounded into a petrolatum-based vehicle (Aquaphor®) as previously described11. MCH was applied once daily to specific lesions or total skin surface (depending on T classification) for 12 months. If new lesions appeared in untreated areas, as is common with initiation of topical MCH therapy 6, patients were converted from spot treatment to regional or whole body treatment. Temporary reduction in daily application frequency (every other day or less frequently) for skin adverse events (AEs) was permitted. Tumor response and toxicity were assessed (by an observer blinded to treatment type) every month during the initial 6 months and every 2 months thereafter. The target enrollment of 260 patients was achieved in this study.
Response Criteria
The primary efficacy endpoint was the Composite Assessment of Index Lesion Severity (CAILS)23,24. Up to five index lesions were identified at baseline and assessed throughout the study in all patients. Because the study also included regional and total skin surface applications, the modified Severity Weighted Assessment Tool (mSWAT)24,25 was employed as a secondary efficacy endpoint in all patients. A determination of the percentage involvement of total body surface area, and, if applicable, assessment of clinically abnormal lymph nodes (≥1.5 cm diameter) was completed at baseline and throughout the twelve-month study. CAILS and mSWAT scores were calculated at baseline (day 1) and each study visit the response was determined using standard oncology criteria for complete response (CR: 100% improvement with score = 0), partial response (PR: ≥50% to <100% reduction from baseline score) and stable disease (SD: <50% reduction from baseline score). Confirmed responses were those observed for ≥ 4 weeks. A patient was considered to have progressive disease (PD) if their CAILS score was ≥25% above baseline. Because the appearance of new lesions is common with the initiation of topical MCH application, investigator discretion and patients’ best interest determined if a patient was withdrawn when PD was documented. Duration of response (DOR) was defined in the clinical trial protocol as the time from the first appearance of the confirmed response to the first assessment where loss of response (LOR) (CAILS score <50% improvement from baseline) or PD was documented. An additional definition for LOR i.e., increase of CAILS score of greater than the sum of the lowest CAILS score (nadir) plus 50% baseline CAILS score, was also utilized based on consensus guidelines recommendations24. As with PD, investigator discretion and patients’ best interest determined if a patient was withdrawn from the study when LOR was documented.
Safety Evaluation
Standard laboratory blood tests (chemistries and complete blood count with differential), physical examinations and AE monitoring were used to evaluate patient safety. Toxicities were evaluated and graded according to the NCI Common Toxicity Criteria of Adverse Events (NCI CTCAE) Version 3.0. AEs were recorded and classified by SMQ (Standardized MedDRA Query) and MedDRA Preferred Terms. Systemic absorption of MCH was measured with a HPLC serum assay from a subset of subjects who agreed to have blood levels drawn on day 1 (0, 1, 3, 6 hours after application) and at 4 weeks. Treatment-limiting AEs were defined as grade 3 or 4 local dermal irritation (on a 4-point scale per protocol and by the NCI CTCAE) that did not resolve to ≤grade 2 within 2 weeks off study drug. Grade 3 or 4 local dermal irritation associated with a positive patch was considered allergic contact dermatitis; others were considered irritant contact dermatitis. For grade 3 or 4 local dermal irritation, treatment frequency was suspended or reduced for up to a maximum of 4 weeks and only resumed after irritation improved to grade 2 or lower. Treatment for skin irritation included topical emollients and systemic antihistamines but use of topical or systemic corticosteroids was prohibited. Patients with positive patch testing (allergic contact dermatitis) associated with grade 3 or 4 reactions were withdrawn from the study. Patients were evaluated for the development of squamous cell carcinomas of the skin for an additional 12 months following treatment.
Statistical Methods
A non-inferiority statistical endpoint was chosen to show that a novel MCH gel formulation is statistically (and clinically) not inferior to a pharmacy-compounded MCH ointment given that their performance would be expected to be similar. All randomized patients were included in the intent-to-treat (ITT) population and were evaluated by CAILS as the primary efficacy endpoint. All patients who were treated for at least six months were included in the protocol-defined efficacy evaluable (EE) population. The safety population included all patients who used any study medication. Non-inferiority of MCH gel to MCH ointment was established if the lower bound of the 95% confidence interval (CI) around the ratio of the RR (CR+PR for MCH gel/MCH ointment) was ≥0.75 Kaplan-Meier (KM) methodology was used to calculate the time to first confirmed response and duration of response curves. Treatment arms were compared using a log-rank statistic.
Results
Patient Characteristics
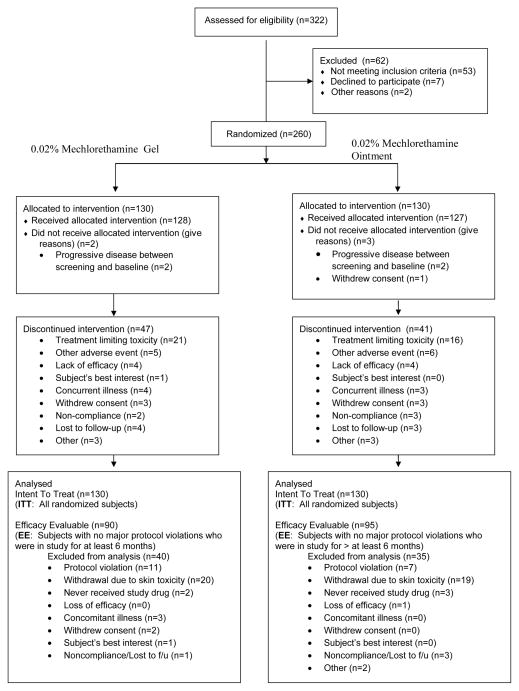
Figure 1 is a flow diagram of subjects assessed, enrolled, randomized and followed throughout the trial. Table 1 summarizes the baseline characteristics of all 260 patients enrolled and demonstrates the similarities in the two treatment arms. Overall, there were 59% men and 41% women with 74% Caucasians, 13% African-Americans and 12% of other race. The median age was 58 (range 11–88). Overall, 54% of the patients had stage IA disease at baseline (59% and 50% for MCH gel and ointment, respectively) and 44% had stage IB disease (40% and 49% for MCH gel and ointment, respectively). Few patients in either arm (2 each) had stage IIA disease at baseline. The median number of prior treatments was 2 (range 1–12).
Figure 1.
Flow diagram of subject progress through the randomized trial.
Table 1.
Patient Characteristics
| Characteristic | MCH Gel (N=130) n (%) | MCH Ointment (N=130) n (%) |
|---|---|---|
| Gender | ||
| Male | 77 (60) | 77 (59) |
| Female | 53 (40) | 53 (41) |
| Race | ||
| Caucasian | 97 (75) | 96 (74) |
| Afro-American | 16 (12) | 19 (15) |
| Other | 17 (13) | 15 (11) |
| Age | ||
| <18 years | 0 (0) | 1 (1) |
| 18–64 years | 93 (72) | 86 (66) |
| 65–74 years | 29 (22) | 33 (25) |
| ≥75 years | 8 (6) | 10 (8) |
| Prior MF Therapies* | ||
| Topical Corticosteroids | 112 (86) | 113 (87) |
| Phototherapy | 50 (39) | 53 (41) |
| Bexarotene (topical & oral) | 23 (18) | 23 (18) |
| Topical NM (>2yrs from study) | 16 (12) | 13 (10) |
| Interferons | 3 (2) | 5 (4) |
| Methotrexate | 3 (2) | 3 (2) |
| Radiation (local & total skin) | 3 (2) | 2 (2) |
| Other* | 14 (11) | 34 (26) |
| MF Stage | ||
| Stage IA | 76 (59) | 65 (50) |
| Stage IB | 52 (40) | 63 (49) |
| Stage IIA | 2 (1) | 2 (1) |
“Other” includes primarily emollients, anti-bacterials, anti-fungals, and retinoids other than bexarotene.
Efficacy Results
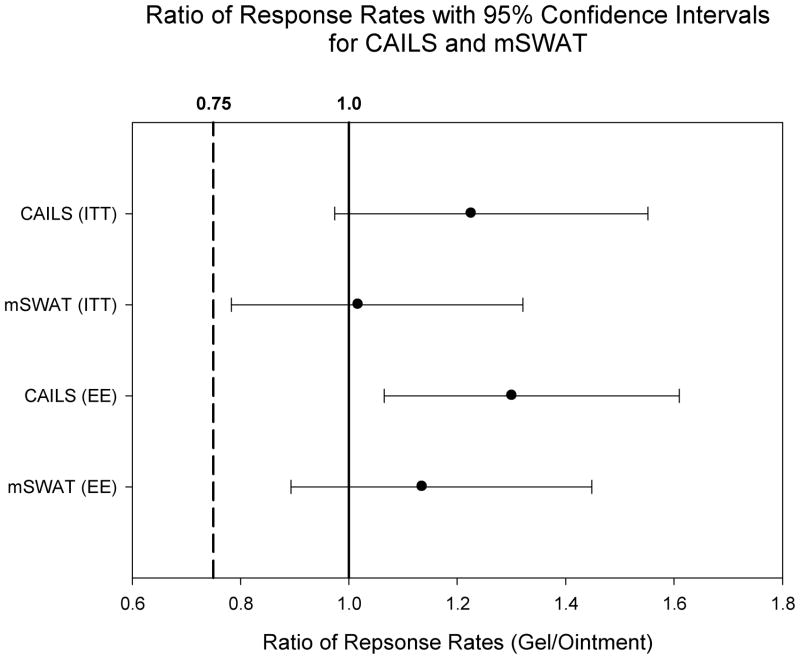
The CAILS RRs are summarized in Table 2. In the ITT population the confirmed RR (CR+PR) was higher for 0.02% MCH gel than 0.02% MCH ointment (59% vs. 48%). The ratio of the RR of MCH gel to ointment was 1.23 (95% CI: 0.97–1.55), meeting the pre-specified criterion for non-inferiority (Figure 2). In the EE population, 77% of patients receiving 0.02% MCH gel vs. 59% of patients receiving 0.02% MCH ointment achieved a confirmed CAILS response (RR ratio 1.30 [95% CI: 1.06–1.61], p=0.011) (Figure 1).
Table 2.
CAILS Treatment Response, n (%)
| ITT Population | ||
|---|---|---|
| CAILS Outcome | MCH Gel (N=130) | MCH Ointment (N=130) |
| Response, N (%) | 76 (59) | 62 (48) |
| Complete | 18 (14) | 15 (12) |
| Partial | 58 (45) | 47 (36) |
| Non-Response, N (%) | 54 (41) | 68 (52) |
| Stable Disease | 42 (32) | 61 (47) |
| Progressive Disease | 5 (4) | 3 (2) |
| Unevaluable | 7 (5)* | 4 (3)* |
| Efficacy Evaluable Population | ||
|---|---|---|
| CAILS Outcome | MCH Gel (N=90) | MCH Ointment (N=95) |
| Response | 69 (77) | 56 (59) |
| Complete | 17 (19) | 14 (15) |
| Partial | 52 (58) | 42 (44) |
| Non-Response | 21 (23) | 39 (41) |
| Stable Disease | 19 (21) | 39 (41) |
| Progressive Disease | 2 (2) | 0 (0) |
includes patients who never received study drug (n=5) or withdrawn without post-baseline assessment for treatment limiting toxicity (n=5) or non-compliance (n=1).
Figure 2.
Ratios of response rates for primary (CAILS) and secondary (mSWAT) efficacy endpoints in ITT and EE populations with 95% confidence intervals greater than non-inferiority threshold (0.75).
Subset analysis by strata revealed relative balance between stratum 1 (stage IA), n = 141 and stratum 2 (stages IB/IIA), n = 119. A 59% overall CAILS RR (in the ITT population) for 0.02% MCH gel versus 40% for 0.02% MCH ointment was seen in stratum 1(RR ratio 1.48 [95% CI: 1.05–2.14]). Stratum 2 subjects revealed a 57% overall RR for gel versus 55% for ointment (RR ratio 1.04 [95% CI: 0.75–1.43]). There were 8 patients with folliculotropic/syringotropic and LCT variants in the study (5 with folliculotropic, 1 with syringotropic, 1 with LCT and 1 with folliculotropic and LCT changes). Of the 6 who completed the study, 4 demonstrated a CAILS response when treated with MCH 0.02%. The responders included 1 PR in the gel arm (folliculotropic) and 3 PRs in the ointment arm (2 folliculotropic and 1 folliculotropic/LCT).
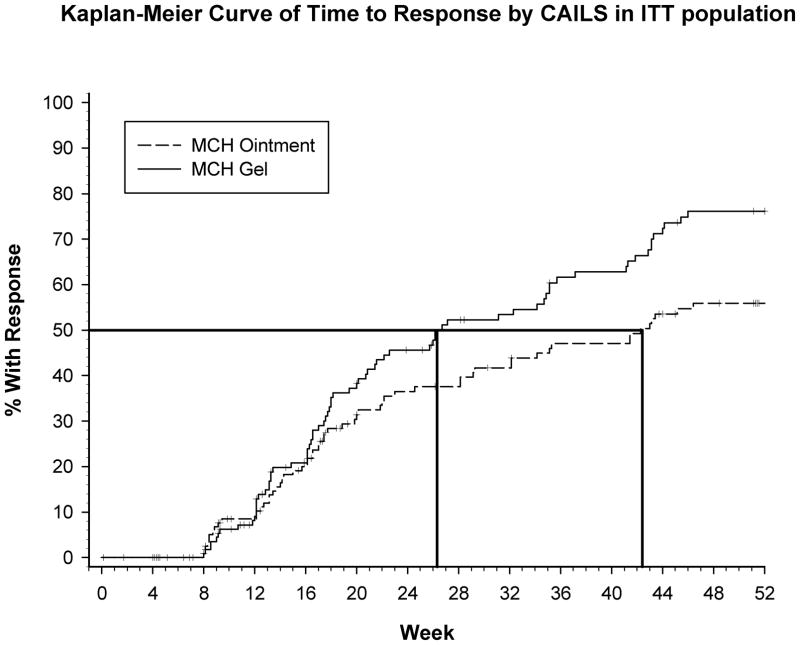
Time to response was evaluated by K-M analysis and revealed that the estimated time to a 50% RR in the MCH gel arm was 26 (95% CI: 20.71 to 35.14) weeks and 42 (95% CI: 29.14 to 53.00) weeks in the MCH ointment arm. Therefore, the gel attained a 50% RR approximately 16 weeks sooner than the ointment. In addition, the RR improved the longer patients were treated with MCH. Approximately 46% of patients treated with gel achieved a confirmed response at 24 weeks and 76% achieved a confirmed response at 52 weeks. Of patients treated with ointment approximately 37% achieved a confirmed response at 24 weeks and approximately 56% achieved a confirmed response at 52 weeks. Finally, patients treated with 0.02% MCH gel had a higher response rate than patients treated with 0.02% MCH ointment beginning at approximately 16 weeks through 52 weeks of treatment (Figure 3). Time to response demonstrated superiority of MCH gel to ointment (p<0.012).
Figure 3.
Kaplan-Meier curve of time to response by CAILS response in ITT population: 50% RR in the MCH gel arm was at 26 weeks and at 42 weeks in the MCH ointment arm.
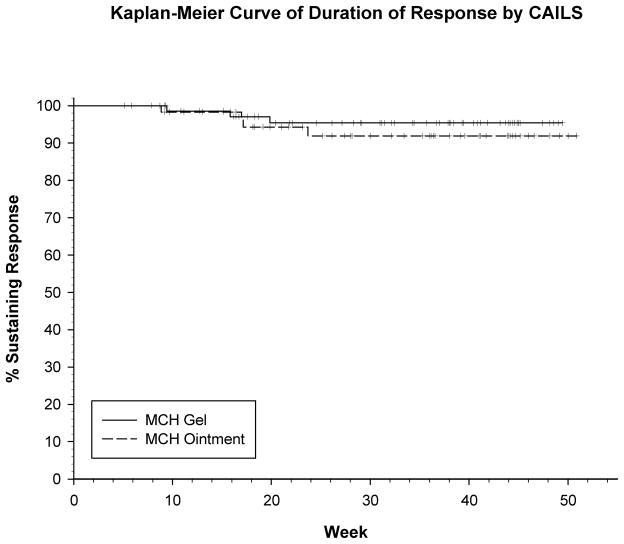
Duration of response (DOR) based on CAILS score in the ITT population was analyzed in those patients who achieved a response (76 on gel; 62 on ointment), utilizing both the protocol (PD and/or LOR of < 50% baseline score) and consensus definition (PD and/or increase of greater than nadir plus 50% baseline score) 24 of DOR. The same duration was seen with both definitions for all but 8 patients (4 in each treatment arm) who had a longer duration using the new consensus definition. Sixty-five of seventy-six (86%) patients on the gel arm and 51/62 (82%) patients on the ointment arm maintained their response through the end of the trial (12 months). Based on the K-M analysis, there was no statistically significant difference between the two treatment arms with respect to DOR (p=0.481 log-rank) and based on the Kaplan-Meier analysis, it is estimated that at least 90% of responses (using new consensus guidelines) will be maintained for 10+ months, the maximum follow-up in the trial (Figure 4).
Figure 4.
Kaplan-Meier curve of duration of response (DOR) by CAILS score in ITT population: at least 90% of responses will be maintained for 10+ months with no statistical significant difference between the two treatment arms (p=0.481 log-rank).
The secondary efficacy endpoint, mSWAT, demonstrated similar results with RR of 47% and 46% for MCH gel and ointment, respectively (RR ratio 1.02 [95% CI = 0.78–1.32]) in the ITT population. In the EE population the RR was 63% and 56% for MCH gel and ointment, respectively (RR ratio 1.14[95% CI = 0.89–1.49]). (Figure 2).
The CR rate ranged from 12–19% in both arms of the ITT and EE populations. Thirty-three percent (33%) and 24% of MCH gel and ointment, respectively, achieved a 90% reduction in CAILS while 52% and 38% reached a 75% reduction in CAILS. Fifteen patients randomized to the MCH gel arm and 10 patients randomized to the MCH ointment arm had PD at some time during the study. However, the majority of patients remained on treatment. Seven of the patients on the MCH gel arm who stayed on treatment achieved a confirmed response. Only eight patients (5-gel; 3-ointment) met the criterion for PD without impact on their T classification, at the time of their last visit.
Safety and Tolerance
No drug-related severe adverse events were reported during this trial. Sixty-two percent and 50% of patients who received MCH gel and ointment, respectively, reported at least one AE that was considered to be related to study drug. The vast majority of AEs in both arms were skin related, characterized mainly as local dermatitis (skin irritation)(Table 3). There was a higher incidence of skin irritation in the gel arm (p=0.040). There were no statistically significant differences in overall incidence of AEs or any other subcategory between the gel and ointment arms.
Table 3.
Adverse Events in the Skin Occurring in ≥5% of Patients
| Skin-related Adverse Event | PG (N=128) n (%) | AP (N=127) n (%) | All Subjects (N=255) n (%) | p Valuea |
|---|---|---|---|---|
| Skin irritation | 32 (25) | 18 (14) | 50 (20) | 0.040 |
| Pruritus | 25 (20) | 20 (16) | 45 (18) | |
| Erythema | 22 (17) | 18 (14) | 40 (16) | |
| Dermatitis contact | 19 (15) | 19 (15) | 38 (15) | |
| Skin hyperpigmentation | 7 (6) | 9 (7) | 16 (6) | |
| Folliculitis | 7 (6) | 5 (4) | 12 (5) |
P value from Fisher’s exact test
The difference in skin irritation in the MCH gel arm is due primarily to an increased incidence of moderate-moderately severe (grade 2 or 3) local dermal irritation. However, there was no difference between the two treatment arms with respect to the number of patients withdrawn from the trial due to protocol defined treatment limiting skin AE: 26/128 (20.3%) on MCH gel vs. 22/127 (17.3%) on MCH ointment, p=0.631. Examination of the data shows that the majority of withdrawals for treatment limiting skin AE occurred within the first few months, and 90% occurred prior to month six.
Though patch testing was not routinely required in this trial, the incidence of treatment limiting skin AE, 21/128 (16.4%) on the MCH gel arm and 16/127 (12.6%) on the MCH ointment arm, can be used as a conservative estimate of allergic contact dermatitis. Of the 21 patients on the gel arm who withdrew from the study due to treatment limiting skin AE, 13 had a positive patch test, and 8 were not tested; of the 16 patients on the ointment arm withdrawn for a treatment limiting skin AE, 11 had a positive patch test, 2 had a negative patch test, and 3 were not tested.
Clinical laboratory monitoring (hematology and serum chemistry parameters at baseline and months 4, 8 and 12) did not demonstrate any systematic pattern of change in any laboratory value measured, consistent with the lack of systemic absorption. Additionally, HPLC serum assays performed on 16 subjects who received 0.02% MCH gel (0, 1, 3, 6 hours after application on day 1 and at week 4) did not reveal any detectable blood levels or evidence of systemic absorption of MCH (data not shown).
Development of secondary non-melanoma skin cancers was monitored throughout the 12-month trial and an additional 12-month follow-up period. Eleven patients (3-gel; 8-ointment) were diagnosed with 20 non-melanoma skin cancers. These included 9 squamous cell carcinomas of the skin (1/9 occurring in a treatment area), 10 basal cell carcinomas (5/10 occurring in a treatment area) and 1 Merkel cell carcinoma (not occurring in a treatment area). Most non-melanoma skin cancers occurred on sun exposed areas and in patients with a prior history of skin cancers or who had received prior skin-directed therapies, including phototherapy, for the treatment of MF.
Discussion
After decades of reported use of topical MCH in MF/CTCL 3,4,6–16, this clinical trial is the first to evaluate the safety and efficacy of topical MCH chemotherapy by comparing a novel 0.02% MCH gel to a compounded 0.02% MCH ointment in stage IA–IIA MF/CTCL. The enrollment of 260 patients represents the single, largest controlled clinical trial in MF/CTCL for any given treatment. A non-inferiority analysis was chosen to show that the 0.02% MCH gel formulation is statistically (and clinically) not inferior to the 0.02% MCH ointment. The overall RR (CR and PR) of the 0.02% MCH gel was 59% compared to 48% for the compounded 0.02% MCH ointment, as measured by the primary efficacy endpoint (CAILS) within the ITT population. The ratio of the RR of MCH gel to ointment was 1.23 (95% CI: 0.97–1.55). The ratio of RR in each stratum was also within the protocol specified limit for non-inferiority (≥0.75). Differences in the response ratio between stratum 1 (stage IA) and stratum 2 (stage IB/IIA) can be related to advanced disease of stratum 2 and/or patient compliance (more effort to treat larger areas) and varied application method (regional or total body) allowed in the protocol. Thus, the clinical trial’s statistical primary endpoint was achieved and demonstrated the efficacy, safety and tolerability of a novel 0.02% MCH gel formulation.
Comparison of this clinical trial’s overall RR for 0.02% MCH gel (59%) with previously reported RRs of topical MCH is difficult. An 83% overall RR for compounded MCH ointment has been previously reported in retrospective case series 16. Several factors might account for the difference in RRs. Retrospective case series have used an efficacy evaluable population (often referred to as an “as treated” population) and do not include all patients that received a particular treatment, specifically excluding subjects who prematurely withdrew from the study. One concentration of topical MCH (0.02%) was used throughout this trial compared to multiple and higher concentrations (0.01%–0.04%) used in reported case series 6. In contrast to reported case series 5,15,16, no concurrent therapies (especially topical corticosteroids) were permitted during this trial and no additional skin-directed and/or systemic therapies were employed in unresponsive or progressive disease. Also, strict termination rules and restrictive management (concurrent use of topical steroids was prohibited) of skin toxicities (irritant and allergic contact dermatitis) were used in this clinical trial. All of the case series reported RRs using the Physician’s Global Assessment (PGA) in an un-blinded manner, whereas in this study the CAILS was utilized by blinded observers.
It is interesting to note that the time to first response analysis (Figure 2) demonstrates that the longer patients were treated with topical MCH in this trial, the greater number of responses was seen and responses were achieved faster in the gel arm. This suggests that the longer a patient is treated topically with 0.02% MCH gel, the more likely they will respond.
There were no serious or unexpected drug-related adverse events (AEs) observed with either the 0.02% MCH gel or ointment. Sixty-two percent (62%) and 50% drug-related skin AEs were seen with MCH gel and ointment, respectively. The vast majority of AEs were characterized mainly as local skin reactions, with signs and symptoms of either irritant contact dermatitis or allergic contact dermatitis (Table 3). The number of patients withdrawn from the trial for a drug-related skin AE was 20% on MCH gel and 17% on MCH ointment with allergic contact dermatitis estimated at 16% and 13%, respectively. These rates compare favorably to the approximate 10% incidence of allergic contact dermatitis reported in the literature for compounded MCH ointments16, considering the restrictive nature of the clinical trial protocol treatment adjustments (for local skin reactions) and termination rules. In this trial, skin AEs were treated with suspension or application reduction within a 4 week period as well as the allowed use of emollients and oral antihistamines. In contrast, clinical practice and retrospective case series utilized varying MCH concentrations (with dose reduction schedules employed), topical corticosteroids over extended periods of time, and no restrictions of length of drug withheld to manage irritant and allergic contact dermatitis.
This clinical trial provided the first opportunity to perform HPLC serum assays for MCH detection after topical application to the skin in MF/CTCL patients. There was no detectable systemic absorption of study drug in the blood in 16 subjects after 0.02% MCH gel application. These data, along with no observed abnormal trends in clinical laboratories throughout the twelve-month treatment period, corroborate numerous case series that recognized no abnormalities related to systemic absorption of topically applied MCH 6,10,11,14,15.
During a 24-month observation period (12 month treatment and 12 month follow-up period) only 6 non-melanoma skin cancers (1-squamous cell carcinoma; 5-basal cell carcinomas) were detected in treatment areas. Within the limitations of the observation period and uncontrolled confounding variables, these data do not support an obvious association between the development of secondary non-melanoma skin cancers and the daily application of topical 0.02% MCH.
In summary, the results of this randomized, controlled, multi-center clinical trial confirm the non-inferiority of a novel 0.02% MCH gel in the treatment of MF/CTCL when compared to a compounded 0.02% MCH petrolatum ointment. The results also corroborate multiple reports and case series in the medical literature spanning over six decades that have affirmed MCH as an effective topical chemotherapy in MF/CTCL 3,4,6–16 and its inclusion as a frontline therapy in treatment guidelines 19,20. A manufactured 0.02% MCH gel addresses the unmet need for good manufacturing product quality assurance that will improve drug availability for MF/CTCL patients.
Acknowledgments
Funding/Support: This study was supported in part by a FDA Orphan Product Development grant (R01 FD003017)(Dr. Lessin) and by Cepartis Therapeutics, Inc., Malvern, PA.
Role of the Sponsor: Cepartis Therapeutics, Inc. monitored conduct of the study and provided data collection and analysis.
We are indebted to Cathie Leister, Statistical Consultant, Cepartis Therapeutics for statistic analysis of the data. We thank our study patients for their participation and the Cutaneous Lymphoma Foundation for its grass roots support of this project.
Footnotes
Financial Disclosure: Dr. Lessin serves as a consultant to Cepartis Therapeutics, Inc. No other authors have relevant financial interests to report.
Author Contributions: Drs. Lessin and Y. Kim had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lessin and Y. Kim. Acquisition of data: Lessin, Duvic, Guitart, Pandya, Strober, Olsen, Hull, Knobler, Rook, E. Kim, Naylor, Adelson, Kimball, Wood, Wu, Sundram, Y. Kim. Analysis and interpretation of data: Lessin, Y. Kim. Drafting of the manuscript: Lessin, Duvic, Guitart, Y. Kim. Critical revision of the manuscript for important intellectual content: Lessin, Duvic, Guitart, Pandya, Strober, Olsen, Hull, Knobler, Rook, E. Kim, Naylor, Adelson, Kimball, Wood, Wu, Sundram, Y. Kim. Obtained funding: Lessin. Administrative, technical, material support: Lessin, Y. Kim, Sundram, Wu. Study supervision: Lessin.
References
- 1.Goodman L, Wintrobe M, Dameshek W, Goodman M, Gilman A, McLennan M. Nitrogen Mustard Therapy: use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. JAMA. 1946;132:126–132. doi: 10.1001/jama.1946.02870380008004. [DOI] [PubMed] [Google Scholar]
- 2.Bunn P, Hoffman S, Norris D, Golitz L, Aeling J. Systemic therapy of cutaneous T-cell lymphoma (mycosis fungoides and the Sezary syndrome) Ann Intern Med. 1994;121(8):592–602. doi: 10.7326/0003-4819-121-8-199410150-00007. [DOI] [PubMed] [Google Scholar]
- 3.Haserick J, Richardson J, Grant D. Remission of lesions in mycosis fungoides following topical application on nitrogen mustard. Cleve Clin Q. 1959;26:144–147. [PubMed] [Google Scholar]
- 4.Sipos K. Painting Treatment of Nitrogen Mustard in Mycosis Fungoides. Dermatologica. 1965;130:3–11. doi: 10.1159/000254511. [DOI] [PubMed] [Google Scholar]
- 5.Ramsay DL, Meller JA, Zackheim HS. Topical treatment of early cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9(5):1031–1056. [PubMed] [Google Scholar]
- 6.Kim YH, Martinez G, Varghese A, Hoppe RT. Topical nitrogen mustard in the management of mycosis fungoides. Arch Dermatol. 2003;139:165–173. doi: 10.1001/archderm.139.2.165. [DOI] [PubMed] [Google Scholar]
- 7.Van Scott EJ. Complete regressions of mycosis fungoides with topical mechlorethamine hydrochloride. JAMA. 1972;222(9):1172. doi: 10.1001/jama.222.9.1172b. [DOI] [PubMed] [Google Scholar]
- 8.Van Scott EJ, Kalmanson JD. Complete remissions of mycosis fungoides lymphoma induced by topical nitrogen mustard (HN2). Control of delayed hypersensitivity to HN2 by desensitization and by induction of specific immunologic tolerance. Cancer. 1973;32(1):18–30. doi: 10.1002/1097-0142(197307)32:1<18::aid-cncr2820320103>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Price NM. Topical mechlorethamine. Cutaneous changes in patients with mycosis fungoides after its administration. Arch Dermatol. 1977;113(10):1387–1389. doi: 10.1001/archderm.113.10.1387. [DOI] [PubMed] [Google Scholar]
- 10.Vonderheid EC, Van Scott EJ, Wallner PE, Johnson WC. A 10-year experience with topical mechlorethamine for mycosis fungoides: comparison with patients treated by total-skin electron-beam radiation therapy. Cancer Treat Rep. 1979;63(4):681–689. [PubMed] [Google Scholar]
- 11.Price NM, Deneau DG, Hoppe RT. The treatment of mycosis fungoides with ointment-based mechlorethamine. Arch Dermatol. 1982;118(4):234–237. [PubMed] [Google Scholar]
- 12.Hamminga B, Noordijk EM, van Vloten WA. Treatment of mycosis fungoides: total-skin electron-beam irradiation vs. topical mechlorethamine therapy. Arch Dermatol. 1982;118(3):150–153. doi: 10.1001/archderm.118.3.150. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay DL, Parnes RE, Dubin N. Response of mycosis fungoides to topical chemotherapy with mechlorethamine. Arch Dermatol. 1984;120(12):1585–1590. [PubMed] [Google Scholar]
- 14.Ramsay D, Halperin P, Zeleniuch-Jacquotte A. Topical nitrogen mustard therapy for early stage mycosis fungoides. J Am Acad Dermatol. 1988;19(4):684–691. doi: 10.1016/s0190-9622(88)70223-6. [DOI] [PubMed] [Google Scholar]
- 15.Vonderheid EC, Tan ET, Kantor AF, Shrager L, Micaily B, Van Scott EJ. Long-term efficacy, curative potential, and carcinogenicity of topical mechlorethamine chemotherapy in cutaneous T cell lymphoma. J Am Acad Dermatol. 1989;20(3):416–428. doi: 10.1016/s0190-9622(89)70051-7. [DOI] [PubMed] [Google Scholar]
- 16.Kim YH, Martinez G, Varghese A, Hoppe RT. Topical nitrogen mustard in the management of mycosis fungoides: update of the Stanford experience. Arch Dermatol. 2003;139(2):165–173. doi: 10.1001/archderm.139.2.165. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y, Jensen R, Watanabe G, Varghese A, Hoppe R. Clinical stage IA (limited patch and plaque) mycosis fungoides. A long-term outcomes analysis. Arch Dermatol. 1996;132(11):1309–1313. [PubMed] [Google Scholar]
- 18.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139(7):857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 19.Whittacker S, Marsden J, Spittle M, Jones R. Joint British Association of Dermatologists and U.K. cutaneous lymphoma group guidelines for management of primary cutaneous T cell lymphoma. Br J Dermatol. 2003;149(6):1095–1107. doi: 10.1111/j.1365-2133.2003.05698.x. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology Version 4.2011. Non-Hodgkin’s lymphomas. Mycosis fungoides/Sezary syndrome. 2011 ( www.nccn.org)
- 21.Duvic M, Olsen EA, Omura GA, et al. A phase III, randomized, double-blind, placebo-controlled study of peldesine (BCX-34) cream as topical therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2001;44(6):940–947. doi: 10.1067/mjd.2001.113478. [DOI] [PubMed] [Google Scholar]
- 22.Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53(6):1053–1063. doi: 10.1016/j.jaad.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 23.Heald P, Mehlmauer M, Martin AG, Crowley CA, Yocum RC, Reich SD. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49(5):801–815. doi: 10.1016/s0190-9622(03)01475-0. [DOI] [PubMed] [Google Scholar]
- 24.Olsen E, Whittaker S, Kim Y, et al. Clinical Endpoints and Response Criteria in Mycosis Fungoides and Sézary Syndrome: a Consensus Statement of the International Society for Cutaneous Lymphomas (ISCL), the United States Consortium for Cutaneous Lymphomas (USCCL) and the Cutaneous Lymphoma Task Force of the European Organization for Research and Treatment of Cancer (EORTC) J Clin Oncol. 2011;29(18):2598–2607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens SR, Ke MS, Parry EJ, Mark J, Cooper KD. Quantifying skin disease burden in mycosis fungoides-type cutaneous T-cell lymphomas: the severity-weighted assessment tool (SWAT) Arch Dermatol. 2002;138(1):42–48. doi: 10.1001/archderm.138.1.42. [DOI] [PubMed] [Google Scholar]
- 26.Breneman D, Duvic M, Kuzel T, Yocum R, Truglia J, Stevens VJ. Phase 1 and 2 trial of bexarotene gel for skin-directed treatment of patients with cutaneous T-cell lymphoma. Arch Dermatol. 2002;138(3):325–332. doi: 10.1001/archderm.138.3.325. [DOI] [PubMed] [Google Scholar]